Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Singlet oxygen generation from Li+@C60 nano-aggregates dispersed by laser irradiation in aqueous solution†
Kei
Ohkubo
*a,
Naoki
Kohno
a,
Yusuke
Yamada
a and
Shunichi
Fukuzumi
*abc
aDepartment of Material and Life Science, Graduate School of Engineering, Osaka University, ALCA and SENTAN, Japan Science and Technology Agency (JST), Suita, Osaka 565-0871, Japan. E-mail: ookubo@chem.eng.osaka-u.ac.jp; fukuzumi@chem.eng.osaka-u.ac.jp
bDepartment of Bioinspired Science, Ewha Womans University, Seoul 120-750, Korea
cFaculty of Science and Engineering, Meijo University, ALCA and SENTAN, Japan Science and Technology Agency (JST), Nagoya, Aichi 468-0073, Japan
First published on 1st April 2015
Abstract
Laser pulse irradiation of a deaerated aqueous solution containing the solid state lithium ion-encapsulated fullerene resulted in the formation of highly dispersed nano-aggregates (Li+@C60)n. Photoirradiation of an O2-saturated D2O solution containing (Li+@C60)n gave singlet oxygen with 55% quantum yield, leading to efficient double-stranded DNA cleavage.
Photodynamic therapy (PDT) has developed as a non-invasive clinical treatment of various dermatological, ophthalmic and cardiovascular diseases.1–6 The tumour cell apoptosis in the PDT treatment is carried out by photoirradiation of the photosensitiser to generate the reactive oxygen species (ROS) such as singlet oxygen (1O2*) and superoxide (O2˙−) in malignant tumour.1–6 The requirements of an ideal photosensitiser are water solubility, low cytotoxicity in the dark, high stability against light, high tumour-specificity, high ability to produce ROS and rapid metabolism.1–6
Fullerenes, especially [60]fullerene (C60), are known as efficient photosensitisers to generate the triplet excited state and ROS with high quantum yields (Φ(3C60*) = 0.98; Φ(1O2*) = 0.96 in C6D6).7 Additionally, fullerenes are remarkably photostable and non-toxic reagents.8 However, pristine C60 is hardly soluble in water (0.4 μg mL−1 at 298 K)9 and biological media to prevent expression of the photoactivity and PDT efficiency.10,11 Therefore, various fullerene derivatives, such as C60 with polyethyleneglycol,12 and γ-cyclodextrin-,13–15 lipid-membrane-16 and porous silicate-incorporated C60,17 have been reported to improve water solubility.18 Introduction of water-soluble substituents has also been reported; however, molecular C60 and substituted C60 have no strong absorption around 600–800 nm. Fullerene dispersion suspended in water is also reported by reprecipitation, solvent replacement, ultrasonication and laser ablation methods.19–22
Recently, a lithium ion-encapsulated fullerene hexafluorophosphate salt (Li+@C60 PF6−) has been reported as an efficient photosensitiser to form the long-lived triplet excited state, which is comparable to that of C60.23 However, neither solubilisation of Li+@C60, C60 or C70 in water nor the photoinduced singlet oxygen generation efficiency has been studied. We report herein highly water-dispersed heterogeneous fullerene nano-aggregates composed of Li+@C60, C60, and C70, which have absorption bands in the visible region as well as an efficient singlet oxygen generation properties.
