Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Efficient room temperature aqueous Sb2S3 synthesis for inorganic–organic sensitized solar cells with 5.1% efficiencies†
Karl C.
Gödel
a,
Yong Chan
Choi
 b,
Bart
Roose
ac,
Aditya
Sadhanala
a,
Henry J.
Snaith
d,
Sang Il
Seok
be,
Ullrich
Steiner
b,
Bart
Roose
ac,
Aditya
Sadhanala
a,
Henry J.
Snaith
d,
Sang Il
Seok
be,
Ullrich
Steiner
 *c and
Sandeep K.
Pathak
ad
*c and
Sandeep K.
Pathak
ad
aCavendish Laboratory, Department of Physics, University of Cambridge, J J Thomson Avenue, Cambridge CB3 0HE, UK
bDivision of Advanced Materials, Korea Research Institute of Chemical Technology, 141 Gajeong-Ro, Yuseong-Gu, Daejeon 305-600, Republic of Korea
cAdolphe Merkle Institute, Rue des Verdiers 4, CH-1700 Fribourg, Switzerland. E-mail: ullrich.steiner@unifr.ch
dClarendon Laboratory, Department of Physics, University of Oxford, Parks Road, Oxford, OX1 3PU, UK
eDepartment of Energy Science, Sungkyunkwan University, 2066 Seoburo, Jangsan-gu, Suwon 440-746, Republic of Korea
First published on 14th April 2015
Abstract
Sb2S3 sensitized solar cells are a promising alternative to devices employing organic dyes. The manufacture of Sb2S3 absorber layers is however slow and cumbersome. Here, we report the modified aqueous chemical bath synthesis of Sb2S3 absorber layers for sensitized solar cells. Our method is based on the hydrolysis of SbCl3 to complex antimony ions decelerating the reaction at ambient conditions, in contrast to the usual low temperature deposition protocol. This simplified deposition route allows the manufacture of sensitized mesoporous-TiO2 solar cells with power conversion efficiencies up to η = 5.1%. Photothermal deflection spectroscopy shows that the sub-bandgap trap-state density is lower in Sb2S3 films deposited with this method, compared to standard deposition protocols.
Antimony sulfide (Sb2S3) is a promising material for several optoelectronic applications. Due to its high absorption coefficient (α ≈ 1.8 × 105 cm−1 at λ = 450 nm) and a suitable direct band-gap of Eg ≈ 1.7 eV, crystalline Sb2S3 (stibnite) is interesting as light absorber for solid-state sensitized solar cells (Fig. 1).1,2 In particular, Sb2S3-based solar cells excel in their stability of operation when compared to other organic–inorganic hybrid devices. Recently, power-conversion efficiencies of η = 6.2% (ref. 3) and η = 7.5% (ref. 4) were achieved using Sb2S3 as the absorber material obtained from chemical bath deposition. Further, the material has been used to improve the stability of methyl-ammonium lead iodide perovskite solar cells.5
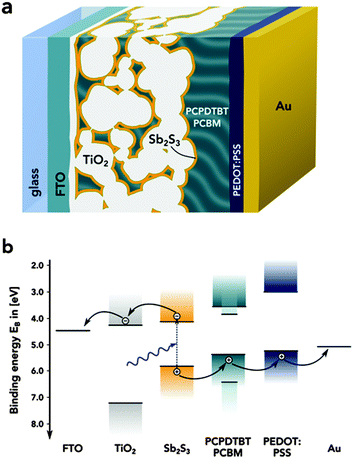 | ||
| Fig. 1 (a) Schematic of the solar cell cross section. (b) Simplified band-diagram of the Sb2S3 sensitised photovoltaic cells. The band edge values were taken from ref. 10. | ||
Antimony sulfide synthesis typically involves deposition in aqueous and non-aqueous chemical baths at low temperatures (low-T deposition).6–9 The standard method is the aqueous chemical bath deposition (CBD) using antimony chloride and sodium thiosulfate. This technique is however problematic since it requires a precise temperature control of the solution when cooling below 10 °C and maintaining the sample at low temperatures. For large-scale applications such a cooling protocol is cumbersome, costly and energy-intensive.
Here, we present an aqueous room temperature (RT) deposition route of Sb2S3 using the same precursor materials as the standard CBD method. We have fabricated Sb2S3-sensitized solar cells using this RT deposition method and demonstrate excellent device performance with efficiencies of up to η = 5.1%.
