 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Renaissance for low shrinking resins: all-in-one solution by bi-functional vinylcyclopropane-amides†
Paul
Pineda Contreras
a,
Christian
Kuttner
b,
Andreas
Fery
b,
Ullrich
Stahlschmidt
c,
Valérie
Jérôme
c,
Ruth
Freitag
c and
Seema
Agarwal
*a
aMacromolecular Chemistry II and Bayreuth Center for Colloids and Interfaces, Universität Bayreuth, Universitätsstrasse 30, 95440 Bayreuth, Germany. E-mail: agarwal@uni-bayreuth.de; Tel: +49-921-553397
bPhysical Chemistry II, Universität Bayreuth, Universitätsstrasse 30, 95440 Bayreuth, Germany
cProcess Biotechnology, Universität Bayreuth, Universitätsstrasse 30, 95440 Bayreuth, Germany
First published on 16th June 2015
Abstract
A low volume shrinking vinylcyclopropane (VCP) monomer, showing both a high reactivity and a low viscosity, was obtained by applying a sterically hindered and isomeric spacer element, incorporating intermolecular amide hydrogen bonds. The resulting properties locate this VCP system in a pronounced range that so far no other efficient and radical polymerizable resin could enter.
Photo-polymerizable resins with a low volume shrinkage are fundamental for coatings,1 electronics,2 microlithography,3 holographic data storages,4 advanced composites,5 and dental applications.6 In these applications, the final properties of the cross-linked network have to match precise specifications adapted for the final end-use. In general, this precision is achieved by the selection of highly specified resin systems.7 Thereby, in many cases the fast and selective curing, the solvent-free formulation and the targeted control of curing over a wide temperature, time, and radiation range made in particular the photo-polymerization technique overwhelming.8 Thus, especially acrylate and methacrylate monomers are favoured in nearly all radically polymerizable resins due to their synthetic ease of access, processability, and chemical variability.9
However, the observed volume shrinkage is pronounced and inevitable due to the transition of the intermolecular interactions from van der Waals spheres for monomers to covalent bonds in the cured resins.10 As a result, a high shrinkage can lead to the reduction in adhesion between the coating layer and the substrate in coating applications, or may cause the formation of contraction gaps between filling and cavity in dental resins, whereby recurrent caries might be the consequence.11
So far, many ongoing developments in dimethacrylate monomers could not adequately resolve the remaining issue of high volume shrinkage.12 Therefore, universally applied resins like bisphenol-A-glycidyl methacrylate (BisGMA) have so far played a pivotal role, as they provided a low volume shrinkage,13 rapid curing, and superior mechanical properties after curing.14 Nevertheless, the core of BisGMA resins is based on bisphenol-A (BPA), which has raised health concerns recently in the food packaging industry, consumer plastics, as well in dental materials about its widespread use, thus the exposure limits for BPA are reduced continuously.15
A promising resin system that sets a great promise on low volume shrinkage consists of vinylcyclopropane (VCP) derivatives, as the radical photo-polymerization results mainly in 1,5-ring-opened polymer units, whereby the volume shrinkage can significantly be reduced compared to methacrylate resins.16 However, so far not a single VCP resin could be successfully introduced to any real-world industrial application. Furthermore, due to the low curing kinetics and efficiency, VCPs have lost interest in academics as well.17
Herein, the first VCP-amide resin (VCPMe3hexAmid) (Scheme 1) with very fast photo-radical polymerization kinetic, a high monomer conversion in less than 2 min in air, low volume shrinkage, low viscosity, an effective refractive index, and good mechanical properties is reported. We studied in detail the preparation of the VCP resin, its enhanced curing behaviour by photo-differential scanning calorimetry (photo-DSC) and dielectric analysis (DEA), its volume shrinkage, viscosity, and cytotoxicity. Furthermore, the change in the optical refractive index during curing by spectroscopic ellipsometry (SE), as well as the mechanical properties and the network structure of the cross-linked network by 13C-CP/MAS (cross polarization/magic angle spinning) solid state NMR spectroscopy were studied to provide a detailed overview of the most relevant resin features.
 | ||
| Scheme 1 Preparation of bi-functional VCPs. DCC = N,N′-dicyclohexyl-carbodiimide; DMAP = 4-dimethylaminopyridine; HOBt = benzotriazol-1-ol. | ||
In order to optimize the reactivity by hydrogen bonding and to prevent any disproportional increase in viscosity a sterically hindered and isomeric amide spacer was used (VCPMe3hexAmid). A VCP-amide monomer, based on a linear C-6 spacer using simple hexamethylenediamine, provided slow crystallization and made itself unsuitable for any comparable detailed bulk polymerization study.
