Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
One-pot selective synthesis of a fullerene bisadduct for organic solar cell applications†
Bolong
Zhang
,
Jegadesan
Subbiah
,
Yu-Ying
Lai
,
Jonathan M.
White
,
David J.
Jones
 and
Wallace W. H.
Wong
*
and
Wallace W. H.
Wong
*
School of Chemistry, University of Melbourne Bio21 Institute, 30 Flemington Rd, Parkville, Victoria 3010, Australia. E-mail: wwhwong@unimelb.edu.au
First published on 20th May 2015
Abstract
A single isomer of fullerene bisadduct, PC61PF, was obtained from commercially available fullerene derivative, PC61BM, in one pot over two steps. The tether-directed remote functionalization approach provided a very simple and fast method to produce a single isomer of fullerene bisadduct with good yield and easy purification. Bulk heterojunction organic solar cells containing the bisadduct was fabricated and tested.
Over the past decade, organic solar cells (OSCs) has been a hot topic of research as an emerging renewable energy technology. In comparison with conventional devices, such as silicon solar cells, OSC technology promises lightweight flexible devices that can be produced at low cost through roll-to-roll printing methods.1 One specific type of OSC is the bulk-heterojunction (BHJ) solar cell which contains a blend of electron donor and acceptor materials in its active layer. Among electron acceptor materials, fullerenes are one of the most attractive compounds, due to their reversible reduction behavior,2 good electron transport properties3 and easily functionalized structures.4 Numerous fullerene derivatives have been reported and used as effective electron acceptor materials in OSC devices, such as [6,6]-phenyl-C61-butyric acid methyl ester (PC61BM)5 and indene C60 bisadduct (IC60BA).6
The power convention efficiency (PCE) of OSCs can be optimized by adjusting the device parameters of open-circuit voltage (Voc), short-circuit current density (Jsc) and fill factor (FF).7 In particular, the Voc is relatively easy to control by tuning the highest occupied molecular orbital (HOMO) energy level of donor material and the lowest unoccupied molecular orbital (LUMO) energy level of acceptor material.8 A common strategy to increase the voltage is to elevate the LUMO energy level of the fullerene acceptor and this is normally achieved by attaching additional substituents to the fullerene core.9 There has been a few examples of fullerene bisadducts employed in OSC devices but these bisadduct materials are typically regioisomer mixtures.10–14 There has been a handful of studies looking at the effect of regioisomers on device performance thus far.15–18 In particular, our group isolated a crystalline single isomer of the indene C70 bisadduct (IC70BA) and examined its device performance.19 While the results were promising, the single isomer was obtained by careful chromatographic separation which meant that the production of material was both time consuming and difficult to scale up.
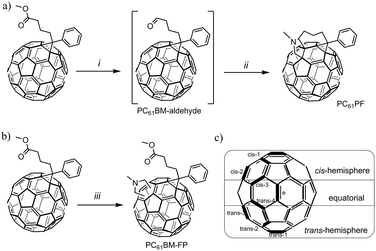
In order to target pure fullerene bisadduct materials in a more efficient manner, we decided to investigate a regioselective synthesis method, namely tether-directed remote functionalization.20,21 In general, this idea aims at increasing the regioselectivity of reactions on the fullerene surface by using a linker between the reactive head groups. This method has been successfully demonstrated on both C60 and C70 substrates and perfect regioselectivity as well as stereoselectivity has been achieved.22–24 In this work, the tether-directed functionalization approach was used to obtain selectively a C60 bisadduct, N-methyl-phenyl-C61-propyl-2-fulleropyrrolidine (PC61PF). A range of characterization methods were performed to show that the final product was a single regioisomer. To compare the properties of the single isomer with an isomeric mixture, a non-tethered PC61BM-based fulleropyrrolidine (PC61BM–FP) was also prepared (Fig. 1). The single isomer of PC61PF as well as PC61BM–FP and PC61BM were used in BHJ OSC devices to examine their photovoltaic performance.