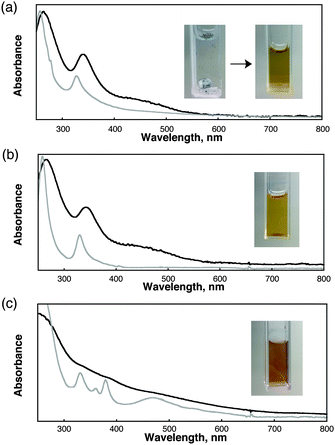
The solubility of the Li+@C60PF6− salt is extremely low in water as shown in the inset pictures in Fig. 1a, where the black powders are deposited at the bottom of the cuvette. Laser pulse irradiation (λ = 532 nm; 500 mW; 10 Hz, 60 min, i.d. = 8 mm) of a deaerated aqueous solution (2.5 mL) containing the dispersed Li+@C60PF6− salt (1.0 mg) resulted in the formation of Li+@C60 nano-aggregates [(Li+@C60)n]. A brown colour supernatant solution containing nano-aggregates was obtained after the centrifugation (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min) and decantation procedures. The UV-vis absorption spectra of nano-aggregates in water are shown in Fig. 1, exhibiting two characteristic absorption bands for (Li+@C60)n in water at 264 and 340 nm, which are red-shifted as compared to that in a dichloromethane solution (257 and 327 nm) by aggregation. A broad shoulder absorption band is also shown at around 400–600 nm, which is characteristic of an intermolecular charge-transfer (CT) transition between fullerenes in the nano-aggregates. A similar CT band was observed for (C60)n. Such a CT band has been reported in the case of a C60 thin film.24 The enhancement of solubility of (Li+@C60)n and (C60)n in water may be obtained by CT interactions.25 The solubilisation of C60 aggregates may occur without the substitution and decomposition of the fullerene cages, which was confirmed by MALDI-TOF-MS spectral measurements indicating the only peak due to non-substituted fullerene.
000 rpm for 10 min) and decantation procedures. The UV-vis absorption spectra of nano-aggregates in water are shown in Fig. 1, exhibiting two characteristic absorption bands for (Li+@C60)n in water at 264 and 340 nm, which are red-shifted as compared to that in a dichloromethane solution (257 and 327 nm) by aggregation. A broad shoulder absorption band is also shown at around 400–600 nm, which is characteristic of an intermolecular charge-transfer (CT) transition between fullerenes in the nano-aggregates. A similar CT band was observed for (C60)n. Such a CT band has been reported in the case of a C60 thin film.24 The enhancement of solubility of (Li+@C60)n and (C60)n in water may be obtained by CT interactions.25 The solubilisation of C60 aggregates may occur without the substitution and decomposition of the fullerene cages, which was confirmed by MALDI-TOF-MS spectral measurements indicating the only peak due to non-substituted fullerene.
The dynamic light scattering (DLS) measurements were performed to evaluate the size of (Li+@C60)n as shown in Fig. 2. The size of the nano-aggregates was significantly decreased to 30 nm by the laser pulse excitations. Thus, one nano-aggregate consists of ca. 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Li+@C60 molecules. When Li+@C60PF6− was replaced by C60 and C70, the highly dispersed nano-aggregates were also obtained by laser pulse irradiation. The sizes of C60 and C70 nano-aggregates were estimated to be 52 and 64 nm, which are larger than (Li+@C60)n. Transmission electron microscopy (TEM) measurements of (Li+@C60)n were performed to evaluate the formation of nano-aggregates, indicating the grape bunch morphology of the nano-aggregates of 30–40 nm size (Fig. 3). The (Li+@C60)n solution was stable at room temperature for three days without re-aggregation.
000 Li+@C60 molecules. When Li+@C60PF6− was replaced by C60 and C70, the highly dispersed nano-aggregates were also obtained by laser pulse irradiation. The sizes of C60 and C70 nano-aggregates were estimated to be 52 and 64 nm, which are larger than (Li+@C60)n. Transmission electron microscopy (TEM) measurements of (Li+@C60)n were performed to evaluate the formation of nano-aggregates, indicating the grape bunch morphology of the nano-aggregates of 30–40 nm size (Fig. 3). The (Li+@C60)n solution was stable at room temperature for three days without re-aggregation.
 | ||
| Fig. 2 Particle size distributions determined by dynamic light scattering (DLS) of (a) (Li+@C60)n, (b) (C60)n and (c) (C70)n. | ||
Photoirradiation of an oxygen-saturated deuterated water (D2O) solution of (Li+@C60)n results in the formation of singlet oxygen, which was detected by 1O2 phosphorescence at 1270 nm (Fig. 4).4 The quantum yields (Φ) of 1O2 generation were determined from the phosphorescence intensity, which was compared to the intensity obtained using rose bengal as a reference compound (Φ = 0.77).26 Relatively high Φ values are obtained and the values are summarised in Table 1, in which the highest Φ value is 0.55 for (Li+@C60)n. The values of nano-aggregates are smaller than those of the corresponding fullerenes in C6D6/C6H5CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) probably because of excited state annihilation (vide infra).