The low-temperature synthesis of Sb2S3 for photovoltaic applications involves the chemical reaction equations7
| 2SbCl3 + 3Na2S2O3 → Sb2(S2O3)3 + 6NaCl |
| Sb2(S2O3)3 + 6H2O → Sb2S3 + 3HSO4− + 3H3O+. |
These reactions have to be slowed down by cooling below 10 °C to avoid immediate precipitation7 and to enable strong adhesion of Sb2S3 to the substrate. Thus, the standard CBD method, termed low-T deposition, requires cooling of the reaction solution, whereas our modified method, RT deposition, can be performed at room temperature. By changing the order of reactant addition at RT, Sb2S3 formation is slowed down and well-adhering films are obtained.
For the RT deposition, a 1.4 M SbCl3 solution in acetone was prepared. Note that the optimal concentration of SbCl3 for the RT method is slightly higher compared to low-T deposition (Fig. S7, ESI†). SbCl3 can be used without dissolution in acetone, changing the reaction behaviour very little (Fig. S8, ESI†). The addition of acetone facilitates however the handling of the highly hygroscopic SbCl3. Deionised water is added under vigorous stirring to reduce the total concentration of SbCl3 to 46 mM. The addition of water hydrolyses SbCl3, which leads to a solid white precipitate. The product of the hydrolysis reaction of SbCl3 is not very well defined. It depends on many parameters such as the dilution of the reaction medium, the pH value of the solution and solvent composition.11,12 The aqueous solution containing the hydrolysed SbCl3 has a pH of 1.4.
The precipitate was filtered and dried and X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR) and energy-dispersive X-ray (EDX) spectroscopy were performed on the white powder (Fig. S10–S12, ESI†). The XRD pattern shows crystalline phases of Sb4O5Cl2, Sb8(OH)6O8Cl2(H2O), Sb8O11Cl2(H2O)6 and Sb3O6(OH). This is in contrast to the reports by Li et al. and Yu et al. describing similar reactions.13,14 They also reporting the formation of a white precipitate, which they identify as antimony oxychloride SbOCl. The XRD pattern of Fig. S10 (ESI†) however shows no evidence of crystalline SbOCl formation. According to Chen et al. hydrolysis at pH 1–2 leads to the formation of Sb4O5Cl2 for mixed solvents such as water and ethanol or water and ethylene glycol.12 We also do not observe an immediate colour change of the precipitate to orange upon the addition of the sulphur source, as reported by Li et al.13 and Yu et al.14
Subsequently, a 1 M aqueous Na2S2O3 solution was added at a final concentration of 0.25 M in the chemical bath. This causes the solution to turn clear as most of the precipitate dissolves, suggesting the formation of a water-soluble complex. After 5–10 min at 20 °C, the solution starts to turn orange, indicating the formation of amorphous Sb2S3. This is accompanied by a pH change of the solution from pH = 3.3 upon sodium thiosulfate (pH = 7.3) addition to pH = 4.3 when antimony sulfide deposition is complete after two hours.
Fig. S2 (ESI†) shows the UV-vis spectra of RT-deposited Sb2S3 films on mesoporous-TiO2, annealed at 300 °C for 5 min.
The annealed antimony sulfide was characterised by powder X-ray diffraction (XRD). Fig. 3a compares the XRD pattern of the low-T and RT deposition methods. Both pattern are very similar and match the stibnite reference pattern.15 Sb2S3 films deposited by the RT method onto mesoporous TiO2 substrates also show the characteristic XRD peaks of crystalline Sb2S3 (Fig. S3, ESI†). A Rietveld analysis of the two patterns using the powder diffraction software ReX16 yields an average Sb2S3 crystallite size of 40 nm and 35 nm for RT and low-T deposition, respectively. The structure of the solar cell studied in this work and a schematic band diagram are shown in Fig. 1.
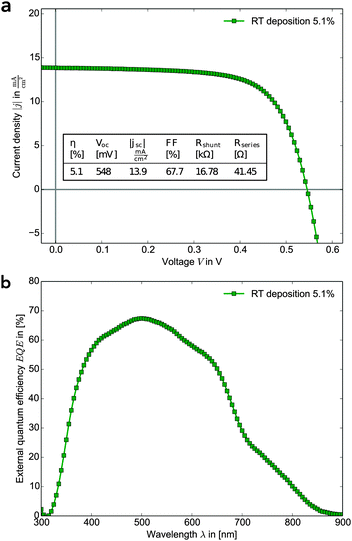
Fig. 2 shows (a) the current–voltage-characteristic and (b) the external quantum efficiency EQE of the best performing solar cell employing RT deposited Sb2S3. Its photovoltaic parameters are summarised in Fig. 2. The shunt and series resistances were obtained by a least square fit of the diode function. The RT method enables the fabrication of solar cells with high reproducibility. The deviations from batch to batch and from device to device were small (Fig. S13, ESI†). The hole transport layer consists of a PCPDTBT–PCBM blend. Charge carriers which are generated by photon absorption in the hole transport layer can also be harvested due to the formation of PCBM electron conducting channels. The PCBM–PCPDTBT blend thus contributes to the current and causes the near-infrared shoulder in the EQE spectrum.17 Earlier cells using P3HT as hole conductor showed lower efficiencies compared to the PCPDTBT:PCBM blend. We therefore concentrated on the donor–acceptor PCPDTBT–PCBM blend. A comparison of a RT solar cell and a solar cell with the same device architecture using the low-temperature deposition method is shown in the ESI† (Fig. S4). The reference cells were prepared in the same laboratory using the identical procedures as the better performing earlier published devices.4 The lower performance may reflect the variation in this system that possibly arises from minor variations in device fabrication. It is however important to point out that the reference devices and devices made with our new methodology were created in parallel and therefore are much less likely to be subject to this type of variation.