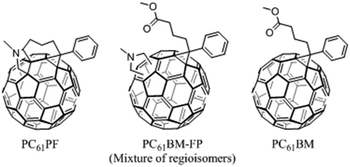 | ||
| Fig. 1 Chemical structures of single isomer, PC61PF, bisadduct isomeric mixture, PC61BM–FP, and widely used PC61BM. | ||
The fullerene bisadduct PC61PF was synthesized from PC61BM in one pot over two steps in 53% yield (Fig. 2a). Firstly, diisobutylaluminium hydride (DIBAL) was added dropwise to a stirred solution of PC61BM at −78 °C in toluene under nitrogen atmosphere. After stirring for one hour, the reaction was quenched with methanol to give the crude aldehyde intermediate. Solvents were removed under vacuum from the reaction vessel and the aldehyde was used in the next step without isolation. The aldehyde was heated with N-methylglycine in 1,2-dichlorobenzene (o-DCB) at 180 °C with to form PC61PF. The 1,3-dipolar cycloaddition step was monitored by thin layer chromatography (SiO2, toluene) to optimize the formation of the fulleropyrrolidine. The PC61PF product was purified by flash chromatography (SiO2, toluene). The purity of the product was analyzed using high performance liquid chromatography with a Cosmosil Buckyprep-D column which indicated the sample contained one major product (see ESI†). The isomeric mixture PC61BM–FP was synthesized from PC61BM and N-methylglycine in 29% yield (Fig. 2b).
The 1,3-dipolar cycloaddition on fullerenes usually results in reaction on the double bonds between six-member rings (namely the [6,6] bonds). In the case of C60 bisadducts, there are eight potential reacting position for the second substituent, which can result in at least eight isomers (Fig. 2c). Due to the length of the linker on PC61PF, possible regioisomers should be limited to the cis-hemisphere of the molecule. Notably, potential products will contain stereoisomers because of the flexibility of the linker.
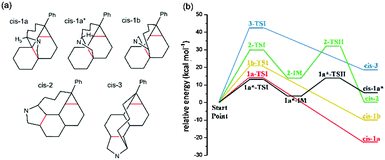
To predict the configuration of the final product, theoretical calculations were carried out with Gaussian 09, applying density functional theory at the B3LYP level. The basis set of 6-311G, with polarization functions (d,p), was used in the calculations. Considering both the length of the three-member carbon chain and the result of theoretical calculation, the possible positions of the pyrrolidine substituent were narrowed down to the cis-1, cis-2 and cis-3 positions of C60. Apart from regioisomers, geometric isomers are also possible at the cis-1 position, namely cis-1a, cis-1a* (the H5 on cis-1a* pointing toward the phenyl group) and cis-1b (Fig. 3a). The relative energy of the transition state of the 1,3-dipolar cycloaddition as well as the energy of the start material and possible products were calculated (Fig. 3b and Table S1, ESI†). The starting point of the calculation was the azomethine intermediate which originated from the reaction between the aldehyde and the amino acid (see ESI† for details). The activation barriers in o-DCB solvent environment are estimated to be 14.03 kcal mol−1 for cis-1a, 21.10 kcal mol−1 for cis-1b and 42.67 kcal mol−1 for cis-3. On the other hand, stepwise cycloaddition is suggested for cis-1a* and cis-2 via the intermediates 1a*-IM and 2-IM with the rate-limiting barriers of 13.39 and 29.76 kcal mol−1, respectively. Based on the computational results, cis-1a and cis-1a* are the kinetic products with the lowest activation energy, while cis-1a is also the thermodynamic product with the deepest product Gibbs free energy. These theoretical calculations were used to guide the structural assignments.
Further data on structure assignment was obtained with UV-vis absorption spectroscopy (Fig. 4). It is known that the UV-vis absorption spectrum of fullerene derivatives contains distinct bands and features for different regioisomers. This is related to the variations of the conjugated surface of the fullerene derivatives. The UV-vis spectrum of PC61PF showed an onset of 730 nm and a peak at 440 nm (Fig. 4). These features are similar to that observed for a reported C60–benzyne bisadduct with cis-1 configuration.25 This observation is in agreement with the assignments in the NMR experiments and in the theoretical calculations.
