Open Access Article
Open Access ArticleOn the handedness of helical aggregates of C3 tricarboxamides: a multichiroptical characterization†
Belén
Nieto-Ortega
a,
Fátima
García
b,
Giovanna
Longhi
c,
Ettore
Castiglioni
c,
Joaquín
Calbo
d,
Sergio
Abbate
c,
Juan T.
López Navarrete
*a,
Francisco J.
Ramírez
a,
Enrique
Ortí
*d,
Luis
Sánchez
*b and
Juan
Casado
*a
aDepartamento de Química Física, Facultad de Ciencias, Universidad de Málaga, Campus de Teatinos s/n, 29071 Málaga, Spain. E-mail: casado@uma.es
bDepartamento de Química Orgánica, Facultad de Ciencias Químicas, Ciudad Universitaria s/n, 28040 Madrid, Spain. E-mail: lusamar@quim.ucm.es
cDipartimento di Medicina Molecolare e Traslazionale, Università di Brescia, 25123 Brescia, Italy. E-mail: sergio.abbate@unibs.it
dInstituto de Ciencia Molecular, Universidad de Valencia, 46980 Paterna, Spain. E-mail: enrique.orti@uv.es
First published on 30th April 2015
Abstract
A complete chiroptical characterization of the supramolecular polymers formed by tricarboxamides (S)-1 and (R)-1 is performed using ECD, VCD and CPL dichroic techniques. The helical aggregates show an intense CPL signal and their absolute P- or M-configuration is assigned with the help of theoretical calculations.
Supramolecular polymers are constituted by monomeric units assembled together by non-covalent forces like H-bonds, π-stacking dispersion forces or electrostatic interactions,1 and can exhibit enhanced mechanical, biological or optoelectronic properties compared to conventional polymers.2 Inspired by the sophistication reached by chiral, natural structures, a number of helical, and consequently chiral, supramolecular polymers have been designed.3 The helical character of the vast majority of the chiral supramolecular polymers reported to date is obtained by the decoration of the constitutive monomeric units with peripheral side chains endowed with stereogenic centres. The rotated stacking of such monomeric units results in the formation of helical, columnar stacks that are usually characterized by a bisignated Cotton effect in the corresponding electronic circular dichroism (ECD) spectra.4 To the best of our knowledge, there has been no example of helical supramolecular polymers in which a complete dichroic characterization of both enantiomers, i.e. the M- and P-type helical structures, had been afforded.
Herein, we report a thorough characterization of the chiral features exhibited by the helical supramolecular polymers formed by the self-assembly of star-shaped oligo(phenylene-ethynylene)-based tricarboxamides (OPE-TAs) (compounds 1 in Fig. 1)5 by using three complementary chiroptical techniques: ECD, vibrational circular dichroism (VCD) and circularly polarized luminescence (CPL). A complete investigation of the intrinsic chiral features from the dichroic responses is possible given that both enantiomers of OPE-TA 1 are available. In addition, the synergy of experimental data and theoretical calculations has allowed the unequivocal assignment of the helical sense (M or P) of the supramolecular aggregates formed by chiral induction from the R- or S-enantiomers of compound 1. The results presented offer a powerful protocol to characterize chiral supramolecular polymers in solution, and contribute to increasing the understanding of the self-assembly mechanism and the transmission of chiral information in the electronic ground state and in the excited states.
