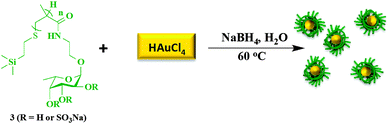Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis and anticancer properties of fucoidan-mimetic glycopolymer coated gold nanoparticles†
Mattias
Tengdelius‡
ab,
Deepanjali
Gurav‡
cd,
Peter
Konradsson
ab,
Peter
Påhlsson
bf,
May
Griffith
bef and
Oommen P.
Oommen
*c
aDivision of Organic Chemistry, Linköping University, SE-581 83, Linköping, Sweden
bIntegrative Regenerative Medicine Center (IGEN), Linköping University, SE-581 83, Linköping, Sweden
cDepartment of Chemistry, Ångström Laboratory, Uppsala University, S-75121 Uppsala, Sweden. E-mail: oommen.op@kemi.uu.se
dDepartment of Chemistry, Savitri Bai Phule Pune University, India
eDivision of Molecular Physics, Department of Physics, Biology and Chemistry (IFM), Linköping University, SE-581 83, Linköping, Sweden
fDivision of Cell Biology, Department of Clinical and Experimental Medicine (IKE), Linköping University, SE-581 83, Linköping, Sweden
First published on 10th April 2015
Abstract
Gold nanoparticles coated with fucoidan-mimetic glycopolymers were synthesized that displayed good colloidal stability and promising anti-cancer properties. Fucoidan mimetic glycopolymers on their own were nontoxic, while glycopolymer coated gold nanoparticles displayed selective cytotoxicity to human colon cancer cell lines (HCT116) while it was non-toxic to mouse fibroblast cells (NIH3T3).
Fucoidan is a fucose-rich sulfated polysaccharide obtained primarily from brown seaweeds. Structurally it resembles heparin, owing to the presence of L-fucose, with varying degrees of sulfation, in addition to the presence of D-xylose, D-galactose, D-mannose and glucuronic acid.1 Fucoidan therefore possesses a range of biological activities that are similar to those of heparin, e.g. it has been reported to be an anti-coagulant, and an anti-thrombotic, anti-angiogenic, anti-proliferative and anti-cancer agent etc.2,3 The multivalent interactions of these polysaccharides with the cell surface receptors are essential for cellular response and bioactivity. Therefore, grafting these sulfated L-fucosides onto a polymeric network mimics the natural polysaccharide, facilitating multiple representation of the active molecule. Unlike the natural polysaccharide, the synthetic functional polymer can be synthesized, purified and scaled up in a controlled manner that can transform this natural product to into a medicinal product that can be advanced into clinical application. We recently reported the synthesis of fucoidan-mimetic glycopolymers (FM-glycopolymers) and showed that these synthetic polymers possess anti-viral properties similar to the natural polysaccharide.4 Herein, we report the synthesis of FM-glycopolymers via a free-radical chain transfer polymerization reaction with improved polydispersity and higher yield compared to our previous report.4
An important criterion for the successful translation of these synthetic polymers into therapeutic molecules is their delivery to the target cells. The anionic polymer generally does not readily undergo endocytosis due to electrostatic repulsion and thermodynamic instability. However, transforming these glycopolymers into a nanoparticle formulation could promote efficient cellular delivery. Gold nanoparticles are versatile platforms for the delivery of therapeutic agents and are known to deliver large anionic polymers like glycosaminoglycans5 and nucleic acids.6 Apart from its carrier function,7 gold nanoparticles have been reported to show anti-cancer properties8 and also function as contrast agents, photothermal agents, and radiosensitizers.9 In this study, we tested the hypothesis that coating gold nanoparticles with FM-glycopolymers may result in a composite particle with synergistic anti-cancer effects. To validate the role of FM-glycopolymer we also developed chondroitin sulfate-A (CS) coated gold nanoparticles (CS–Au–NPs). CS is a natural glycosaminoglycan, which is an integral part of the extracellular matrix and is a structural component of human cartilage. Similar to fucoidan, CS is a sulfated polysaccharide, however it is known to be non-toxic.
