Open Access Article
Open Access ArticleAnion-induced AgI self-assemblies with electron deficient aromatic ligands: anion–π-system interactions as a driving force for templated coordination networks†
Damir A.
Safin
a,
Amélie
Pialat
a,
Alicea A.
Leitch
a,
Nikolay A.
Tumanov
b,
Ilia
Korobkov
a,
Yaroslav
Filinchuk
b,
Jaclyn L.
Brusso
a and
Muralee
Murugesu
*a
aDepartment of Chemistry, University of Ottawa, 10 Marie Curie Private, Ottawa, ON, Canada K1N 6N5. E-mail: m.murugesu@uottawa.ca; Fax: +1 (613) 562 5170; Tel: +1 (613) 562 5800 ext. 2733
bInstitute of Condensed Matter and Nanosciences, Université catholique de Louvain, Place L. Pasteur 1, 1348 Louvain-la-Neuve, Belgium
First published on 27th April 2015
Abstract
Three novel 1D, 2D and 3D coordination polymers were successfully isolated using nitrogen based 3,6-bis(2′-pyrimidyl)-1,2,4,5-tetrazine (BPymTz) and 2,4,6-tris(2-pyrimidyl)-1,3,5-triazine (TPymT) ligands with AgI ions. The formation of these supramolecular assemblies was templated through anion–π-system interactions.
In the last decade, a new type of non-covalent force, commonly referred to as an anion–π-system interaction, has received significant interest due to its fundamental role in biological and chemical applications.1 While the electron donating nature of anions would typically result in repulsion with aromatic π-systems, in this instance that is not the case as the electron deficiency of the aromatic system enables these interactions. Furthermore, the interactions between electron deficient aromatic systems and anions have been shown to be energetically favourable (∼20–70 kJ mol−1) based on theoretical studies.2 Consequently, harnessing these interactions can facilitate the creation of coordination networks where the nature of the anions (e.g., size, shape, charge) can be utilized as a driving force for structure formation. While this approach has been successfully employed for the self-assembly of molecular aggregates,3 it is rarely used in the synthesis of highly dimensional non-discrete structures.3h
Recently, it was reported that s-triazine and 1,2,4,5-tetrazine rings are efficient receptors for interaction with a variety of anions. In particular, the nature of the anions (PF6−, BF4−, ClO4−) in the corresponding AgI salts controls the self-assembly of the metal cation with 2,4,6-tris(2-pyridyl)-1,3,5-triazine.4 In other studies involving first-row transition metals (NiII, ZnII, MnII, FeII, CuII) with 3,6-bis(2-pyridyl)-1,2,4,5-tetrazine or 3,6-bis(2′-pyrimidyl)-1,2,4,5-tetrazine (BPymTz), the anions chosen (SbF6−, PF6−, BF4−, ClO4−, Br3−, I−) played a crucial role in the supramolecular aggregate formation.3f,5 With this in mind, we intend to exploit anion–π-system interactions towards the development of highly ordered supramolecular structures. To that end, BPymTz6 and 2,4,6-tris(2-pyrimidyl)-1,3,5-triazine (TPymT)7 are attractive candidates for such interactions as they not only contain π-acidic aromatic moieties (viz. s-triazine and 1,2,4,5-tetrazine), but also possess coordination environments similar to 2,2′-bipyridine and terpyridine (Chart 1).1b–f,5,8 Another remarkable feature of these rigid ligands is that they possess multiple chelating pockets on either side, promoting multidimensional growth rather than discrete molecules.
