Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Accessing heavy allyl-analogous [(TerN)2E]− (E = Sb, Bi) ions and their reactivity towards ECl3†
Alexander
Hinz
a,
Axel
Schulz
*ab and
Alexander
Villinger
a
aInstitut für Chemie, Universität Rostock, Albert-Einstein-Str. 3a, 18059 Rostock, Germany. E-mail: axel.schulz@uni-rostock.de
bAbteilung Materialdesign, Leibniz-Institut für Katalyse e.V. an der Universität Rostock, Albert-Einstein-Str. 29a, 18059 Rostock, Germany
First published on 12th June 2015
Abstract
The attempted preparation of the biradicaloid [E(μ-NTer)]2 (E = Sb, Bi) yielded salts of the anion [(TerN)2E]−. These heteroatom allyl analogues could be further utilized in the reaction with pnictogen(III) chlorides to form the first 1,3-dichloro-1-bisma-3-stiba-2,4-diazane [ClSb(μ-NTer)2BiCl].
In recent research, allyl-analogous group 15 anions of the type [(R–E1)2E2]− (E1,2 = N, P, As) have attracted great interest (Scheme 1). Triazenides, the lightest examples of such anions, were identified as redox-active ligands by Kaim et al. only in 2013.1 For their structural aspects, Niemeyer et al. investigated several unsymmetrically substituted triazenides and found intriguing aggregation behaviour that shows dependence on the cation.2–4 In contrast, the heavier homologue, [(R–N)2P]−, was targeted less often. The NPN anion was studied by Niecke et al. in the early 1990s5,6 and were recently utilized in the preparation of an arsa-phospha-diazane.7 Roesky et al. were the first to investigate [(R–N)2As]−, which was obtained as salt of Zn2+ and Cd2+ upon deprotonation of the acid of [(R–N)2As]− with metal amine bases [MN(SiMe3)22] (M = Zn, Cd).8 Substitution of the pnictogens bearing the organic substituents from R–N to R–P lead to the formation of highly sensitive compounds, of which several metal complexes were investigated by the groups of Niecke (NPP),9 Lerner (PPP),10 Jutzi (PPP, PAsP),11 Scheer (PPP, PAsP),12,13 and Wright (PAsP).14 These highly reactive anions can be stabilized by bulky substituents, as for example m-terphenyls, which were systematically studied by Power et al. They have been employed frequently in both transition metal and main group element chemistry. The use of m-terphenyls enabled the study of several unusual bonding situations,15–17 ranging from Cr–Cr quintuple bonds18 to M–E triple bonds (Re
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ge, Mo
Ge, Mo![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si, W
Si, W![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Sn)19–21 and several examples of multiple bonds between heavy main group elements.22,23 These heavy element multiply bonded species can exhibit biradical character, as was shown for the digermyne in the reactions with small molecules.24–26 Complementing the variety of highly reactive species stabilized by m-terphenyls, Power et al. were able to synthesise heavy group 14 carbene analogs.27
Sn)19–21 and several examples of multiple bonds between heavy main group elements.22,23 These heavy element multiply bonded species can exhibit biradical character, as was shown for the digermyne in the reactions with small molecules.24–26 Complementing the variety of highly reactive species stabilized by m-terphenyls, Power et al. were able to synthesise heavy group 14 carbene analogs.27
 | ||
| Scheme 1 Selected examples for known group 15 allyl-analogues (Ter = 2,6-bis(2,4,6-trimethylphenyl)-phenyl, Dip = 2,6-diisopropyl-phenyl, Mes* = 2,4,6-tri-tertbutyl-phenyl). | ||
In the course of our investigations concerning small group 15 heterocycles, we succeeded in the preparation of the singlet biradicaloids [P(μ-NTer)]2 and [As(μ-NTer)]2 (Ter = 2,6-bis(2,4,6-trimethylphenyl)-phenyl).28–31 The chemistry of biradicaloids was recently reviewed by Abe and Breher.32,33 Computations predicted a considerably larger biradical character for the heavier congeners, [Sb(μ-NTer)]2 and [Bi(μ-NTer)]2,34,35 rendering them attractive preparative targets.36,37
In this contribution, we present the first examples of group 15 heteroatom allyl-analogues featuring the heaviest pnictogens (Sb and Bi) and m-terphenyl substituents and investigated their reactivity towards pnictogen(III) halides.