The VCPMe3hexAmid was synthesized in high purity and yield according to Scheme 1, the structural characterization using NMR spectroscopy showed the formation of an isomeric mixture due to the stereo- and constitution specificity of the VCP- and spacer-molecules. A more complete description of the VCP resin is presented in the ESI† in Fig. S1–S7.
The VCPMe3hexAmid was exposed to a commercial light-emitting diode (LED) source for studying the photo-curing kinetics and the absolute monomer conversion, which was determined by time-dependent extraction of the resulting material using deuterated chloroform. A mixture of camphorquinone (CQ) and ethyl 4-(dimethylamino)-benzoate (EDMAB) in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 was used for photo-polymerization at 35 °C. The very fast curing kinetics of VCPMe3hexAmid was evident from a conversion of ∼80% within 30 s and very high overall conversions of around 96% (Fig. 1a). The experiments were carried out both in inert gas as well as in air. The very fast curing-kinetics was retained even in air.
2 was used for photo-polymerization at 35 °C. The very fast curing kinetics of VCPMe3hexAmid was evident from a conversion of ∼80% within 30 s and very high overall conversions of around 96% (Fig. 1a). The experiments were carried out both in inert gas as well as in air. The very fast curing-kinetics was retained even in air.
These results were compared with that of the bi-functional VCP-ester derivative (VCPhexEster) (Scheme 1b, Fig. 1a). VCPhexEster yielded monomer conversions of ∼30% after 30 min of curing, while VCPMe3hexAmid reached the same conversion in less than 20 s. Furthermore, VCPhexEster could not reach a high overall monomer conversion even after prolonged curing times up to several hours (the maximum conversion in nitrogen was 42% in 12 h). Therefore VCPhexEster is several orders of magnitude less reactive than the VCPMe3hexAmid. Furthermore, the polymerization heat flow during the curing process was monitored by carrying out photo-polymerization in a DSC-cell for both VCPhexEster and VCPMe3hexAmid (Fig. 1b). Recording the relatively low heat flow changes during the curing of VCPhexEster also illustrated the general low reactivity of ester-based VCP resin.
The difference in reactivity of VCPMe3hexAmid and VCPhexEster could be due to the molecular structure of VCPMe3hexAmid allowing intermolecular hydrogen bonding via amide units, leading to a partial preorganization of monomer molecules (Fig. 2a). This could also lead to a profitable orientation of the singly occupied molecular orbital (SOMO) of the ring-opened VCP-structure to further monomer units or rather their π-orbitals, which enhances the curing process (Fig. 2b). The shift of the amide protons of VCPMe3hexAmid to higher fields with increasing temperature in 1H-NMR experiments (see Fig. S8 and S9 in the ESI†) and the meaningful difference in the monomer viscosities, VCPMe3hexAmid was significantly more viscous (2.4 Pa s) than VCPhexEster (0.08 Pa s), verified the H-bonding. Furthermore, photo-DSC curing experiments, with an additional amount of potassium thiocyanate (KSCN) as a chaotropic agent, have been carried out to underline the hydrogen bonding effect (Fig. 2c). Thereby, chaotropic agents can disrupt the H-bonding forces.18 An unambiguous effect toward increasing KSCN amounts to the curing behavior was observed, yielding 19% lower heat flow values (ΔHmax) and 37% lower rates of polymerization (RP), respectively, for 1 wt% of a KSCN solution (392 mg mL−1 in DMSO) and 1 wt% pure DMSO (RP values were determined from the slope of the linear fit after reaching Hmax). The enhanced RP for acrylate monomers capable of having H-bonding is documented in the literature,19 however this effect appears to be not so pronounced as for VCPs.