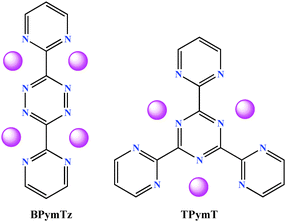 | ||
| Chart 1 Ligands employed for the isolation of coordination networks in this study. Pink balls highlight multi-metal coordination sites. | ||
Using this strategy, we herein report the self-assembly of a supramolecular anion-templated (e.g., PF6− and OTf−) ribbon-like 1D chain, {[Ag2(BPymTz)2](PF6)2·2CH3CN}n (1), and a honeycomb-like 2D network, {[Ag4(BPymTz)3]⊂(OTf)2(CH3CN)2·2(OTf)·CH3CN}n (2), based on BPymTz and AgI ions. In addition, we also report the first self-assembled TPymT-based 3D framework, {[AgTPymT]⊂(ClO4)}n (3), with an unprecedented 2-fold interpenetrating coordination network. In all of the presented complexes, the anion–π-system interactions between PF6−, OTf−, ClO4− and the electron deficient aromatic ligands play a crucial role in the self-assembly of the superstructures (Chart 2).
The reaction of BPymTz with one equivalent of AgPF6 or AgOTf in CH3CN affords purple crystals of 1 or dark grey crystals of 2, respectively (Fig. S1 and S2, and for details see the ESI†). The reaction of TPymT with three equivalents of AgClO4 in hot H2O affords 3 as orange block-like crystals (Fig. S3 and for details see the ESI†). The FTIR spectra of 1–3 exhibit a strong band of the PF6−, OTf− or ClO4− anions at 835, 1245 and 1082 cm−1, respectively, together with all the characteristic bands of the parent ligands BPymTz and TPymT.6,9
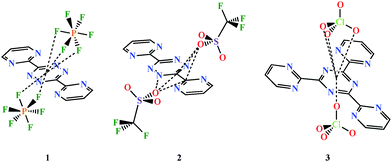
Compounds 1 and 2 crystallize in the orthorhombic Pnma and monoclinic P21/n space groups, respectively. The asymmetric unit of 1 consists of one AgI ion, two halves of BPymTz, two PF6− anions, and one molecule of CH3CN. The asymmetric unit of 2 contains four AgI ions, three molecules of BPymTz, four OTf− anions, and three molecules of CH3CN. In both complexes, each AgI is coordinated to three ligands via the “bipy” coordination pockets of BPymTz affording a distorted trigonal prismatic environment (Fig. 1), while each BPymTz is coordinated to four AgI ions, thus promoting the formation of the extended networks. Furthermore, the AgI⋯AgI distances along and across each BPymTz range from 4.25 to 4.28 Å and 6.37 to 6.51 Å for 1 and 2, respectively. The Ag–N(tetrazine) bond distances are within the range of 2.41–2.61 Å, while the Ag–N(pyrimidine) bond lengths are slightly shorter (i.e., 2.35–2.50 Å). In both structures the N(tetrazine)–Ag–N(pyrimidine) angles are ∼65° and the BPymTz ligands are essentially planar, with a slight torsion angle between the tetrazine and pyrimidine rings (<12°).
The structure of 1 can be described as a 1D cationic zig-zag ribbon running parallel to the a axis (Fig. 1), which consists of multiple pairs of AgI ions held together via bridging of the BPymTz ligands. Each pair has an additional terminal ligand facing away from either side of the ribbon. The space between the two ribbons is filled with PF6− anions and CH3CN molecules. The structure is further stabilized by PF6−–π-system interactions (Chart 2) and C–H⋯F hydrogen bonds with CH3CN, which occur between adjacent 1D chains. The closest AgI⋯AgI distance between the two ribbons is 7.34 Å. Each PF6− anion interacts with the centroids of the tetrazine moiety of the ligand, affording the π-anion linkage with the distances ranging between 2.93 and 3.22 Å.3h,10 This templating effect of the PF6− anion drives the overall formation of the extended 1D ribbon-like superstructure.