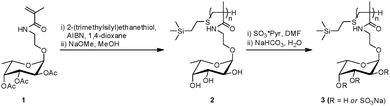
To synthesize a FM-glycopolymer, we utilized our previously reported fucoside monomer (2-methacrylamidoethyl 2,3,4-tri-O-acetyl-α-L-fucopyranoside)41 as a suitable starting material. Monomer 1 was polymerized in dioxane through a classical free-radical chain transfer polymerization with 2-(trimethylsilyl)ethanethiol as the chain transfer agent and AIBN as the radical initiator (Scheme 1). The peracetylated polymer was precipitated in ether, collected through filtration and de-protected in NaOMe/MeOH to give glycopolymer 2 (53% monomer conversion over two steps) upon purification by dialysis against deionized water. 1H NMR spectroscopy (Fig. 1) showed the presence of the trimethylsilyl protons at 0.0 ppm and was otherwise in good agreement with the results from our previously reported FM-glycopolymers.4 Free-radical polymerization with thiols acting as chain transfer agents yields polymers with polydispersities ranging from 1.3 to 4.7.10–12 GPC analysis of glycopolymer 2 (Fig. 1B) showed a number-average molecular weight of 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 Da and a satisfactory polydispersity of 1.32.
600 Da and a satisfactory polydispersity of 1.32.
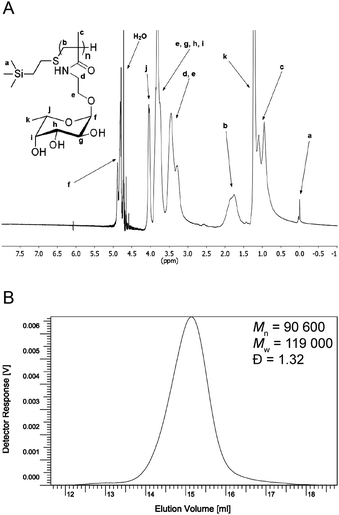 | ||
| Fig. 1 (A) Characterization of glycopolymer 2. 1H NMR in D2O, 300 MHz. (B) GPC elugram of glycopolymer 2. | ||
Glycopolymer 2 was subsequently partially O-sulfated using a sulfur trioxide–pyridine complex in DMF and treated with sodium bicarbonate to furnish the sodium salt product FM-glycopolymer 3. The obtained product was purified by dialysis against deionized water. 1H NMR analysis showed a downfield shift of the fucoside protons neighboring the sulfate esters as described in our previous protocol 4 thus confirming a successful O-sulfation. Elemental analysis showed the carbon content to be 24.9 wt% and the sulfur content to be 14.4 wt%, which corresponds to a hydroxyl group to sulfate ester conversion of 87%.
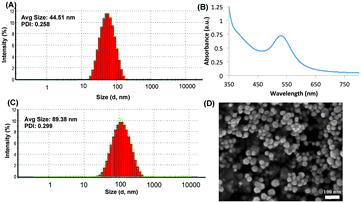
After successfully synthesizing the FM-glycopolymer, we synthesized gold nanoparticles using NaBH4 as the reducing agent and the FM-glycopolymer as the capping agent (Scheme 2). The ratio of HAuCl4, NaBH4 and FM-glycopolymer was carefully optimized to deliver uniform particles with monomodal distribution. A molar ratio of 0.5 equivalents of NaBH4, 1 equivalent of HAuCl4 and 2 equivalents of glycopolymer (with respect to L-fucoside repeat units) was found to be efficient in producing spherical nanoparticles with an average size of 44 nm as measured by dynamic light scattering (DLS) experiments (Fig. 2A). As a control experiment we synthesized CS–Au–NPs using NaBH4 as the reducing agent, which formed particles of 40 nm size (data not shown). Gold nanoparticles (Au–NPs) are generally stable as a colloidal suspension and a lyophilization procedure generally destroys the particle yielding macro-sized aggregates. However, for clinical translation nanoparticles that could be stored in the dry state could be extremely advantageous, as this would prolong storage and would give control over particle dispersion/concentration.