Due to the fact that with time curing reactions are exothermic and could lead to undesirable local heating and an increase in temperature, additional experiments were carried out to monitor temperature changes during polymerization of VCPMe3hexAmid by the DEA technique. Advantageously, the temperature increase ΔT during curing was very low (slightly above 4 °C) for VCPMe3hexAmid (Fig. 3). Furthermore, for VCPMe3hexAmid the curing began immediately with an increase in the ion-viscosity (μi) reaching the top of a plateau in about 1–2 min, whereas the continued exposure did not further affect μi. Thus, the curing was practically completed within the first 2 min. The slight increase in μi upon switching off the light source was due to a decrease in temperature from 39 °C (the maximum attained during curing) to around 35 °C (start temperature). No attempts were made to carry out similar experiments with VCPhexEster due to its very slow and incomplete polymerization as shown above. In contrast to VCPMe3hexAmid, the well-known photo-cross-linkable resin urethane-dimethacrylate (UDMA) showed during curing a rise in temperature of 13 °C to about 48 °C under similar reaction conditions. Especially with regard to biological coatings and dental applications any reduction in an excessive exothermic temperature rise reduces the hazard potential significantly.20
The volume shrinkage as measured by Archimedes's method amounted to 4.9% for pure VCPMe3hexAmid. The same measurement for VCPhexEster unfortunately could not be done due to incomplete conversions. Therefore, a comparison of our synthesized VCPMe3hexAmid to widely used methacrylate resins, like UDMA, dodecanediol-dimethacrylate (1,12-DMA), and a mixture of BisGMA and triethylene-glycol-dimethacrylate (TEGDMA), was made.
VCPMe3hexAmid showed a reduction by 45% in volume shrinkage to UDMA, 17% to a 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 mixture of BisGMA
4 mixture of BisGMA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TEGDMA and similar to BisGMA. Furthermore, upon comparing the resin viscosity in dependence of volume shrinkage (Fig. 4a), a unique advantage of VCPMe3hexAmid could be observed. Both BisGMA with 1200 Pa s21 and UDMA with 13.8 Pa s are too viscous to be used on their own. In contrast, VCPMe3hexAmid exhibits with 2.4 Pa s a clearly lower viscosity and simultaneously low volume shrinkage. The optical refractive index n of the cured VCPMe3hexAmid averaged to 1.522 as measured by SE and lies in the range of other existing systems, e.g. UDMA with 1.508 (Fig. 4b). The regression line for the changes in the refractive indices Δn20D in dependence of the volume shrinkage ΔV showed a simple and accurate correlation (R2 = 0.985) that the effect of the volume shrinkage mainly induced the refraction alteration.
TEGDMA and similar to BisGMA. Furthermore, upon comparing the resin viscosity in dependence of volume shrinkage (Fig. 4a), a unique advantage of VCPMe3hexAmid could be observed. Both BisGMA with 1200 Pa s21 and UDMA with 13.8 Pa s are too viscous to be used on their own. In contrast, VCPMe3hexAmid exhibits with 2.4 Pa s a clearly lower viscosity and simultaneously low volume shrinkage. The optical refractive index n of the cured VCPMe3hexAmid averaged to 1.522 as measured by SE and lies in the range of other existing systems, e.g. UDMA with 1.508 (Fig. 4b). The regression line for the changes in the refractive indices Δn20D in dependence of the volume shrinkage ΔV showed a simple and accurate correlation (R2 = 0.985) that the effect of the volume shrinkage mainly induced the refraction alteration.
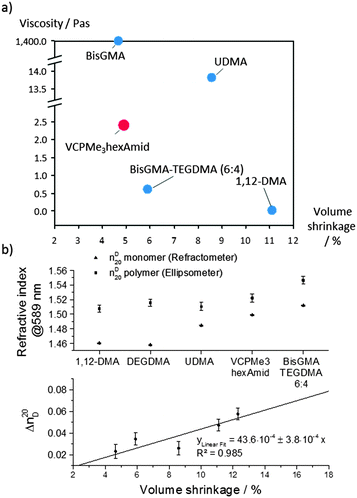 | ||
| Fig. 4 Classification of resin properties in comparison to commercially available methacrylate resins. (a) Ashby plot of specific monomer viscosities as a function of the volume shrinkage. (b) Upper graph: refractive indices (@589 nm), respectively, for the monomer and polymer networks. Lower graph: graphical evaluation of the refractive index alteration Δn20D from the volume shrinkage of the respectively polymers. Please find in the ESI† and Table S1 a summary of the properties of the materials shown here. | ||
Furthermore, VCPMe3hexAmid showed similar LD50 values (0.067 ± 0.005 mM) to those of UDMA (0.071 ± 0.009 mM) in case it has to be recommended for dental applications (see the ESI† for cytotoxicity determination, Fig. S15 and S16).