The starting materials, [ClSb(μ-NTer)]2 and [ClBi(μ-NTer)]2, are already known38,39 and were reduced with utilizing magnesium in THF (Scheme 2), KC8 in toluene or benzene, and the benzophenone ketyl radical in THF (cf. ESI†). Upon reduction with magnesium, the solution of the dichloro-cyclo-dipnictadiazanes 1E turned dark blue for [(Ter–N)2Sb]MgCl·THF (2Sb) and dark green for [(Ter–N)2Bi]MgCl·THF (2Bi) from initially yellow and red, respectively. After workup, crystalline material of black appearance was obtained in both cases. Even though both compounds are thermally stable (2Sb: Tdec = 228, 2Bi: Tdec = 246 °C), they are very sensitive towards air and water. The use of KC8 instead of magnesium as a reducing agent did not afford the heavy biradicaloids either. When utilized in a stoichiometric ratio, a dark greenish mixture of products was obtained. After fractional crystallisation, only considerable amounts of the starting material were obtained in numerous experiments, even though its colour ranged from nearly colourless via the expected yellow of [ClSb(μ-NTer)]2, but even orange, green and black crystals were grown due to small amounts of highly coloured impurities. When an excess of KC8 was used, colourless [K(μ-N(H)Ter)]2 was obtained (cf. ESI†). Similar over-reduction occurred, when [ClBi(μ-NTer)]2 and Mg were stirred for three days, affording colourless imido complex [TerN(MgCl)2]2·4THF (cf. ESI†).
IR data for both 2Sb and 2Bi are very similar. The slight differences observed barely exceed the measurement accuracy. The in-plane N–E–N deformation vibration was observed at 876 cm−1 for 2Sb and at 870 cm−1 for 2Bi, respectively. The E–N stretch is observed at 686 (2Sb) and 676 cm−1 (2Bi) in good agreement with computed data (cf. ESI†).40
The heavy allyl analogs 2Sb and 2Bi crystallise isotypically from benzene in the monoclinic space group P21/n. The molecular structures feature short N–E bonds, displaying considerable double bond character (Fig. 1, 2Sb: 1.963, 1.971; 2Bi: 2.075, 2.086, ∑rcov(N = Sb) = 1.93, ∑rcov(N = Bi) = 2.01, cf. [ClSb(μ-NTer)]2 2.051(2), 2.063(2); [ClBi(μ-NTer)]2 2.151(2), 2.169(3) Å).41 There are acute N–E–N angles in both species 2 (2Sb: 85.97(6); 2Bi: 82.78(8)°) due to an s-type lone pair of the heavy pnictogen and bond orbitals mainly composed of p-atomic orbitals (cf.Table 1).
| 2P | 2As | 2Sb | 2Bi | |
|---|---|---|---|---|
| q(E) | +1.22 | +1.23 | +1.33 | +1.40 |
| q(N) | −1.09 | −1.09 | −1.13 | −1.15 |
| s character of E lone pair [%] | 70 | 77 | 82 | 86 |
| HOMO–LUMO gap [kJ mol−1] | 361 | 306 | 267 | 251 |
| λ max [nm] | 401 | 474 | 581 | 662 |
The intensive blue colour can be rationalized with the aid of DFT computations. The computed frontier orbitals are in good agreement with the ones sketched by Niecke et al. for [(R–N)2P]− ions.6 The HOMO → LUMO excitation represents an π → π* transition, with the π* orbital featuring large coefficients for a p atomic orbital at the E center perpendicular to the N2E plane (Fig. 2, 2Sb: λmax = 585; 2Bi: λmax = 697; computed: 2Sb: λmax = 581; 2Bi: λmax = 662 nm).
 | ||
| Fig. 2 Computed molecular orbitals of 2Sb (HOMO left and LUMO right).42 | ||
NBO analysis of the series of [(R–N)2E]− anions42 revealed clear trends. The E lone pair is of increasing s character in the sequence P (70%) < As (77%) < Sb (82%) < Bi (86%). In contrast, the N lone pairs possess considerable p orbital contributions (sp1.7 hybrids). The NEN moiety is strongly polarized for which the charge distribution is a good estimate (E = P: q(N) −1.09, q(P) +1.22; E = As: q(N) −1.09, q(As) +1.23; E = Sb: q(N) −1.13, q(Sb) +1.33; E = Bi: q(N) −1.15, q(Bi); +1.40 e). The HOMO–LUMO gap decreases in the series towards heavier pnictogens (cf.Table 1).