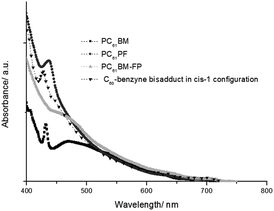 | ||
| Fig. 4 The UV-vis absorption spectrum of PC61BM, PC61PF, the mixture of PC61BM–FP and C60–benzyne bisadducts in cis-1 configuration. | ||
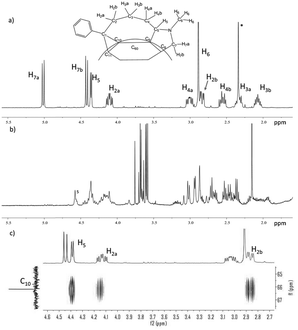
The 1H-NMR spectrum of the single isomer of PC61PF was well-resolved (Fig. 5a). Most interestingly, the six protons on the propyl linker all have different chemical shifts in the 1 to 5 ppm region of the NMR spectrum. These separated proton resonances strongly supported the fact that the propyl linker was locked in a fused cyclic system with the protons residing in different environments above the fullerene surface. This was also indicative of the regioselectivity of the reaction. Further indication of selectivity was evident by comparing the 1H NMR spectrum of the bisadduct mixture PC61BM–PF to that of the PC61PF sample (Fig. 5a and b). Full assignments of the proton and carbon resonances of the substituents on PC61PF was achieved using correlation spectroscopy and heteronuclear single quantum coherence spectroscopy 2D-NMR experiments (see ESI†). With these assignments, it was possible to use heteronuclear multiple-bond correlation spectroscopy (HMBC) experiment to aid in the structural determination. By focusing on the C10 carbon, it was apparent that there was coupling with protons H5, H2a and H2b (Fig. 5c). This correlation suggested a structural configuration that matched the cis-1a isomer. In addition, the configuration of the cis-1b isomer would lead to coupling of carbon C10 with protons H7a and H7b. This was not observed in the HMBC experiment.
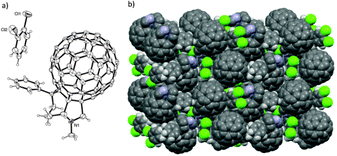
The relative configuration of the PC61PF material was confirmed by single crystal X-ray analysis (Fig. 5). Good quality single crystals were obtained by recrystallization from o-DCB and petroleum spirit 40–60 °C. Crystals of PC61PF consisted of a racemic mixture of the cis-1a isomer with o-DCB solvent incorporated in the crystal structure. The packing diagram of crystalline PC61PF showed alternating rows of enantiomeric pairs with o-DCB filling gaps between PC61PF molecules. (Fig. 6). The nearest distance between fullerenes is 3.38 Å.
With the molecular structure of PC61PF solved, relevant data on its materials properties were acquired. To estimate the LUMO energy level of the material, cyclic voltammetry was performed (Fig. S14, ESI†). Data was also obtained for the PC61BM–FP mixture and PC61BM for comparison (Table 1). The fullerene bisadducts PC61PF and PC61BM–FP had relatively higher LUMO energy levels of −3.76 eV and −3.66 eV respectively compared to that of PC61BM at −3.85 eV. This was the predicted outcome and improved open circuit voltages were expected for devices using the bisadducts as the electron acceptor material. Thermal analysis, such as differential scanning calorimetry and thermo gravimetric analysis, were also performed on the materials (Fig. S9 to S13, ESI†). No phase transitions were observed up to 300 °C and the decomposition temperature (5% weight loss) for fullerene compounds PC61PF and PC61BM was more than 400 °C (Table 1).