As the polymer network structure could influence the overall properties, high-resolution solid state 13C-CP/MAS NMR was used for studying the network structure by comparing the participation of double bonds in the cross-linking process. Almost all vinyl double bonds participated in the cross-linking reaction as evidenced by the presence of a very significant peak at 129.3 ppm originating from the carbon signal of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond of the ring-opened VCP-unit. Only a very small shoulder at 119.7 ppm (dedicated to unreacted vinyl double bonds) was identified, implying an extraordinary high cross-linking density of the cured VCPMe3hexAmid. A closer examination of the network structure is presented in the ESI† and Scheme S4 and Fig. S10; with respect to the polymerization mechanism of VCPs the formation of cyclobutane structures besides the 1,5-ring-opened units should also be taken into consideration, but unfortunately it could not be analyzed using present techniques.16
C double bond of the ring-opened VCP-unit. Only a very small shoulder at 119.7 ppm (dedicated to unreacted vinyl double bonds) was identified, implying an extraordinary high cross-linking density of the cured VCPMe3hexAmid. A closer examination of the network structure is presented in the ESI† and Scheme S4 and Fig. S10; with respect to the polymerization mechanism of VCPs the formation of cyclobutane structures besides the 1,5-ring-opened units should also be taken into consideration, but unfortunately it could not be analyzed using present techniques.16
Cured VCPMe3hexAmid specimens showed E-moduli of 130.9 ± 0.7 MPa which increased to 181.9 ± 0.5 MPa after additional storage at 100 °C (the complete evaluation is provided in the ESI† and Fig. S12) and Vickers hardness (HV) values of 1.52 ± 0.02 HV. These values are in the range of commercially available and cured UDMA.
In summary, after ∼10 years of silence in academics and patent registrations the first highly reactive and low volume shrinking VCP resin was obtained, which can serve as a model monomer for further resin systems. The introduction of H-bond forming amide groups into the monomer structure induced partial preorganization, leading to high propagation efficiency during curing. Any disproportionate increase in resin viscosity could be prevented by using a sterically hindered, isomeric spacer element. The resulting VCPMe3hexAmid represents for the first time a highly efficient and fast curing VCP resin. Moreover, the most valuable properties conversion, mechanical strength, viscosity, and optical refractive index were all carefully balanced in optimum ranges. Since radical polymerizable, low-shrinking resins remain a challenge for several applications, this novel resin could be of immense interest, offering a real and sustainable alternative to the currently used dimethacrylate resins. So far, the outstanding possibilities offered by VCP resins have not yet been exploited. Therefore a broad application range for both industry and academia may be imagined.
This work was supported by the German Federal Ministry for Economic Affairs and Energy (BMWi) (http://www.zim-bmwi.de). We thank Dr A. Bublewitz, Dr A. Theis and the Kettenbach GmbH for providing further resources and support. The authors are indebted to Prof. P. Strohriegel and Dr R. Giesa for providing access to photo-DSC and DMTA, to Prof. J. Senker and Dr R. Siegel for solid-state NMR measurements, and further to NETZSCH company and Dr S. Schmölzer for DEA measurements. We also thank C. Goldhahn and P. Schmode for their experimental support as research students.
Notes and references
- (a) Y. Jian, Y. He, L. Zhao, A. Kowalczyk, W. Yang and J. Nie, Adv. Polym. Technol., 2013, 32, 21331 CrossRef PubMed; (b) E. Andrzejewska, Prog. Polym. Sci., 2001, 26, 605 CrossRef CAS.
- (a) J. G. Kloosterboer, Adv. Polym. Sci., Springer, Berlin Heidelberg, 1988, vol. 84, pp. 1–61 Search PubMed; (b) Y. Fuchs, O. Soppera and K. Haupt, Anal. Chim. Acta, 2012, 717, 7 CrossRef CAS PubMed.
- C. Decker, Macromol. Rapid Commun., 2002, 23, 1067 CrossRef CAS PubMed.
- J. Guo, M. R. Gleeson and J. T. Sheridan, Phys. Res. Int., 2012, 2012, 1 CrossRef PubMed.