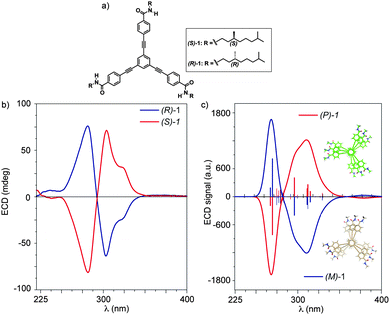
The chemical structure of tricarboxamides 1 facilitates their self-assembly in columnar stacks by the formation of a triple array of H-bonds between the amide functional groups reinforced by the π-stacking of the aromatic moieties. To accommodate this triple array of H-bonding interactions, the self-assembling units rotate ∼18° along the vertical stacking axis resulting in helical aggregates that grow up following a cooperative mechanism.5,6 In analogy with other self-assembling discotics,7 the handedness of the aggregates is dictated by the absolute configuration of the stereogenic centres located at the side alkyl chains, which gives rise to a clear bisignated Cotton effect in the ECD spectra.5 The ECD spectra of enantiomers (S)-1 and (R)-1 are mirror-like images (Fig. 1b), and the whole dichroic response is cancelled either by heating the diluted solution or by adding a good solvent like chloroform that destroys the H-bond triple array sustaining the helical aggregate (Fig. S1 in the ESI†).5 The −/+ (281 nm)/(303, 323 nm) pattern experimentally observed for (S)-1 and the reverse +/− pattern recorded for (R)-1 were assigned to right- and left-handed helices, respectively, by comparison with previously reported cases,8 but without any other intrinsic evidence.5
To corroborate the helical sense of the aggregate, the ECD spectra of right-handed aggregates incorporating an increasing number of OPE-TA units were first calculated at the semiempirical PM6/CIS level (Fig. S2, ESI†). At this level of theory, a −/+ band is predicted at around 300 nm, which grows up in intensity with the number of monomeric units. The −/+ pattern obtained is in agreement with previous calculations performed on related systems.9 Time-dependent density functional theory (TD-DFT) calculations were performed in a second step for the trimer aggregate in order to get a more accurate insight into the nature of the electronic transitions that give rise to the bisignated band observed in the ECD spectra (see the ESI† for computational details). Two intense, double electronic excitations S0 → S56/57 (312 nm) and S0 → S96/97 (288 nm) arise in the 300 nm region with oscillator strengths (f) of 1.89 and 1.77, respectively (Fig. S3, ESI†). These transitions correspond to π → π* monoexcitations involving the conjugated skeleton of the OPE-TA units and show a large multiconfigurational character. Among the different one-electron excitations contributing to these transitions, the most relevant are those associated with molecular orbitals mostly located on the molecules placed at the bottom (S56/57) and on the top (S96/97) of the columnar aggregate. Although other excitations are computed in the 320–400 nm range, their oscillator strengths are negligible (f < 0.01) and they have no influence on the shape of the convoluted UV-vis theoretical spectrum (Fig. S3, ESI†). Fig. 1c shows the theoretical simulation of the ECD spectrum calculated for right-handed and left-handed trimers of 1. The right-handed P-trimer presents a −/+ pattern of the ECD signal in the 280–320 nm region that perfectly correlates with the pattern of the Cotton band experimentally recorded for the aggregate generated from (S)-1 (Fig. 1b). In a similar way, the theoretical ECD spectrum of the left-handed M-trimer is a mirror image of that obtained for the P-trimer and corresponds to that observed for the aggregate of (R)-1 (Fig. 1). Consequently, the complementary information provided by ECD experiments and theoretical spectra allows the unequivocal assignment of the absolute configuration of the helical structures generated in the supramolecular polymerization of tricarboxamides 1: (S)-1 gives rise to right-handed P-aggregates whereas (R)-1 results in left-handed M-aggregates.
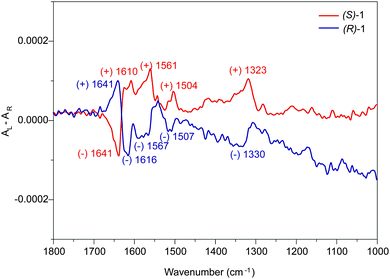
After assigning the ECD pattern to the corresponding helical structures, we have further investigated the chiroptical features of such chiral entities by using VCD spectroscopy. This technique is the chiral branch of the common infrared absorption spectroscopy, and provides molecular-level information of the structure of the chemical groups that propagate the helical motif in the aggregate and are mostly involved in the amplification of the VCD absorbance.10 The VCD spectra were registered for 3 × 10−3 M solutions of (S)-1 and (R)-1 in MCH. At this concentration, the columnar aggregates are intertwined to form an organogel. Fig. 2 shows the VCD spectra of the organogels formed by (S)-1 and (R)-1. As expected, the VCD spectra of both enantiomers exhibit mirror-like patterns. The spectra are dominated by a bisignated band at 1628 cm−1 corresponding to the amide I vibrational mode, ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).5 The measured wavenumber is significantly downshifted with respect to the usual value for a free amide group (∼1690 cm−1) revealing the existence of a strong C
O).5 The measured wavenumber is significantly downshifted with respect to the usual value for a free amide group (∼1690 cm−1) revealing the existence of a strong C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H–N hydrogen-bond network.11 The opposite dichroic patterns obtained in the VCD spectrum for the ν(C
O⋯H–N hydrogen-bond network.11 The opposite dichroic patterns obtained in the VCD spectrum for the ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) band of (S)-1 and (R)-1 are identical to those of the Cotton band in the ECD spectrum (Fig. 1b). This suggests that the relative (local) dihedral angle formed by H-bonded amides in the stack is similar to the (overall) angle formed between the principal arms of the trisamides.