The structure of 2 can be described as 2D distorted honeycomb-like layers running parallel to the a axis (Fig. 1). This transformation from a 1D (1) to a 2D (2) system is driven by the change in the counter anion from PF6− to OTf− and confirms that anion–π-system interactions play a key role in the resulting supramolecular self-assembly. In 2, each distorted honeycomb-like motif is constructed from six BPymTz ligands linking twelve AgI ions (Fig. 1). The honeycomb-like cavities contain two OTf− anions and two CH3CN molecules with the remaining OTf− anions and CH3CN molecules situated between adjacent 2D layers. The OTf− anions within a honeycomb-like cavity interact with three tetrazine rings with the distances between the oxygen atoms of OTf− and the centroids of the tetrazine rings ranging from 2.93 to 3.68 Å.10 The CH3CN molecules inside the cavity exhibit C–H⋯O and C–H⋯F contacts with the OTf− anions. The neighbouring honeycomb-like layers are interlinked through the C–H⋯O and C–H⋯F interactions. The closest AgI⋯AgI distance between two honeycomb-like 2D layers is 12.27 Å.
In order to determine if the oligomeric structures also exist in solution, electrospray mass spectra (ES MS) studies were performed on CH3CN solutions of 1 and 2 prior to crystallization. The spectra exhibit a number of peaks due to the formation of different species during the ionization process. For 1, several oligomers of silver ions BPymTz and PF6− were detected, which exhibited the expected isotopomer distributions [Ag2(BPymTz)2(PF6)]+m/z 836.8, [Ag3(BPymTz)3(PF6)2]+m/z 1328.7 and [Ag4(BPymTz)3(PF6)3]+m/z 1582.5 (Fig. S4, for details see the ESI†). Similar observations were made for complex 2, with the detection of the oligomers [Ag2(BPymTz)(OTf)]+m/z 602.7, [Ag3(BPymTz)2(OTf)2]+m/z 1098.5 and [Ag4(BPymTz)2(OTf)3]+m/z 1354.3 (Fig. S5, and for details see the ESI†). As previously demonstrated,11 oligomers of silver ions with organic ligands and anions can be detected in solution by means of ES MS. Although this technique is not illustrative of the distribution of species in solution, as it can only detect charged species in the gas phase, it does provide interesting information on the self-assembly process before crystallization.
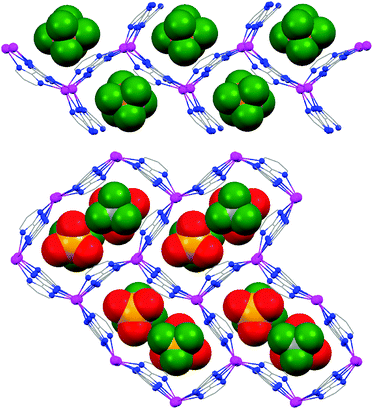
Crystals of 3 crystallize in the cubic space group I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3d with the unit cell parameters of 20.2313(6) Å. Based on single crystal X-ray analysis, TPymT coordinates to three AgI ions acting as a tris-terpyridine ligand. Each AgI ion has a distorted hexacoordinate environment formed by the coordination pockets of two neighbouring TPymT ligands (Fig. 2). The average Ag–N(triazine) bond distance is 2.30 Å, while the Ag–N(pyrimidine) bond lengths are 2.51 and 2.58 Å, which are significantly elongated with respect to the Ag–N(triazine) bond. Within the planar trinuclear unit, the AgI⋯AgI distance is 6.20 Å, which is 0.24–0.29 Å shorter than those found in the discrete trinuclear analogue [Ag3(TPymT)(H2O)(NO3)3]·H2O (4).12 Furthermore, each of the trinuclear [Ag3TPymT]3+ fragments is twisted by ∼70° relative to one another imposed by the distorted octahedral AgI coordination environment.
3d with the unit cell parameters of 20.2313(6) Å. Based on single crystal X-ray analysis, TPymT coordinates to three AgI ions acting as a tris-terpyridine ligand. Each AgI ion has a distorted hexacoordinate environment formed by the coordination pockets of two neighbouring TPymT ligands (Fig. 2). The average Ag–N(triazine) bond distance is 2.30 Å, while the Ag–N(pyrimidine) bond lengths are 2.51 and 2.58 Å, which are significantly elongated with respect to the Ag–N(triazine) bond. Within the planar trinuclear unit, the AgI⋯AgI distance is 6.20 Å, which is 0.24–0.29 Å shorter than those found in the discrete trinuclear analogue [Ag3(TPymT)(H2O)(NO3)3]·H2O (4).12 Furthermore, each of the trinuclear [Ag3TPymT]3+ fragments is twisted by ∼70° relative to one another imposed by the distorted octahedral AgI coordination environment.