1 v/v) probably because of excited state annihilation (vide infra).
 | ||
| Fig. 4 Emission spectra of 1O2 obtained by photoirradiation (λ = 532 nm) of O2-saturated D2O solutions containing (Li+@C60)n, (C60)n and (C70)n at 298 K. | ||
Femtosecond and nanosecond time-resolved transient absorption spectral measurements were performed to clarify the excited state dynamics and reaction mechanisms for the formation of singlet oxygen from (Li+@C60)n. Ultrafast photodynamics for intersystem crossing (ISC) from the singlet to the triplet excited state of (Li+@C60)n was observed by femtosecond laser flash photolysis. The transient absorption band at 960 nm taken at 10 ps after the femtosecond laser pulse excitation at 393 nm is assigned to the singlet excited state of Li+@C60 [1(Li+@C60)*] in nano-aggregates (Fig. 5), which is relatively broadened as compared to the singlet–singlet absorption of Li+@C60 in PhCN.27 The decay of absorbance at 900 nm obeyed a two-exponential curve. The faster component could be assigned to the singlet–singlet annihilation in the (Li+@C60)n nano-aggregates because the ratio of the faster component increased with increasing the excitation laser power intensity without changing the rate constant (3.8 × 1012 s−1) (Fig. S2 in the ESI†). The residual absorption band at 900 nm slowly decayed with the appearance of the absorption band at 700 nm due to the triplet excited state of the Li+@C60 dimer.28 The decay rate constant of the slower part was determined to be 6.6 × 108 s−1, which is virtually the same as that of the formation of the triplet excited state of Li+@C60 (7.0 × 108 s−1) (see Fig. S3 in the ESI†). This value is slightly slower than the value of homogeneous Li+@C60 in PhCN (8.9 × 108 s−1).27
The triplet excited state of (Li+@C60)n is also detected by the transient absorption spectral measurements observed in a strictly deaerated aqueous solution after nanosecond laser excitation at 355 nm. The transient absorption band taken at 20 ns are due to the triplet–triplet (T–T) transition (see Fig. S3a in ESI†). The band is significantly broadened compared to the case of Li+@C60 in PhCN. The T–T absorption maximum of (Li+@C60)n is virtually the same as that of Li+@C60 (λmax = 750 nm).27 This suggests the aggregation with strong π stacking between the fullerene cages in (Li+@C60)n. The decay of T–T absorption obeyed the first-order kinetics. The lifetime of the transient species was determined to be 32 ns (Fig. S4 in the ESI†). There was no contribution of the T–T annihilation, because the triplet lifetime remained constant at different laser power intensities (Fig. S4b in ESI†). The short triplet lifetime may result from the strong π stacking between the fullerene cages in (Li+@C60)n. On the other hand, no T–T absorption spectrum was observed when (Li+@C60)n was replaced by (C60)n and (C60)n under otherwise the same experimental conditions.28 The π stacking in (Li+@C60)n is much weaker than those of (C60)n and (C60)n because (Li+@C60)n contains equivalent number of PF6− counter anions in the nano-aggregates to avoid π–π interaction between the fullerene cages.29
The triplet excited state of (Li+@C60)n can be an active species for formation of singlet oxygen by energy transfer with molecular O2. We also examined the DNA-cleavage activity of (Li+@C60)n in the presence of O2 using the widely used assay with the supercoiled double-stranded plasmid DNA, pBR322, because singlet oxygen is formed by the photoirradiation of (Li+@C60)n in aqueous solution. The agarose gel electrophoresis was performed after 10 h photoirradiation of pBR 322 with a xenon lamp (λ > 380 nm) in the presence of (Li+@C60)n in comparison with the control experiments as shown in Fig. 6a. Photoirradiation of (Li+@C60)n in the presence of O2 is significantly effective for DNA cleavage due to the singlet oxygen generation due to the observation of a large amount of cleaved DNA (Form II). The DNA cleavage activity of (Li+@C60)n is much higher than that of (C60)n as shown in Fig. 6b, suggesting that a cationic (Li+@C60)n may electrostatically access the minor grove in the double-stranded DNA.
In conclusion, highly dispersed (Li+@C60)n produced by laser irradiation of Li+@C60 acts as an efficient photosensitiser for generation of singlet oxygen in aqueous solution. The excited states of (Li+@C60)n have been successfully detected by femto- and nanosecond transient absorption spectroscopies. We believe that water-soluble (Li+@C60)n can be employed as a convenient PDT photosensitiser in the near future.