O) band of (S)-1 and (R)-1 are identical to those of the Cotton band in the ECD spectrum (Fig. 1b). This suggests that the relative (local) dihedral angle formed by H-bonded amides in the stack is similar to the (overall) angle formed between the principal arms of the trisamides.
The supramolecular polymerization of tricarboxamides 1, directed by the triple array of H-bonds between the amide functional groups and the π–π interaction of the aromatic units, produces strongly packed and highly dense π-stacks. The structures of these helical aggregates are ideal for the formation of highly delocalized excitons potentially capable of diffusing over rather large distances, hence making these structures suitable candidates to exhibit chiroptical luminescence.
To investigate the excited-state, the emission spectra of tricarboxamides 1 were registered under different experimental conditions (Fig. 3a and S4, ESI†). In diluted CHCl3, a good solvent in which tricarboxamides 1 are molecularly dissolved and the formation of aggregates becomes difficult (Fig. S1, ESI†),5c the fluorescence spectrum exhibits an intense broad band with maximum emission at 360 nm and shoulders at 370 and 389 nm. TD-DFT calculations confirm the assignment of this band to the emission of 1 in the monomeric form since the transition from the first singlet excited state (S1) to the ground state presents a vibrational structure (Fig. S5, ESI†), in perfect agreement with that observed experimentally (Fig. 3a). The change of the solvent to MCH, a bad solvent that strongly facilitates the aggregation of 1,5c results in a bathochromic shift of the emission and a broad band centred at 405 nm is observed (Fig. 3a).
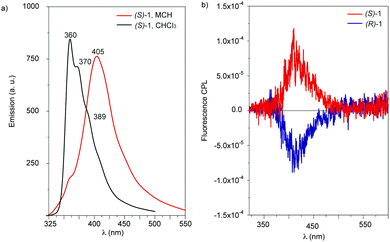 | ||
| Fig. 3 (a) Emission spectra of (S)-1 in MCH and CHCl3 (5 × 10−5 M, λexc = 297 nm; 298 K). (b) CPL spectra of of (S)-1 and (R)-1 in MCH (5 × 10−5 M, λexc = 297 nm; 298 K). | ||
Circularly polarized light is obviously inherently chiral and has been considered one of the potential sources for the origin of homochirality in nature,12 an issue in which supramolecular polymers are being successfully applied.3 General requisites to attain well-resolved CPL signals are intense fluorescence and well-defined sources of chiroptical activity. However, aggregation often quenches fluorescence and, therefore, attaining aggregated chiral systems with CPL activity is very unusual. The number of reports in which the CPL emission is triggered by supramolecular assembly is much reduced.13 In fact, CPL activity is exhibited by selected lanthanide complexes and conjugated polymers.14,15
Considering that tricarboxamides 1 are medium-active fluorophores, one can expect a negligible CPL effect for the aggregates generated upon their self-assembly. To our surprise, this is not the case for the investigated discotics. Prior to measuring the CPL spectra of compounds 1, the stability of the emission was checked by recording the fluorescence spectra of 1 in pure MCH and also in mixtures of MCH/CHCl3 at 95% and 90% within a time interval of 150 s (Fig. S4, ESI†). The aggregates in pure MCH show great kinetic stability, which is a pre-condition to conduct CPL measurements since they usually take long acquisition times.
As in the case of the ECD and VCD spectra, the CPL spectra of (S)-1 and (R)-1 (MCH, 5 × 10−5 M and λexc = 297 nm) are mirror-like images with polarized light emissions that cover the range 375–500 nm. The maximal signal, positive for (S)-1 and negative for (R)-1, is found at around 408 nm (Fig. 3b). This CPL pattern, which is cancelled in CHCl3 (Fig. S6, ESI†), coincides with the sign of the longest wavelength component of the ECD spectra for the corresponding enantiomeric system. This suggests that the excitation process and the subsequent emission from the first singlet excited state do not change the organization and handedness of the assembly. The intensity of the emitted circularly polarized light, determined by the dissymmetry factor, glum, which is defined by the equation glum = 2(IL − IR)/(IL + IR), has been calculated to be +2.8 × 10−3 and −2.2 × 10−3 for (S)-1 and (R)-1, respectively. In very good agreement with that observed in ECD, no CPL response is achieved for solutions of (S)-1 and (R)-1 in CHCl3 (Fig. S6, ESI†). Assuming the delocalized character of the generated excitons in the stack and the chiral features of the associated emission, we can conclude that CPL is directly dictated by the handedness of the supramolecular aggregates. Similar results have been obtained in experiments using different excitation wavelengths (Fig. S7, ESI†), which validate the reliability of the CPL spectra. Consequently, the reduced quenching effect exerted by aggregation on the emission, together with the helical arrangement of the supramolecular polymers formed from tricarboxamides 1, results in a clear CPL signal.