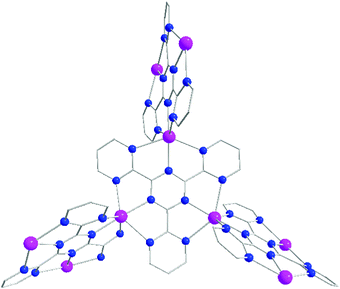 | ||
| Fig. 2 Ball and stick molecular structure of the [Ag9TPymT4]9+ segment in the structure of 3 (hydrogen atoms and ClO4− anions were omitted for clarity). Color code: C = grey, N = blue, Ag = magenta. | ||
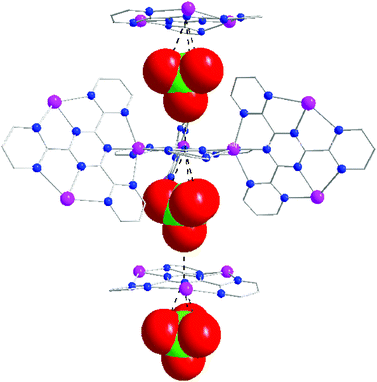
Similar to complexes 1 and 2, the formation of the interpenetrating 3D network of 3 is driven by anion–π-system interactions, as evidenced by the contacts between ClO4− with the neighbouring electron deficient s-triazine core of TPymT. The ClO4− anions in 3 are situated directly above the central triazine rings (Fig. 3) with distances between the oxygen atoms of ClO4− and the centroids of the triazine rings being 3.21 and 3.87 Å.13 Through these interactions, a 1D chain of alternating anion–π-system moieties are formed, thus stabilizing the network.
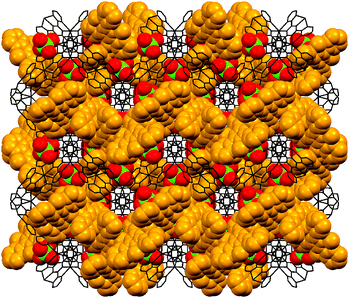
Since each TPymT coordinates to three AgI ions and each AgI ion coordinates to two TPymT ligands, this leads to a complex 2-fold interpenetrating 3D coordination network (Fig. 4). This network can be described as a (10,3)-a topology, with each TPymT ligand as a three-connected node and each AgI cation as a linker. The same topology was also observed in the ZnSiF6 complex of 2,4,6-tri(4-pyridyl)-1,3,5-triazine and the AgClO4 complex of 1,4,5,8,9,12-hexaazatriphenylene.14 A comparison of the structures 3 and 4 establishes the vital templating role of the ClO4− anion vs. NO3−. The former serves as a driving force in the formation of the 2-fold interpenetrating 3D coordination network, while the latter yields a discrete trinuclear molecule.12
The bulk sample of 3 was studied by means of powder X-ray diffraction (Fig. S6 in the ESI†). The experimental X-ray powder pattern is in agreement with the calculated powder pattern obtained from a single crystal X-ray structure. On the other hand, powder X-ray diffraction analysis of complexes 1 and 2 shows some discrepancies between the predicted and experimental patterns due to extensive evaporation of solvent molecules from the isolated crystals (Fig. S7 and S8 in the ESI†). Nonetheless, several single crystals of each 1 and 2 were analysed via single crystal XRD and found to be isostructural.