X-ray photoelectron spectroscopy (XPS) measurements were carried out on Sb2S3 films formed by both deposition techniques, shown in Fig. 3b.
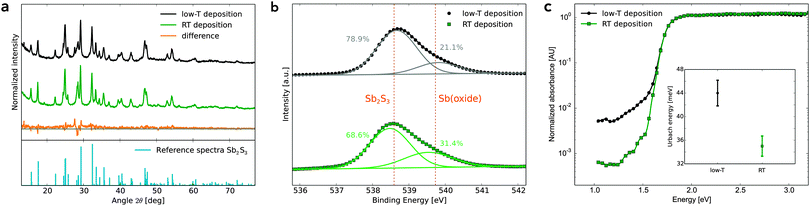 | ||
| Fig. 3 (a) XRD pattern of crystalline Sb2S3, synthesised via the low temperature deposition method (upper trace) and the RT technique (center trace). The lower trace shows the difference of the two patterns. The stibnite reference pattern was taken from ref. 15. (b) XPS Sb3d3/2 peak of films from both deposition methods. The solid lines show a least squares fit of a superposition of two Gaussian curves. (c) PDS measurements showing the sub band-gap energy levels for both deposition methods. The inset shows the corresponding Urbach energies. | ||
To compare the oxide content of the samples, the antimony Sb3d3/2 peak was examined, because the oxygen O1s peak directly overlaps with the antimony Sb3d5/2 peak. The Sb3d3/2 peak can be modelled using a superposition of two Gaussians, one at ≈538.5 eV representing Sb2S3 and one at ≈539.5 eV for SbOx, most likely Sb2O3.18 The RT sample has a marginally higher oxide content compared to the low-T material. This probably causes the lower conductivity seen in these films (Fig. S6, ESI†).
One of the biggest challenges of using antimony sulfide as absorber in sensitized solar cells is the high density of electronic traps in this material, i.e. the number of energy states which lie in the band-gap of Sb2S3.4,19 These trap-states lead to a significant loss in potential and to charge carrier recombination in the solar cell. To explore this, we employed photo-thermal deflection spectroscopy (PDS) to determine the trap-state density and the energetic disorder of Sb2S3. PDS is a highly sensitive absorption measurement technique, which can detect absorbance values down to 10−5 AU. Thus, PDS is able to accurately measure weak absorption in the bandgap. Fig. 3c shows the PDS spectra of Sb2S3 samples on mesoporous TiO2 for both deposition methods. The corresponding Urbach energies are given in the inset.
The absorption in the RT-deposited sample was significantly lower at energies below the band-gap of Sb2S3 compared to the low-T sample, by nearly one order of magnitude at energies below 1.5 eV. This indicates a clear reduction in the density of deep-trap states for the RT deposited Sb2S3. The difference in the open-circuit voltage for low-T and RT deposited Sb2S3 in optimized solar cells are however similar (Fig. S4, ESI†). As the band-gap of antimony oxide is higher than that of Sb2S3,20 the PDS spectrum cannot show a potential increase of deep traps caused by the higher content of antimony oxide in the RT-deposited sample.
We have demonstrated an aqueous deposition technique of antimony sulfide for sensitized solar cells, which can be carried out at room temperature. The chemical bath deposition method is based on the same precursor materials but uses the hydrolysis of SbCl3 to complex antimony ions. The resulting Sb2S3 films were investigated using UV-vis spectroscopy, XRD, PDS and XPS. PDS shows a reduction in sub-band gap trap states in RT-deposited Sb2S3. Manufactured devices achieved a maximum power conversion efficiency η = 5.1% for Sb2S3 sensitized solar cells using the RT deposition method. A more detailed optimization of the deposition step, interfacial surface treatments21,22 or doping of the Sb2S323 could lead to a further improvement in solar cell performance. This work is therefore an important step in the development of low-cost, stable and highly efficient solar cells.