In summary, a complete chiroptical characterization of the supramolecular polymers formed by the self-assembly of chiral tricarboxamides (S)-1 and (R)-1 has been reported. The chiral information embedded in the self-assembling units is efficiently transferred to the aggregate as demonstrated by ECD, VCD and CPL measurements. Thus, whilst compounds 1 do not show any dichroic response in a good solvent like CHCl3, the aggregation in MCH yields columnar stacks with enhanced ECD and CPL activities. Additionally, the combination of experimental evidence and theoretical calculations allows the unambiguous assignment of the absolute helicity associated with the dichroic response for (S)-1 and (R)-1: the former, with a −/+ pattern in ECD and a positive response in CPL, corresponds to a P-type helix, whereas the latter, with a +/− pattern in ECD and a negative signal in CPL, forms a M-type helix. To the best of our knowledge, this is one of the few cases where CPL has been measured for two self-assembling enantiomers. The data presented in this work contribute to the understanding about the molecular self-assembly mechanism and the transmission of the structural information to the excited states, the latter is a key factor for the design of luminescent superstructures with modular properties.
This work was supported by the MINECO of Spain (CTQ2011-22581, CTQ2012-31914 and CTQ2012-33733), Comunidad de Madrid (NanoBIOSOMA, S2013/MIT-2807), Generalitat Valenciana (Prometeo/2012/053), Junta de Andalucía (P09-FQM-4708), and European FEDER funds (CTQ2012-31914). B.N.-O is indebted to University of Malaga, Programa de Fortalecimiento de las Capacidades en I+D+i, FEDER funds. F. G. and J. C. thank the MECD for FPU predoctoral grants.
Notes and references
- L. Brunsveld, B. J. B. Folmer, E. W. Meijer and R. P. Sijbesma, Chem. Commun., 2001, 4071 CAS; F. Aparicio, F. García and L. Sánchez, in Enciclopedia of Polymer Science and Technology, ed. M. Peterca, Wiley, 2012, p. 549 Search PubMed.
- (a) T. Aida, E. W. Meijer and S. I. Stupp, Science, 2012, 335, 813 CrossRef CAS PubMed; (b) S. S. Babu, S. Prasanthkumar and A. Ajayaghosh, Angew. Chem., Int. Ed., 2012, 51, 1766 CrossRef CAS PubMed.
- A. R. A. Palmans and E. W. Meijer, Angew. Chem., Int. Ed., 2007, 46, 8948 CrossRef CAS PubMed.
- (a) G. Pescitelli, L. Di Bari and N. Berova, Chem. Soc. Rev., 2014, 43, 5211 RSC; (b) C. C. Lee, C. Grenier, E. W. Meijer and A. P. H. J. Schenning, Chem. Soc. Rev., 2009, 38, 371 Search PubMed.
- (a) F. García, P. M. Viruela, E. Ortí and L. Sánchez, Chem. – Eur. J., 2011, 17, 7755 CrossRef PubMed; (b) F. García and L. Sánchez, J. Am. Chem. Soc., 2012, 134, 734 CrossRef PubMed; (c) F. García, P. A. Korevaar, A. Verlee, E. W. Meijer, A. R. A. Palmans and L. Sánchez, Chem. Commun., 2013, 49, 8674 RSC.
- (a) T. F. A. De Greef, M. M. J. Smulders, M. Wolffs, A. P. H. J. Schenning, R. P. Sijbesma and E. W. Meijer, Chem. Rev., 2009, 109, 5687 CrossRef CAS PubMed; (b) Z. Chen, A. Lohr, C. R. Saha-Mçller and F. Würthner, Chem. Soc. Rev., 2009, 38, 564 RSC.