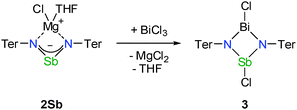
Further investigations targeted the metathesis reaction of 2Sb and 2Bi with pnictogen(III) halides. The reaction of 2Sb and 2Bi with the chloride of the same pnictogen yielded the starting material, [ClSb(μ-NTer)]2 and [ClBi(μ-NTer)]2, respectively. This might be a useful approach to dichloro-cyclo-dipnictadiazanes, if another synthesis of the allyl-analogous anions 2E can be found. When 2Sb or 2Bi was reacted with one equivalent of a AsCl3 (Scheme 3), no clean reaction was observed. Upon employing an excess of AsCl3, substitution of the heavy pnictogen occurred, and [ClAs(μ-NTer)]2 was obtained. However, by conversion of 2Sb with BiCl3 it was possible to synthesize the first 1,3-dichloro-1-bisma-3-stiba-2,4-diazane (3), [ClSb(μ-NTer)2BiCl], in good yield as bright red crystals (Scheme 4).
Compound 3 crystallized isotypic to [ClSb(μ-NTer)]2 and [ClBi(μ-NTer)]2 in the orthorhombic space group Pbca. The molecular structure features the trans isomer with N–E and E–Cl single bonds (Fig. 3, 2Sb: N–Sb 2.037, 2.038, 2Bi: N–Bi 2.193, 2.183; 3: Sb1–Cl2 2.44(2), Bi1–Cl1 2.55(2) Å) in good agreement with the values observed for [ClSb(μ-NTer)]2 and [ClBi(μ-NTer)]2 (cf. Sb–Cl 2.4321(4), Bi–Cl 2.537(2) Å).
The bisma-stiba-diazane 3 is thermally stable (Tdec = 266 °C) and can be prepared in bulk. The solution 1H and 13C NMR spectra indicate the exclusive existence of the trans isomer, in which all ortho-CH3 groups are equivalent in solution at ambient temperature due to intramolecular dynamics. The ν(E–Cl) vibrations were found at 280 and 265 cm−1, which compares well with the known values for [ClSb(μ-NTer)]2 (284, 258 cm−1) and [ClBi(μ-NTer)]2 (274, 256 cm−1). The in-phase combination of E–N–E deformation vibrations was observed at 188 cm−1 for 3 (Sb, Bi), while the respective vibration was found at 216 cm−1 in [ClSb(μ-NTer)]2 and at 150 cm−1 in [ClBi(μ-NTer)]2. At higher wavenumbers, the combination of in-phase N–E–N deformation vibrations was assigned (3: 888; [ClSb(μ-NTer)]2 894; [ClBi(μ-NTer)]2 876 cm−1).
In conclusion, the reduction of the heavy dichloro-dipnictadiazanes did not lead to the desired biradicaloids, which we assess to be beyond the reach of synthetic methods at the moment. Possibly, other substituents feature suitable steric and electronic properties will enable the stabilisation of the heavy biradicaloids [Sb(μ-NTer)]2 and [Bi(μ-NTer)]2, which remains a challenge. However, the synthesis of 2Sb and 2Bi filled a gap in main group chemistry in the heavy group 15 allyl-analogues and enabled the synthesis of the first dichloro-cyclo-bisma-stiba-diazane, which may prove a suitable starting material for the generation of reactive cations, radicals or Sb/Bi nitrides.43 The metathesis reactivity of cyclic N–E bonded species was previously utilised in the preparation of [ClSb(μ-NTer)]2 and [ClBi(μ-NTer)]2 from [Sn(μ-NTer)]2, a preparative method which we could extend to metathesis from [ClSb(μ-NTer)]2 and [ClBi(μ-NTer)]2 to the lighter congener [ClAs(μ-NTer)]2.