In summary, 1D, 2D and 3D supramolecular coordination networks were successfully isolated through the use of anion–π-system interactions. The π-acidic aromatic moieties in the ligands BPymTz and TPymT provide an ideal platform to induce such interactions. This, coupled with the multiple coordination sites in the ligands, promotes multidimensional growth. In particular, the selective use of PF6− and OTf− leads to the formation of a 1D ribbon-like chain (1) and a 2D honeycomb-like layered structure (2), respectively, through coordination of BPymTz with AgI. Furthermore, exploiting such interactions between a rationally designed ligand with three-fold symmetry (TPymT) and ClO4− enables the formation of a two-fold interpenetrating compact 3D coordination network (3). These three examples provide clear evidence of the pivotal role played by different anions (size and shape) and their interactions with the ligand, which directs the superstructure formation. This strategy offers a unique approach in the development of novel supramolecular assemblies through the use of counter anions as strategic synthons.
This work was financially supported by the NSERC-DG, CFI, ORF, and ERA. We acknowledge the FSR (UCL) for the incoming postdoctoral fellowship co-funded by the Marie Curie actions of the European Commission granted to N. A. Tumanov. We thank SNBL at the ESRF for the beamtime allocation. R. J. Holmberg is also kindly acknowledged for the STM and X-ray powder pattern measurements.
Notes and references
- (a) H.-J. Schneider, Angew. Chem., Int. Ed., 1991, 30, 1417 CrossRef PubMed; (b) P. Gamez, T. J. Mooibroek, S. J. Teat and J. Reedijk, Acc. Chem. Res., 2007, 40, 435 CrossRef CAS PubMed; (c) B. L. Schottel, H. T. Chifotides and K. R. Dunbar, Chem. Soc. Rev., 2008, 37, 68 RSC; (d) C. Caltagirone and P. A. Gale, Chem. Soc. Rev., 2009, 38, 520 RSC; (e) A. Robertazzi, F. Krull, E.-W. Knapp and P. Gamez, CrystEngComm, 2011, 13, 3293 RSC; (f) C. Estarellas, A. Frontera, D. Quiñonero and P. M. Deyà, Angew. Chem., Int. Ed., 2011, 50, 415 CrossRef CAS PubMed; (g) A. Frontera, P. Gamez, M. Mascal, T. J. Mooibroek and J. Reedijk, Angew. Chem., Int. Ed., 2011, 50, 9564 CrossRef CAS PubMed; (h) H. T. Chifotides and K. R. Dunbar, Acc. Chem. Res., 2012, 46, 894 CrossRef PubMed; (i) I. V. Krieger, J. S. Freundlich, V. B. Gawandi, J. B. Roberts, V. B. Gawandi, Q. Sun, J. L. Owen, M. T. Fraile, S. I. Huss, J.-L. Lavandera, T. R. Loerger and J. C. Sacchettini, Chem. Biol., 2012, 19, 1556 CrossRef CAS PubMed; (j) K. Bowman-James, A. Bianchi and E. García-España, Anion Coordination Chemistry, Wiley-VCH, 2012 Search PubMed.
- (a) M. Mascal, A. Armstrong and M. D. Bartberger, J. Am. Chem. Soc., 2002, 124, 6274 CrossRef CAS PubMed; (b) I. Alkorta, I. Rozas and J. Elguero, J. Am. Chem. Soc., 2002, 124, 8593 CrossRef CAS PubMed; (c) D. Quiñonero, C. Garau, C. Rotger, A. Frontera, P. Ballester, A. Costa and P. M. Deyà, Angew. Chem., Int. Ed., 2002, 41, 3389 CrossRef.