This work was supported by Grants-in-Aid (no. 26620154 and 26288037 to K.O. and no. 24350069 and 25600025 to Y.Y.) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT); ALCA and SENTAN projects from JST, Japan (to S.F.). We acknowledge Research Centre for Ultra-Precision Science & Technology in Osaka University for TEM measurements.
Notes and references
- R. Bonnett, Chemical Aspects of Photodynamic Therapy, Gordon and Breach Science Publishers, Amsterdam, 2000 Search PubMed.
- M. Hurtgen, A. Debuigne, M. Hoebecke, C. Passirani, N. Lautram, A. Mouithys-Mickalad, P.-H. Guelluy, C. Jérome and C. Detrembleur, Macromol. Biosci., 2013, 13, 106–115 CrossRef CAS PubMed.
- K. Lang, J. Mosinger and D. M. Wagnerova, Coord. Chem. Rev., 2004, 248, 321–350 CrossRef CAS PubMed.
- (a) S. Gross, A. Gilead, A. Scherz, M. Neeman and Y. Salomon, Nat. Med., 2003, 9, 1327–1331 CrossRef CAS PubMed; (b) D. E. J. G. J. Dolmans, D. Fukumura and R. K. Jain, Nat. Rev. Cancer, 2003, 3, 380–387 CrossRef CAS PubMed.
- (a) Y.-Y. Huang, S. K. Sharma, R. Yin, T. Agrawal, L. Y. Chiang and M. R. Hamblin, J. Biomed. Nanotechnol., 2014, 10, 1918–1936 CrossRef CAS PubMed; (b) Q. Li, H. Ruan and H. Li, Pharm. Nanotechnol., 2014, 2, 25–64 Search PubMed.
- (a) T. J. Dougherty, C. J. Gomer, B. W. Henderson, G. Jori, D. Kessel, M. Korbelik, J. Moan and Q. Peng, J. Natl. Cancer Inst., 1998, 90, 889–905 CrossRef CAS PubMed; (b) W. M. Sharman, C. M. Allen and J. E. van Lier, Drug Discovery Today, 1999, 4, 507–517 CrossRef CAS.
- (a) J. W. Arbogast, A. P. Darmanyan, C. S. Foote, F. N. Diederich, Y. Rubin, M. M. Alvarez, S. J. Anz and R. L. Whetten, J. Phys. Chem., 1991, 95, 11–12 CrossRef CAS; (b) J. W. Arbogast and C. S. Foote, J. Am. Chem. Soc., 1991, 113, 8886–8889 CrossRef CAS.
- (a) M. Wang, L. Huang, S. K. Sharma, S. Jeon, S. Thota, F. F. Sperandio, S. Nayka, J. Chang, M. R. Hamblin and L. Y. Chiang, J. Med. Chem., 2012, 55, 4274–4285 CrossRef CAS PubMed; (b) N. Gharbi, M. Pressac, M. Hadchouel, H. Szwarc, S. R. Wilson and F. Moussa, Nano Lett., 2005, 5, 2578–2585 CrossRef CAS PubMed.
- (a) D. Heymann, Fullerene Sci. Technol., 1996, 4, 509–515 CrossRef CAS; (b) Y. Marcus, A. L. Smith, M. V. Korobov, A. L. Mirakyan, N. V. Avramenko and E. B. Stukalin, J. Phys. Chem. B, 2001, 105, 2499–2506 CrossRef CAS.
- S. K. Sharma, L. Y. Chiang and M. R. Hamblin, Nanomedicine, 2011, 6, 1813–1825 CrossRef CAS PubMed.
- P. Mroz, G. P. Tegos, H. Gali, T. Wharton, T. Sarna and M. R. Hamblin, Photochem. Photobiol. Sci., 2007, 6, 1139–1149 CAS.
- (a) T. Andersson, K. Nilsson, M. Sundahl, G. Westman and O. Wennerström, J. Chem. Soc., Chem. Commun., 1992, 604–606 RSC; (b) K. Komatsu, K. Fujiwara, Y. Murata and T. Braun, J. Chem. Soc., Perkin Trans. 1, 1999, 2963–2966 RSC.