We acknowledge Adam Brown's help with the XPS measurements. K.C.G. would like to thank the Cambridge Trust, the Mott Fund for Physics of the Environment and Corpus Christi College Cambridge for funding. A.S. acknowledges funding from the Engineering and Physical Sciences Research Council (EPSRC).
References
- M. Y. Versavel and J. A. Haber, Thin Solid Films, 2007, 515, 7171–7176 CrossRef CAS PubMed.
- S.-J. Moon, Y. Itzhaik, J.-H. Yum, S. M. Zakeeruddin, G. Hodes and M. Grätzel, J. Phys. Chem. Lett., 2010, 1, 1524–1527 CrossRef CAS.
- S. H. Im, C.-S. Lim, J. A. Chang, Y. H. Lee, N. Maiti, H.-J. Kim, M. K. Nazeeruddin, M. Grätzel and S. I. Seok, Nano Lett., 2011, 11, 4789–4793 CrossRef CAS PubMed.
- Y. C. Choi, D. U. Lee, J. H. Noh, E. K. Kim and S. I. Seok, Adv. Funct. Mater., 2014, 24, 3587–3592 CrossRef CAS PubMed.
- S. Ito, S. Tanaka, K. Manabe and H. Nishino, J. Phys. Chem. C, 2014, 118, 16995–17000 CAS.
- I. Grozdanov, Semicond. Sci. Technol., 1994, 9, 1234 CrossRef CAS.
- M. T. S. Nair, Y. Pena, J. Campos, V. M. Garcia and P. K. Nair, J. Electrochem. Soc., 1998, 145, 2113–2120 CrossRef CAS PubMed.
- S. Messina, M. Nair and P. Nair, Thin Solid Films, 2007, 515, 5777–5782 CrossRef CAS PubMed.
- N. Maiti, S. H. Im, C.-S. Lim and S. I. Seok, Dalton Trans., 2012, 41, 11569–11572 RSC.
- J. A. Chang, J. H. Rhee, S. H. Im, Y. H. Lee, H.-j. Kim, S. I. Seok, M. K. Nazeeruddin and M. Gratzel, Nano Lett., 2010, 10, 2609–2612 CrossRef CAS PubMed.
- A. Koliadima, A. Henglein and E. Matijević, Colloid Polym. Sci., 1997, 275, 972–978 CAS.
- X. Y. Chen, H. S. Huh and S. W. Lee, J. Solid State Chem., 2008, 181, 2127–2132 CrossRef CAS PubMed.
- C. Li, X. Yang, Y. Liu, Z. Zhao and Y. Qian, J. Cryst. Growth, 2003, 255, 342–347 CrossRef CAS.
- Y. Yu, R. H. Wang, Q. Chen and L.-M. Peng, J. Phys. Chem. B, 2005, 109, 23312–23315 CrossRef CAS PubMed.
- A. Kyono and M. Kimata, Am. Mineral., 2004, 89, 932–940 CAS.
- M. Bortolotti, L. Lutterotti and I. Lonardelli, J. Appl. Crystallogr., 2009, 42, 538–539 CrossRef CAS.
- J. A. Chang, S. H. Im, Y. H. Lee, H.-J. Kim, C.-S. Lim, J. H. Heo and S. I. Seok, Nano Lett., 2012, 12, 1863–1867 CrossRef CAS PubMed.
- V. P. Zakaznova-Herzog, S. L. Harmer, H. W. Nesbitt, G. M. Bancroft, R. Flemming and A. R. Pratt, Surf. Sci., 2006, 600, 348–356 CrossRef CAS PubMed.
- A. Darga, D. Mencaraglia, C. Longeaud, T. J. Savenije, B. O'Regan, S. Bourdais, T. Muto, B. Delatouche and G. Dennler, J. Phys. Chem. C, 2013, 117, 20525–20530 CAS.
- Z. Deng, F. Tang, D. Chen, X. Meng, L. Cao and B. Zou, J. Phys. Chem. B, 2006, 110, 18225–18230 CrossRef CAS PubMed.
- K. Tsujimoto, D.-C. Nguyen, S. Ito, H. Nishino, H. Matsuyoshi, A. Konno, G. R. A. Kumara and K. Tennakone, J. Phys. Chem. C, 2012, 116, 13465–13471 CAS.
- T. Fukumoto, T. Moehl, Y. Niwa, M. K. Nazeeruddin, M. Grätzel and L. Etgar, Adv. Energy Mater., 2013, 3, 29–33 CrossRef CAS PubMed.
- S. Ito, K. Tsujimoto, D.-C. Nguyen, K. Manabe and H. Nishino, Int. J. Hydrogen Energy, 2013, 38, 16749–16754 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5cc01966d |
| This journal is © The Royal Society of Chemistry 2015 |