- (a) R. Hasenknopf, J.-M. Lehn, B. O. Kneisel, G. Baum and D. Fenske, Angew. Chem., Int. Ed., 1996, 35, 1838 CrossRef PubMed; (b) R. Hasenknopf, J.-M. Lehn, N. Boumediene, A. Dupont-Gervais, A. Van Dorsselaer, B. Kneisel and D. Fenske, J. Am. Chem. Soc., 1997, 119, 10956 CrossRef; (c) P. D. Beer and P. A. Gale, Angew. Chem., Int. Ed., 2001, 40, 486 CrossRef CAS; (d) M. J. Hannon, C. L. Painting, E. A. Plummer, L. J. Childs and N. W. Alcock, Chem. – Eur. J., 2002, 8, 2225 CrossRef CAS; (e) R. Vilar, Angew. Chem., Int. Ed., 2003, 42, 1460 CrossRef CAS PubMed; (f) C. S. Campos-Fernández, B. L. Schottel, H. T. Chifotides, J. K. Bera, J. Bacsa, J. M. Koomen, D. H. Russell and K. R. Dunbar, J. Am. Chem. Soc., 2005, 127, 12909 CrossRef PubMed; (g) M. Shatruk, A. Chouai and K. R. Dunbar, Dalton Trans., 2006, 2184 RSC; (h) B. L. Schottel, H. T. Chifotides, M. Shatruk, A. Chouai, L. M. Pérez, J. Bacsa and K. R. Dunbar, J. Am. Chem. Soc., 2006, 128, 5895 CrossRef CAS PubMed.
- (a) X.-P. Zhou, D. Li, T. Wu and X. Zhang, Dalton Trans., 2006, 2435 RSC; (b) X.-P. Zhou, D. Li, T. Wu and X. Zhang, Inorg. Chem., 2006, 45, 7119 CrossRef CAS PubMed.
- I. D. Giles, H. T. Chifotides, M. Shatruk and K. R. Dunbar, Chem. Commun., 2011, 47, 12604 RSC.
- W. Kaim and J. Fees, Z. Naturforsch., 1995, 50b, 123 Search PubMed.
- F. H. Case and E. Koft, J. Am. Chem. Soc., 1959, 81, 905 CrossRef CAS.
- (a) S. Demeshko, S. Dechert and F. Meyer, J. Am. Chem. Soc., 2004, 126, 4508 CrossRef CAS PubMed; (b) P. de Hoog, P. Gamez, I. Mutikainen, U. Turpeinen and J. Reedijk, Angew. Chem., Int. Ed., 2004, 43, 5815 CrossRef CAS PubMed.
- D. A. Safin, Y. Xu, I. Korobkov, D. L. Bryce and M. Murugesu, CrystEngComm, 2013, 15, 10419 RSC.
- (a) B. L. Schottel, J. Bacsa and K. R. Dunbar, Chem. Commun., 2005, 46 RSC; (b) K. Chainok, S. M. Neville, C. M. Forsyth, W. J. Gee, K. S. Murray and S. R. Batten, CrystEngComm, 2012, 14, 3717 RSC.
- (a) A. M. Bond, R. Colton, Y. A. Mah and J. C. Traeger, Inorg. Chem., 1994, 33, 2548 CrossRef CAS; (b) K. Nomiya, Y. Kondoh, H. Nagano and M. Oda, J. Chem. Soc., Chem. Commun., 1995, 1679 RSC; (c) E. C. Constable, C. E. Housecroft, B. M. Kariuki, N. Kelly and C. B. Smith, Inorg. Chem. Commun., 2002, 5, 199 CrossRef CAS; (d) F. Bachechi, A. Burini, R. Galassi, B. R. Pietroni and M. Ricciutelli, Inorg. Chim. Acta, 2004, 357, 4349 CAS.
- D. A. Safin, A. Pialat, I. Korobkov and M. Murugesu, Chem. – Eur. J., 2015, 21, 6144 CrossRef CAS PubMed.
- P. U. Maheswari, B. Modec, A. Pevec, B. Kozlevcar, C. Massera, P. Gamez and J. Reedijk, Inorg. Chem., 2006, 45, 6637 CrossRef CAS PubMed.
- (a) B. F. Abrahams, S. R. Batten, H. Hamit, B. F. Hoskins and R. Robson, Chem. Commun., 1996, 1313 RSC; (b) B. F. Abrahams, P. A. Jackson and R. Robson, Angew. Chem., Int. Ed., 1998, 37, 2656 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional data and Fig. S1–S8. CCDC 1028560, 1028561 and 1027767. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5cc01597a |
| This journal is © The Royal Society of Chemistry 2015 |