DFG (SCHU 1170/11-1) is gratefully acknowledged for financial support. The authors thank M. Sc. Jonas Bresien for setting up and maintaining Gaussian and NBO software on the cluster computer as well as our own workstation computers.
Notes and references
- F. Ehret, M. Bubrin, S. Záliš and W. Kaim, Angew. Chem., 2013, 125, 4771–4773 ( Angew. Chem., Int. Ed. , 2013 , 52 , 4673–4675 ) CrossRef PubMed.
- S.-O. Hauber, F. Lissner, G. B. Deacon and M. Niemeyer, Angew. Chem., 2005, 117, 6021–6025 ( Angew. Chem., Int. Ed. , 2005 , 44 , 5871–5875 ) CrossRef PubMed.
- H. Lee, S.-O. Hauber, D. Vinduš and M. Niemeyer, Inorg. Chem., 2008, 47, 4401–4412 CrossRef CAS PubMed.
- S. L. Hyui and M. Niemeyer, Inorg. Chem., 2006, 45, 6126–6128 CrossRef PubMed.
- E. Niecke, R. Detsch and M. Nieger, Chem. Ber., 1990, 123, 797–799 CrossRef CAS PubMed.
- R. Detsch, E. Niecke, M. Nieger and W. W. Schoeller, Chem. Ber., 1992, 125, 1119–1124 CrossRef CAS PubMed.
- A. Hinz, A. Schulz and A. Villinger, Angew. Chem., 2014, 127, 678–682 ( Angew. Chem., Int. Ed. , 2014 , 54 , 668–672 ) CrossRef PubMed.
- U. Wirringa, H. W. Roesky, M. Noltemeyer and H.-G. Schmidt, Angew. Chem., 1993, 105, 1680–1681 ( Angew. Chem., Int. Ed. , 1993 , 32 , 1628–1630 ) CrossRef CAS PubMed.
- E. Niecke, B. Kramer and M. Nieger, Organometallics, 1991, 10, 10–11 CrossRef CAS.
- H.-W. Lerner, I. Sänger, F. Schödel, A. Lorbach, M. Bolte and M. Wagner, Dalton Trans., 2008, 787–792 RSC.
- P. Jutzi and U. Meyer, J. Organomet. Chem., 1987, 333, C18–C20 CrossRef CAS.
- M. Scheer, C. Kuntz, M. Stubenhofer, M. Linseis, R. F. Winter and M. Sierka, Angew. Chem., 2009, 121, 2638–2642 ( Angew. Chem., Int. Ed. , 2009 , 48 , 2600–2604 ) CrossRef PubMed.
- M. Stubenhofer, C. Kuntz, M. Bodensteiner, U. Zenneck, M. Sierka and M. Scheer, Chem. – Eur. J., 2010, 16, 7488–7495 CrossRef CAS PubMed.
- L. S. H. Dixon, L. K. Allen, R. J. Less and D. S. Wright, Chem. Commun., 2014, 50, 3007–3009 RSC.
- E. Rivard and P. P. Power, Inorg. Chem., 2007, 46, 10047–10064 CrossRef CAS PubMed.
- J. A C. Clyburne and N. McMullen, Coord. Chem. Rev., 2000, 210, 73–99 CrossRef CAS.
- P. P. Power, Nature, 2010, 463, 171–177 CrossRef CAS PubMed.
- T. Nguyen, A. D. Sutton, M. Brynda, J. C. Fettinger, G. J. Long and P. P. Power, Science, 2005, 310, 844–847 CrossRef CAS PubMed.
- A. C. Filippou, U. Chakraborty and G. Schnakenburg, Chem. – Eur. J., 2013, 19, 5676–5686 CrossRef CAS PubMed.
- A. C. Filippou, O. Chernov and G. Schnakenburg, Angew. Chem., 2011, 123, 1154–1158 ( Angew. Chem., Int. Ed. , 2011 , 50 , 1122–1126 ) CrossRef PubMed.
- A. C. Filippou, A. I. Philippopoulos and G. Schnakenburg, Organometallics, 2003, 22, 3339–3341 CrossRef CAS.
- B. Twamley, C. D. Sofield, M. M. Olmstead and P. P. Power, J. Am. Chem. Soc., 1999, 121, 3357–3367 CrossRef CAS.