| Electron acceptor materials |
E
red1/2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (eV) a (eV) |
E
LUMO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (eV) b (eV) |
UV-vis abs λmax![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c (nm) c (nm) |
T
d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d (°C) d (°C) |
|---|---|---|---|---|
| a Half-wave reduction potential from cyclic voltammetry. b E LUMO = −(Ered1/2 + 4.8) eV. c Absorption coefficient (×103 M−1 cm−1) in brackets. d Decomposition temperature (5% weight loss). | ||||
| PC61PF | −1.04 | −3.76 | 437 (8.7) | 428 |
| PC61BM–FP | −1.15 | −3.65 | 463 (4.7) | 379 |
| PC61BM | −0.95 | −3.85 | 432 (4.1) | 432 |
The final part of the study was to compare the solar cell performance of the single isomer PC61PF material with PC61BM and the isomer mixture PC61BM–FP. Bulk heterojunction devices were made in the following geometry: ITO/PEDOT:PSS/active layer/Ca/Al, in which the active layer was a blend of poly(3-hexylthiophene) P3HT and each acceptor materials with the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by weight (Fig. S15, ESI†). The processing solvent was o-DCB and the thicknesses of the devices was optimized by adjusting solution concentration and spin coating speed (see ESI†). The device performance parameters are summarized in Table 2. It is clear that the Voc of devices increased significantly, from 0.58 to 0.70 V, when using PC61PF instead PC61BM as acceptor material (Fig. S15, ESI†). This is in agreement with previous literature reports of using fullerene bisadduct materials to boost the Voc.19 Comparing the devices containing PC61PF and the isomer mixture PC61BM–FP, both Jsc and FF were higher for the PC61PF devices (Table 2). A reason for this observation is the pure single isomer material PC61PF should have better charge transport properties than the isomer mixture PC61BM–FP (vide infra). The external quantum efficiency (EQE) spectrum of the devices were obtained to investigate the photo-current contribution of the active layers in OPV devices. The better performing device containing PC61PF showed significant photocurrent enhancement from 300 nm to 630 nm comparing to the devices containing PC61BM–FP (Fig. S15, ESI†). The maximum EQE of device containing PC61PF was 63% at 500 nm which was higher than the maximum EQE of 59% in the devices containing PC61BM–FP. The EQE data of the PC61BM device showed slightly higher efficiency across the spectrum. This could be attributed to the higher charge mobility of the PC61BM material (vide infra). It is interesting to note that the higher ELUMO measured in electrochemical experiments for PC61BM–FP was not reflected in the Voc of devices. Two factors may account for this observation. The PC61BM–FP material consisted of a mixture of isomers and there could be energy level variations between the various species present in the sample. Another way to look at these results is to consider the variation in device optimization. Assuming the electrochemical data gave a true reflection on the ELUMO of the PC61BM–FP material, then the Voc of device should be higher. Device optimizations, such as blend ratios and processing methods, have been shown to affect Voc. It may be possible to improve the Voc of the PC61BM–FP device through extensive device optimization.
1 by weight (Fig. S15, ESI†). The processing solvent was o-DCB and the thicknesses of the devices was optimized by adjusting solution concentration and spin coating speed (see ESI†). The device performance parameters are summarized in Table 2. It is clear that the Voc of devices increased significantly, from 0.58 to 0.70 V, when using PC61PF instead PC61BM as acceptor material (Fig. S15, ESI†). This is in agreement with previous literature reports of using fullerene bisadduct materials to boost the Voc.19 Comparing the devices containing PC61PF and the isomer mixture PC61BM–FP, both Jsc and FF were higher for the PC61PF devices (Table 2). A reason for this observation is the pure single isomer material PC61PF should have better charge transport properties than the isomer mixture PC61BM–FP (vide infra). The external quantum efficiency (EQE) spectrum of the devices were obtained to investigate the photo-current contribution of the active layers in OPV devices. The