- (a) W. Jin, T. Fukushima, M. Niki, A. Kosaka, N. Ishii and T. Aida, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 10801 CrossRef CAS PubMed; (b) M. M. J. Smulders, A. P. H. J. Schenning and E. W. Meijer, J. Am. Chem. Soc., 2008, 130, 606 CrossRef CAS PubMed; (c) S. Ghosh, X.-Q. Li, V. Stepanenko and F. Würthner, Chem. – Eur. J., 2008, 14, 11343 CrossRef CAS PubMed; (d) C. Kulkarni, R. Manirathinam and S. J. George, Chem. – Eur. J., 2013, 19, 11270 CrossRef CAS PubMed.
- (a) E. L. Eliel, S. H. Wilen and L. N. Mander, Stereochemistry of Organic Compounds, Wiley, New York, 1994 Search PubMed; (b) G. Pescitelli, L. Di Bari and N. Berova, Chem. Soc. Rev., 2011, 40, 4063 RSC.
- Referable studies have been reported for C3-symmetric discotics: (a) M. M. J. Smulders, T. Buffeteau, D. Cavagnat, M. Wolffs, A. P. H. J. Schenning and E. W. Meijer, Chirality, 2008, 20, 2016 CrossRef PubMed; (b) B. Nieto-Ortega, J. Casado, J. T. López Navarrete, G. Hennrich and F. J. Ramírez, J. Chem. Theory Comput., 2011, 7, 3314 CrossRef CAS; (c) Y. Nakano, T. Hirose, P. J. M. Stals, E. W. Meijer and A. R. A. Palmans, Chem. Sci., 2012, 3, 148 RSC.
- (a) G. Yang and Y. Xu, in Electronic and Magnetic Properties of Chiral Molecules and Supramolecular Architectures, ed. R. Naaman, D. N. Beratan and D. Waldeck, Springer, Berlin, 2011, vol. 298, p. 189 Search PubMed; (b) F. Aparicio, B. Nieto-Ortega, F. Nájera, F. J. Ramírez, J. T. López Navarrete, J. Casado and L. Sánchez, Angew. Chem., Int. Ed., 2014, 53, 1373 CrossRef CAS PubMed.
- K. Hanabusa, C. Koto, M. Kimura, H. Shirai and A. Kakel, Chem. Lett., 1997, 429 CrossRef CAS.
- (a) D. K. Kondepudi and K. Asakura, Acc. Chem. Res., 2001, 34, 946 CrossRef CAS PubMed; (b) B. L. Feringa and R. A. van Delden, Angew. Chem., Int. Ed., 1999, 38, 3418 CrossRef.
- (a) F. C. Spano, S. C. J. Meskers, E. Hennebicq and D. Beljonne, J. Am. Chem. Soc., 2007, 129, 7044 CrossRef CAS PubMed; (b) R. Randazzo, A. Mammana, A. D'Urso, R. Lauceri and R. Purrello, Angew. Chem., Int. Ed., 2008, 47, 9879 CrossRef CAS PubMed; (c) T. Ikeda, T. Masuda, T. Hirao, J. Yuasa, H. Tsumatori, T. Kawai and T. Haino, Chem. Commun., 2012, 48, 6025 RSC.
- (a) F. S. Richardson and J. P. Riehl, Chem. Rev., 1977, 77, 773 CrossRef CAS; (b) D. Parker, R. S. Dickins, H. Puschmann, C. Crossland and J. A. K. Howard, Chem. Rev., 2002, 102, 1977 CrossRef CAS PubMed; (c) J. C. G. Bünzli and C. Piguet, Chem. Soc. Rev., 2005, 34, 1048 RSC.
- (a) E. Peeters, M. P. T. Christiaans, R. A. J. Janssen, H. F. M. Schoo, H. P. J. M. Dekkers and E. W. Meijer, J. Am. Chem. Soc., 1997, 119, 9909 CrossRef CAS; (b) H. Goto and K. Akagi, Angew. Chem., Int. Ed., 2005, 44, 4322 CrossRef CAS PubMed; (c) F. Sannicolò, P. R. Mussini, T. Benincori, R. Cirilli, S. Abbate, S. Arnaboldi, S. Casolo, E. Castiglioni, G. Longhi, R. Martinazzo, M. Panigati, M. Pappini, E. Quartapelle Procopio and S. Rizzo, Chem. – Eur. J., 2014, 20, 15298 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1–S7 and theoretical computational details. See DOI: 10.1039/c5cc03054d |
| This journal is © The Royal Society of Chemistry 2015 |