- J. Lange, S. Toll, J.-A. E. Månson and A. Hult, Polymer, 1997, 38, 809 CrossRef CAS.
- (a) N. Moszner and T. Hirt, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 4369 CrossRef CAS PubMed; (b) K. L. van Landuyt, J. Snauwaert, J. de Munck, M. Peumans, Y. Yoshida, A. Poitevin, E. Coutinho, K. Suzuki, P. Lambrechts and B. van Meerbeek, Biomaterials, 2007, 28, 3757 CrossRef CAS PubMed.
- K. S. Anseth, C. N. Bowman and N. A. Peppas, J. Polym. Sci., Part A: Polym. Chem., 1994, 32, 139 CrossRef CAS PubMed.
- (a) A. Lendlein, H. Jiang, O. Jünger and R. Langer, Nature, 2005, 434, 879 CrossRef CAS PubMed; (b) C. Fo Tsang, Microelectron. Int., 2000, 17, 27 CrossRef.
- (a) C. Decker, Prog. Polym. Sci., 1996, 21, 593 CrossRef CAS; (b) K. L. van Landuyt, J. Snauwaert, J. de Munck, M. Peumans, Y. Yoshida, A. Poitevin, E. Coutinho, K. Suzuki, P. Lambrechts and B. van Meerbeek, Biomaterials, 2007, 28, 3757 CrossRef CAS PubMed.
- F. Rueggeberg and K. Tamareselvy, Dent. Mater., 1995, 11, 265 CrossRef CAS.
- B. Lu, P. Xiao, M. Sun and J. Nie, J. Appl. Polym. Sci., 2007, 104, 1126 CrossRef CAS PubMed.
- J. W. Stansbury, M. Trujillo-Lemon, H. Lu, X. Ding, Y. Lin and J. Ge, Dent. Mater., 2005, 21, 56 CrossRef CAS PubMed.
- R. L. Bowen, US 3066112, 1959.
- (a) R. L. Bowen, J. Am. Dent. Assoc., JADA, 1963, 66, 57 CrossRef CAS; (b) A. Peutzfeldt, Eur. J. Oral Sci., 1997, 105, 97 CrossRef CAS PubMed.
- (a) C. Liao and K. Kannan, Environ. Sci. Technol., 2011, 45, 9372 CrossRef CAS PubMed; (b) L. Chen and B. Suh, JSM Dent, 2013, 1, 1004 Search PubMed; (c) C. Bolognesi, L. Castle, J.-P. Cravedi, K.-H. Engel, P. Fowler, R. Franz, K. Grob, R. Gürtler, T. Husøy, W. Mennes, M. R. Milana, A. Penninks, F. Roland, V. Silano, A. Smith, M. de Fátima Tavares Poças, C. Tlustos, F. Toldrá, D. Wölfle and H. Zorn, EFSA J., 2015, 13, 3978 Search PubMed.
- P. Pineda Contreras, P. Tyagi and S. Agarwal, Polym. Chem., 2015, 6, 2297 RSC.
- N. Moszner, Macromol. Symp., 2004, 217, 63 CrossRef CAS PubMed.
- F. Liu, J. Seuring and S. Agarwal, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 4920 CrossRef CAS PubMed.
- (a) J. F. G. A. Jansen, A. A. Dias, M. Dorschu and B. Coussens, Macromolecules, 2003, 36, 3861 CrossRef CAS; (b) S. H. Dickens, J. W. Stansbury, K. M. Choi and C. J. E. Floyd, Macromolecules, 2003, 36, 6043 CrossRef CAS; (c) I. Sideridou, V. Tserki and G. Papanastasiou, Biomaterials, 2002, 23, 1819 CrossRef CAS.
- (a) A. C. Shortall and E. Harrington, J. Oral Rehabil., 1998, 25, 908–913 CrossRef CAS; (b) Z. Czech and R. Milker, J. Appl. Polym. Sci., 2003, 87, 182 CrossRef CAS PubMed.
- S. G. Pereira, T. G. Nunes and S. Kalachandra, Biomaterials, 2002, 23, 3799 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Materials, methods, synthesis, and supplementary characterization. See DOI: 10.1039/c5cc03901k |
| This journal is © The Royal Society of Chemistry 2015 |