better performing device containing PC61PF showed significant photocurrent enhancement from 300 nm to 630 nm comparing to the devices containing PC61BM–FP (Fig. S15, ESI†). The maximum EQE of device containing PC61PF was 63% at 500 nm which was higher than the maximum EQE of 59% in the devices containing PC61BM–FP. The EQE data of the PC61BM device showed slightly higher efficiency across the spectrum. This could be attributed to the higher charge mobility of the PC61BM material (vide infra). It is interesting to note that the higher ELUMO measured in electrochemical experiments for PC61BM–FP was not reflected in the Voc of devices. Two factors may account for this observation. The PC61BM–FP material consisted of a mixture of isomers and there could be energy level variations between the various species present in the sample. Another way to look at these results is to consider the variation in device optimization. Assuming the electrochemical data gave a true reflection on the ELUMO of the PC61BM–FP material, then the Voc of device should be higher. Device optimizations, such as blend ratios and processing methods, have been shown to affect Voc. It may be possible to improve the Voc of the PC61BM–FP device through extensive device optimization.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 blend ratio by weight)
1 blend ratio by weight)
| Active layer | J sc (mA cm−2) | V oc (V) | FF (%) | PCE (%) | Electron mobility 10−4 (cm2 V−1 s−1) |
|---|---|---|---|---|---|
| P3HT | 8.5 ± 0.3 | 0.58 ± 0.01 | 63 ± 2 | 3.1 ± 0.2 | 8.50 |
| PC61BM | |||||
| P3HT | 8.1 ± 0.3 | 0.70 ± 0.02 | 58 ± 3 | 3.3 ± 0.3 | 3.80 |
| PC61PF | |||||
| P3HT | 7.7 ± 0.4 | 0.69 ± 0.02 | 50 ± 3 | 2.7 ± 0.2 | 1.35 |
| PC61BM–FP |
To examine the charge transport behavior of the acceptor materials in the bulk heterojunction, the space charge limited current (SCLC) measurements were obtained (Fig. S16, ESI†). The electron mobility in devices containing PC61PF and PC61BM–FP were calculated to be 3.80 × 10−4 and 1.35 × 10−4 cm2 V−1 s−1 respectively. This result showed that the single isomer of fullerene bisadduct had better electron mobility than the isomer mixture PC61BM–FP in these BHJ devices. In addition, the hole mobility of the P3HT from SCLC was previously measured at 3.30 × 10−4 cm2 V−1 s−1 in BHJ devices.26 This meant that more balanced charge transport occurred in the P3HT–PC61PF blend films. The surface morphology of the films were examined using atomic force microscopy. Nanoscale features were observed in the topography and phase images and were similar in all P3HT:fullerene blend films (Fig. S17, ESI†). This means the difference in the solar cell device performance between the PC61PF and PC61BM–FP materials can be attributed to their electron mobility in the BHJ blends.
Regioselective synthesis of a single C60 bisadduct isomer, PC61PF, was achieved efficiently in a one-pot two step procedure from commercially available PC61BM. The tether-directed functionalization strategy was applied successfully to control the regioselectivity of the final product in the cis-1 configuration. In BHJ solar cell devices, the single isomer PC61PF outperformed the isomeric mixture PC61BM–FP and was on par with PC61BM. Further research in modifying the properties, such as solubility and crystallinity, of PC61PF derivatives by changing the amino acid in the synthesis process is currently in progress.
This work was made possible by support from the Australian Renewable Energy Agency which funds the project grants within the Australian Centre for Advanced Photovoltaics. WWHW is supported by an Australian Research Council Future Fellowship (FT130100500). Responsibility for the views, information, or advice herein is not accepted by the Australian Government.
Notes and references
- R. Søndergaard, M. Hösel, D. Angmo, T. T. Larsen-Olsen and F. C. Krebs, Mater. Today, 2012, 15, 36–49 CrossRef.
- L. Echegoyen and L. E. Echegoyen, Acc. Chem. Res., 1998, 31, 593–601 CrossRef CAS.
- P. Bracher and D. Schuster, in Fullerenes: From Synthesis to Optoelectronic Properties, ed. D. Guldi and N. Martin, Springer, Netherlands, 2002, ch. 6, vol. 4, pp. 163–212 Search PubMed.
- F. Langa and J.-F. Nierengarten, Fullerenes: principles and applications, Royal Society of Chemistry, 2007 Search PubMed.