- Y. Yamakoshi, S. Aoua, T.-M. D. Nguyen, Y. Iwamoto and T. Ohnishi, Faraday Discuss., 2014, 173, 287–296 CAS.
- W. Zhang, X. Gong, C. Liu, Y. Piao, Y. Sun and G. Diao, J. Mater. Chem. B, 2014, 2, 5107–5115 RSC.
- I. Nakanishi, S. Fukuzumi, T. Konishi, K. Ohkubo, M. Fujitsuka, O. Ito and N. Miyata, J. Phys. Chem. B, 2002, 106, 2372–2380 CrossRef CAS.
- A. Ikeda, Y. Doi, M. Hashizume, J.-i. Kikuchi and T. Konishi, J. Am. Chem. Soc., 2007, 129, 4140–4141 CrossRef CAS PubMed.
- S.-R. Chae, A. R. Badireddy, J. F. Budarz, S. Lin, Y. Xiao, M. Therezien and M. R. Wiesner, ACS Nano, 2010, 4, 5011–5018 CrossRef CAS PubMed.
- (a) T. D. Ros and M. Prato, Chem. Commun., 1999, 663–669 RSC; (b) E. Nakamura and H. Isobe, Acc. Chem. Res., 2003, 36, 807–815 CrossRef CAS PubMed.
- G. V. Andrievsky, M. V. Kosevich, O. M. Vovk, V. S. Shelkovsky and L. A. Vashchenko, J. Chem. Soc., Chem. Commun., 1995, 1281–1282 RSC.
- S. Deguchi, R. G. Alargova and K. Tsujii, Langmuir, 2001, 17, 6013–6017 CrossRef CAS.
- W.-B. Ko, J.-Y. Heo, J.-H. Nam and K.-B. Lee, Ultrasonics, 2004, 41, 727–730 CrossRef CAS PubMed.
- (a) T. Asahi, T. Sugiyama and H. Masuhara, Acc. Chem. Res., 2008, 41, 1790–1798 CrossRef CAS PubMed; (b) T. Sugiyama, S.-i. Ryo, I. Oh, T. Asahi and H. Masuhara, J. Photochem. Photobiol., A, 2009, 207, 7–12 CrossRef CAS PubMed; (c) H. Tabata, M. Akamatsu, M. Fujii and S. Hayashi, Jpn. J. Appl. Phys., 2007, 46, 4338–4343 CrossRef CAS.
- Y. Kawashima, K. Ohkubo and S. Fukuzumi, Chem. – Asian J., 2015, 10, 44–54 CrossRef CAS PubMed.
- S. Kazaoui, R. Ross and N. Minami, Solid State Commun., 1994, 90, 623–628 CrossRef CAS.
- Enhancement of solubility by charge-transfer formation: see (a) A. P. O'Mullane, N. Fay, A. Nafady and A. M. Bond, J. Am. Chem. Soc., 2007, 129, 2066–2073 CrossRef PubMed; (b) I. Natori, S. Natori and K. Ogino, Macromolecules, 2009, 42, 1964–1969 CrossRef CAS; (c) H. Destaillats and R. Fernández-Prini, J. Chem. Soc., Faraday Trans., 1998, 94, 871–878 RSC.
- R. W. Redmond and J. N. Gamlin, Photochem. Photobiol., 1999, 70, 391–475 CrossRef CAS.
- (a) Y. Kawashima, K. Ohkubo and S. Fukuzumi, J. Phys. Chem. A, 2012, 116, 8942–8948 CrossRef CAS PubMed; (b) K. Ohkubo, Y. Kawashima and S. Fukuzumi, Chem. Commun., 2012, 48, 4314–4316 RSC.
- Y. Ishibashi, M. Arinishi, T. Katayama, H. Miyasaka and T. Asahi, Chem. Lett., 2012, 41, 1104–1106 CrossRef CAS.
- Solid state Li+@C60 PF6− is known to be a rock-salt type crystal structure. see: S. Aoyagi, Y. Sado, E. Nishibori, H. Sawa, H. Okada, H. Tobita, Y. Kasama, R. Kitaura and H. Shinohara, Angew. Chem., Int. Ed., 2012, 51, 3377–3381 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental and spectroscopic details. See DOI: 10.1039/c5cc01885d |
| This journal is © The Royal Society of Chemistry 2015 |