We anticipated that the gold particles coated with FM-glycopolymers (FMG–Au–NPs) would possess good colloidal stability, promoted by their inherent electrostatic repulsion. To test this hypothesis, we subjected the FMG–Au–NPs to lyophilization. Upon re-dispersion of the lyophilized sample in water we observed a change in particle size from 44 nm to 90 nm with monomodal distribution indicating re-assembly as a result of lyophilization (Fig. 2C). Similarly, CS–Au–NPs provided nanoparticles of 99 nm with good polydispersity (Fig. S1 in ESI†). The UV-Vis analysis showed that the FMG–Au–NP possessed λmax at 530 nm, with single surface plasma resonance confirming that the particles were spherical in shape (Fig. 2B). This was further corroborated by SEM analysis of FMG–Au–NPs, which showed spherical nanoparticles ranging from 20–55 nm size distributions (Fig. 2D). This difference in size distribution (as compared to DLS) is presumably due to hydration of the hygroscopic FMG coating of particles during DLS measurements, which is absent in dry samples used for SEM.
To evaluate the percentage of the polymer presented on the gold nanoparticle surface of FMG–Au–NPs and CS–Au–NPs, we performed thermogravimetric analysis (TGA). We observed that FMG–Au–NPs and CS–Au–NPs contained 65.55% and 83.16% of polymer respectively (Fig. 3).
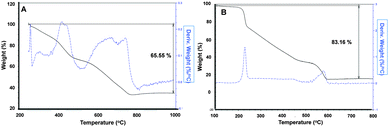 | ||
| Fig. 3 (A) Thermogravimetric analysis profile of FMG–Au–NPs. (B) Thermogravimetric analysis profile of CS–Au–NPs. | ||
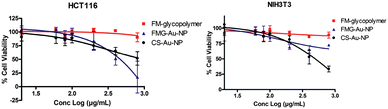
We then assessed the differential cytotoxic effects of FMG–Au–NPs and the FM-glycopolymer on a human colon cancer cell line (HCT116) and compared it with a non-cancer mouse fibroblast cell line (NIH3T3). We performed the cytotoxic evaluation using an ApoTox-Glo™ Triplex Assay kit that assays for viability, cytotoxicity and apoptosis, following the manufacturer's protocol. We measured the cell viability and estimated the inhibition coefficient with 50% cell death (IC50) for FMG–Au–NPs and FM-glycopolymers by logarithmic curve fitting of cell viability (%) using the Graphpad Prism software (Fig. 4). Dose dependent cytotoxicity for FMG–Au–NPs was observed with the HCT116 cells while the FG-glycopolymer alone had no effect (Fig. 4). The IC50 value for FMG–Au–NPs against HCT116 cells was found to be 457.08 μg mL−1, which is lower than fucoidan polysaccharides derived from Ascophyllum nodosum13 and Saccharina cichorioides14 indicating higher cytotoxicity.
Neither FM-glycopolymer nor FMG–Au–NPs was cytotoxic to NIH3T3 cells. This clearly indicates that FMG–Au–NPs preferentially triggered apoptosis in the HCT116 cells. Unmodified Au–NP (citrate coated) is known to inhibit cancer cell proliferation8 however, it is known to be nontoxic to HCT116 cells up to a concentration of 1000 μg mL−1.15 In order to decipher the role of FMG in FMG–Au–NPs we investigate the cytotoxicity of analogous NPs, namely CS–Au–NPs. Interestingly, CS–Au–NPs showed significantly lower cytotoxicity to cancer cells (HCT116), as compared to the non-cancerous fibroblast cells (NIH3T3; IC50 = 494.31 μg mL−1). This clearly demonstrates that FMG–Au–NPs possess selective cytotoxicity to cancer cells over normal cells.
Kim et al. earlier reported that fucoidan derived from Fucus vesiculosus inhibited the growth of HCT116 cells and induces apoptosis via death-receptor-mediated and mitochondria-mediated apoptotic pathways.16 However, Chen et al. recently concluded that the anti-tumor activity of fucoidan on HCT116 cells is exerted by modulation of the ER stress cascades.17 Fucoidan from Ascophyllum nodosum was shown to induce 57% apoptosis in 48 h at doses of 1000 μg mL−1,13 whereas fucoidan from Saccharina cichorioides showed a modest 15% reduction in HCT116 cell numbers at 800 μg mL−1 in 24 h.14 To further understand the mechanism by which FMG–Au–NPs exerted cytotoxicity to the HCT116 cells, we measured the activity of caspase 3/7, an endoprotease responsible for cell disassembly during apoptosis using the Caspase-Glo® Assay which provides a luminescent signal proportional to the level of caspase activity in each cell line (Fig. S2 in ESI†). Interestingly, neither FM-glycopolymers nor FMG–Au–NPs triggered any significant caspase activity in HCT116 and NIH3T3 cell lines, suggesting that FMG–Au–NPs likely acted through caspase 3/7-independent mechanisms of apoptosis.18
In conclusion, we have described an efficient synthesis of a fucoidan-mimetic glycopolymer with good polydispersity and high yield. We exploited this glycopolymer for tailoring FM-glycopolymer coated gold nanoparticles. These nanoparticles when fully hydrated were found to have an average size of 90 nm by DLS and displayed 20–55 nm when dried for SEM analysis. FMG–Au–NPs possessed good colloidal stability even after lyophilization due to electrostatic repulsion. FMG–Au–NPs showed differential cytotoxicity toward colon cancer cells and were non-toxic to a fibroblast cell line. The FM-glycopolymer alone, however, was non-cytotoxic. This versatile biomimetic nanoparticle can be further explored for other biomedical applications where fucoidan is known to exhibit interesting bioactivity.