- G. Yu, J. Gao, J. Hummelen, F. Wudl and A. Heeger, Science, 1995, 270, 1789–1790 CAS.
- Y. He, H.-Y. Chen, J. Hou and Y. Li, J. Am. Chem. Soc., 2010, 132, 1377–1382 CrossRef CAS PubMed.
- H. Hoppe and N. S. Sariciftci, J. Mater. Res., 2004, 19, 1924–1945 CrossRef CAS.
- G. Dennler, M. C. Scharber and C. J. Brabec, Adv. Mater., 2009, 21, 1323–1338 CrossRef CAS PubMed.
- M. J. van Eis, P. Seiler, L. A. Muslinkina, M. Badertscher, E. Pretsch, F. Diederich, R. J. Alvarado, L. Echegoyen and I. P. Nunez, Helv. Chim. Acta, 2002, 85, 2009–2055 CrossRef CAS.
- Y.-Y. Lai, Y.-J. Cheng and C.-S. Hsu, Energy Environ. Sci., 2014, 7, 1866–1883 CAS.
- C.-Z. Li, H.-L. Yip and A. K. Y. Jen, J. Mater. Chem., 2012, 22, 4161–4177 RSC.
- H.-L. Yip and A. K. Y. Jen, Energy Environ. Sci., 2012, 5, 5994–6011 CAS.
- Y. Li, Chem. – Asian J., 2013, 8, 2316–2328 CrossRef CAS PubMed.
- T. Umeyama and H. Imahori, J. Mater. Chem. A, 2014, 2, 11545–11560 CAS.
- X. Meng, G. Zhao, Q. Xu, Z. a. Tan, Z. Zhang, L. Jiang, C. Shu, C. Wang and Y. Li, Adv. Funct. Mater., 2014, 24, 158–163 CrossRef CAS PubMed.
- Y. Matsuo, J. Kawai, H. Inada, T. Nakagawa, H. Ota, S. Otsubo and E. Nakamura, Adv. Mater., 2013, 25, 6266–6269 CrossRef CAS PubMed.
- S. Kitaura, K. Kurotobi, M. Sato, Y. Takano, T. Umeyama and H. Imahori, Chem. Commun., 2012, 48, 8550–8552 RSC.
- M.-H. Liao, Y.-Y. Lai, Y.-Y. Lai, Y.-T. Chen, C.-E. Tsai, W.-W. Liang and Y.-J. Cheng, ACS Appl. Mater. Interfaces, 2014, 6, 996–1004 CAS.
- W. W. H. Wong, J. Subbiah, J. M. White, H. Seyler, B. L. Zhang, D. J. Jones and A. B. Holmes, Chem. Mater., 2014, 26, 1686–1689 CrossRef CAS.
- R. Breslow, Acc. Chem. Res., 1980, 13, 170–177 CrossRef CAS.
- L. Isaacs, R. F. Haldimann and F. Diederich, Angew. Chem., Int. Ed., 1994, 33, 2339–2342 CrossRef PubMed.
- C. Thilgen and F. Diederich, C. R. Chim., 2006, 9, 868–880 CrossRef CAS PubMed.
- W. W. H. Wong and F. Diederich, Chem. – Eur. J., 2006, 12, 3463–3471 CrossRef CAS PubMed.
- S. Sergeyev and F. Diederich, Angew. Chem., Int. Ed., 2004, 43, 1738–1740 CrossRef CAS PubMed.
- Y. Nakamura, N. Takano, T. Nishimura, E. Yashima, M. Sato, T. Kudo and J. Nishimura, Org. Lett., 2001, 3, 1193–1196 CrossRef CAS PubMed.
- C. Goh, R. J. Kline, M. D. McGehee, E. N. Kadnikova and J. M. J. Fréchet, Appl. Phys. Lett., 2005, 86, 122110 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details on the synthesis procedure and characterization of the materials as well as device fabrication and testing. CCDC 1057267. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5cc02701b |
| This journal is © The Royal Society of Chemistry 2015 |