- J. D. Protasiewicz, M. P. Washington, V. B. Gudimetla, J. L. Payton and M. Cather Simpson, Inorg. Chim. Acta, 2010, 364, 39–45 CrossRef CAS PubMed.
- G. H. Spikes, J. C. Fettinger and P. P. Power, J. Am. Chem. Soc., 2005, 127, 12232–12233 CrossRef CAS PubMed.
- C. Cui, M. M. Olmstead, J. C. Fettinger, G. H. Spikes and P. P. Power, J. Am. Chem. Soc., 2005, 127, 17530–17541 CrossRef CAS PubMed.
- X. Wang, Y. Peng, M. M. Olmstead, J. C. Fettinger and P. P. Power, J. Am. Chem. Soc., 2009, 131, 14164–14165 CrossRef CAS PubMed.
- P. Wilfling, K. Schittelkopf, M. Flock, R. H. Herber, P. P. Power and R. C. Fischer, Organometallics, 2015, 34, 2222–2232 CrossRef CAS.
- T. Beweries, R. Kuzora, U. Rosenthal, A. Schulz and A. Villinger, Angew. Chem., 2011, 123, 9136–9140 ( Angew. Chem., Int. Ed. , 2011 , 50 , 8974–8978 ) CrossRef PubMed.
- S. Demeshko, C. Godemann, R. Kuzora, A. Schulz and A. Villinger, Angew. Chem., 2013, 125, 2159–2162 ( Angew. Chem., Int. Ed. , 2013 , 52 , 2105–2108 ) CrossRef PubMed.
- F. Reiß, A. Schulz, A. Villinger and N. Weding, Dalton Trans., 2010, 39, 9962–9972 RSC.
- M. Lehmann, A. Schulz and A. Villinger, Eur. J. Inorg. Chem., 2010, 5501–5508 CrossRef CAS PubMed.
- M. Abe, Chem. Rev., 2013, 113, 7011–7088 CrossRef CAS PubMed.
- F. Breher, Coord. Chem. Rev., 2007, 251, 1007–1043 CrossRef CAS PubMed.
- According to CASSCF(4,6) computations, the biradical character β of [ClSb(μ-NTer)]2 and [ClBi(μ-NTer)]2 amounts to 43% and 47%, respectively.
- E. Miliordos, K. Ruedenberg and S. S. Xantheas, Angew. Chem., 2013, 125, 5848–5851 ( Angew. Chem., Int. Ed. , 2013 , 52 , 5736–5739 ) CrossRef PubMed.
- G. He, O. Shynkaruk, M. W. Lui and E. Rivard, Chem. Rev., 2014, 114, 7815–7880 CrossRef CAS PubMed.
- M. S. Balakrishna, D. J. Eisler and T. Chivers, Chem. Soc. Rev., 2007, 36, 650–664 RSC.
- M. Lehmann, A. Schulz and A. Villinger, Angew. Chem., 2012, 124, 8211–8215 ( Angew. Chem., Int. Ed. , 2012 , 51 , 8087–8091 ) CrossRef PubMed.
- D. Michalik, A. Schulz and A. Villinger, Angew. Chem., 2010, 122, 7737–7740 ( Angew. Chem., Int. Ed. , 2010 , 49 , 7575–7577 ) CrossRef PubMed.
- Displacement vectors for the mentioned vibrations can be found in the ESI.†.
- P. Pyykkö and M. Atsumi, Chem. – Eur. J., 2009, 15, 12770–12779 CrossRef PubMed.
- Model compounds: [(Ph–N)2E]MgCl·THF and [ClSb(μ-NPh)2BiCl]. PBE1PBE, 6-31G(d,p) for all elements except for Sb and Bi. These were computed with ECP (ECP46MDF 4 46, ECP78MDF 4 78) and a corresponding basis set.
- A. Brückner, A. Hinz, J. Priebe, A. Schulz and A. Villinger, Angew. Chem., 2015, 127, 7534–7538 ( Angew. Chem., Int. Ed. , 2015 , 54 , 7426–7430 ) Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental and computational details and information on X-ray structure elucidation. CCDC 1059323 (2Sb), 1059324 (2Bi), and 1059325 (3). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5cc04355g |
| This journal is © The Royal Society of Chemistry 2015 |