This work was supported by grants from Swedish Strategic Research ‘StemTherapy’. We also acknowledge Scilife platform of Karolinska high-throughput center, Dr Jianping Liu from Karolinska Institute for cytotoxicity studies, and Dr Anumol Ashokkumar for SEM analysis. We also thank Dr Vaishali S. Shinde for assisting DG.
Notes and references
- P. G. Thomas and R. Rengasamy, J. Med. Food, 2008, 11, 638–642 CrossRef PubMed.
- J. Y. Kwak, Mar. Drugs, 2014, 12, 851–870 CrossRef CAS PubMed.
- W. A. J. P. Wijesinghe and Y. J. Jeon, Carbohydr. Polym., 2012, 88, 13–20 CrossRef CAS PubMed.
- M. Tengdelius, C. J. Lee, M. Grenegård, M. Griffith, P. Påhlsson and P. Konradsson, Biomacromolecules, 2014, 15, 2359–2368 CAS.
- H. Lee, K. Lee, I. K. Kim and T. G. Park, Biomaterials, 2008, 29, 4709–4718 CrossRef CAS PubMed.
- Y.-W. Lin, C.-W. Liu and H.-T. Chang, Anal. Methods, 2009, 1, 14–24 RSC.
- R. Mout, D. F. Moyano, S. Rana and V. M. Rotello, Chem. Soc. Rev., 2012, 41, 2539–2544 RSC.
- R. R. Arvizo, S. Saha, E. Wang, J. D. Robertson, R. Bhattacharya and P. Mukherjee, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 6700–6705 CrossRef CAS PubMed.
- S. Jain, D. G. Hirst and J. M. O'Sullivan, Br. J. Radiol., 2012, 85, 101–113 CrossRef CAS PubMed.
- L. M. Gugliotta, A. Salazar, J. R. Vega and G. R. Meira, Polymer, 2001, 42, 2719–2726 CrossRef CAS.
- G. Maatz, A. Maciollek and H. Ritter, Beilstein J. Org. Chem., 2012, 8, 1929–1935 CrossRef CAS PubMed.
- S. Reinelt, D. Steinke and H. Ritter, Beilstein J. Org. Chem., 2014, 10, 680–691 CrossRef PubMed.
- S. A. Foley, B. Mulloy and M. G. Tuohy, J. Nat. Prod., 2011, 74, 1851–1861 CrossRef CAS PubMed.
- O. S. Vishchuk, S. P. Ermakova and T. N. Zvyagintseva, Mar. Drugs, 2013, 11, 194–212 CrossRef CAS PubMed.
- M. M. Joseph, S. R. Aravind, S. Varghese, S. Mini and T. T. Sreelekha, Colloids Surf., B, 2013, 104, 32–39 CrossRef CAS PubMed.
- E. J. Kim, S. Y. Park, J. Y. Lee and J. H. Y. Park, BMC Gastroenterol., 2010, 10, 96 CrossRef PubMed.
- S. Chen, Y. Zhao, Y. Zhang and D. Zhang, PLoS One, 2014, 9, e108157 Search PubMed.
- L. E. Broker, F. A. Kruyt and G. Giaccone, Clin. Cancer Res., 2005, 11, 3155–3162 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis of glycopolymers, synthesis of gold nanoparticles, cytotoxicity studies, caspase activity. See DOI: 10.1039/c5cc02387d |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2015 |