Open Access Article
Open Access ArticleFormation of a nanometer-thick water layer at high humidity on a dynamic crystalline material composed of multi-interactive molecules†
Yumi
Yakiyama‡
a,
Gil Ryeong
Lee‡
a,
Sung Yeon
Kim
a,
Yoshitaka
Matsushita
b,
Yasushi
Morita
c,
Moon Jeong
Park
ad and
Masaki
Kawano
*a
aThe Division of Advanced Materials Science, Pohang University of Science and Technology (POSTECH), Cheongam-ro 77, Namgu, Pohang 790-784, Korea. E-mail: mkawano@postech.ac.kr; Fax: +82 54 279 8736; Tel: +82 54 279 8740
bResearch Network and Facility Services Division, National Institute for Materials Science (NIMS), Sengen 1-2-1, Tsukuba, Ibaraki 305-0047, Japan
cDepartment of Applied Chemistry, Faculty of Engineering, Aichi Institute of Technology, Yachigusa 1247, Yakusa, Toyota 470-0392, Japan
dDepartment of Chemistry, Pohang University of Science and Technology (POSTECH), Cheongam-ro 77, Namgu, Pohang 790-784, Korea
First published on 11th March 2015
Abstract
Crystalline powders self-assembled from interactive discrete molecules reversibly transformed from a porous structure to a 2D one with a nanometer-thick H2O layer by hydration/dehydration. Multi-point weak intermolecular interactions contributed to maintenance of each phase. This structure transformation induced a humidity-dependent ion conductivity change from insulator to 3.4 × 10−3 S cm−1.
Intermolecular interactions participate in emerging functions in functional materials1 and biological systems.2 Especially, multi-interactive interactions contribute to stabilizing key meta-stable intermediates in biological processes, because the multiple interactions can deepen the local minimum potential well to trap kinetic states.3 Therefore, we aimed to implement multi-interactivity into a ligand to achieve kinetically self-assembled networks.4 We designed a multi-interactive ligand, tri(4-pyridyl)hexaazaphenalene anion (TPHAP−), and succeeded in trapping meta-stable coordination networks.5 In the process, we unexpectedly prepared crystalline self-assembled materials composed of K+TPHAP− under highly hydrated conditions. Here we report a 1.2 nm-thick H2O layer formed of discrete molecules, and the dynamic structure change revealed by X-ray powder diffraction (XRPD) analysis.6 The ion conductivity σ of the hydrated material changed from insulator to 3.4 × 10−3 S cm−1 depending on the amount of intercalated H2O.
The TPHAP anion has a D3h symmetrical aromatic plane7 (Scheme 1) that can form π–π stacking interaction, and nine nitrogen atoms that can form hydrogen bonds, coordination bonds, or both.5 Such multi-interactivity of TPHAP enabled the selective trapping of a kinetic network followed by surface-mediated dynamic structure transformation.5a We also prepared various coordination networks composed of Zn2+ ions and TPHAP anions from the same crystallization set up by changing only solvents or additives because of the multi-interactivity of TPHAP.5b,c The interactive nature of TPHAP was also observed in a 1D-channel structure of a K+TPHAP− single crystal 1. In the crystal, H2O molecules were encapsulated by OH⋯N hydrogen bonds with the N atoms on the TPHAP− skeleton (Fig. S1, ESI†). Indeed, the single crystal of K+TPHAP− is highly hygroscopic: 1 g of powder of K+TPHAP− adsorbs >70 mL of H2O (at 25 °C, 30% RH) while the potassium salt of the t-butyl group substituted hexaazaphenalene (HAP) anion does not show any hygroscopic nature.7b K+TPHAP− powder retained its crystallinity after hydration.
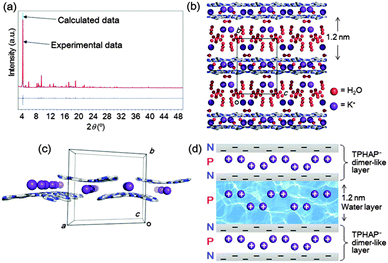
Therefore, we measured the XRPD pattern of fully-hydrated powder 2 which was prepared by hydration of K+TPHAP− single crystals 1 at 95% RH and 25 °C for 1 day (Fig. 1). The XRPD pattern of 2 showed a drastic and irreversible change from initial K+TPHAP− single crystalline powder 1 while maintaining a surprisingly high crystallinity. We also identified how the structure changed during drying. We gradually dried fully-hydrated powder 2 by keeping it at 20% RH and 20 °C for 20 s, then quickly measured its XRPD pattern in an airtight cell to avoid further drying during the measurement. This quick drying operation was repeated three times. After the first 20 s, the XRPD pattern was almost intact; the next 20 s of drying induced drastic changes in the XRPD pattern, and a further 20 s of drying (total 1 min) produced a new phase close to the final state, i.e., 1 day dried powder 3 (Fig. S2, ESI†). Notably, the sharp powder pattern of 2 was recovered by rehydration of 3. This hydration/dehydration process between 2 and 3 was reversible.
To reveal the reversible structure transformation observed during the hydration/dehydration process, we performed XRPD structure analyses of fully-hydrated powder 2, 1 min dried powder, and 1 day dried powder 3. The structures were determined by the simulated annealing method in DASH,8 followed by Rietveld refinement using RIETAN-FP9 to refine the position of each atom with soft bond-angle restraints for a TPHAP− group. As a result, we revealed that 1.2 nm H2O layers form in the fully-hydrated 2 (Scheme 1) and that 1D H2O channels form in 3 by dehydration. Powder structure analysis of 1 min dried powder revealed a severely disordered structure which was an intermediate state before reaching the state of 1 day dried 3 (Fig. S2, ESI†). It should be noted that although the powder patterns of the 1 min dried powder and 3 look very similar, their unit cells are very different (1 min dried powder: a = 20.656(6) Å, b = 15.978(4) Å, c = 16.80(1) Å, β = 100.68(3)°, V = 5449(4) Å3, P2/a; 3: a = 20.90(1) Å, b = 15.93(1) Å, c = 9.711(5) Å, β = 104.65(3)°, V = 3128(3) Å3, P2/a).
The powder analysis of 2 revealed four equally possible models that have monoclinic P2/n systems that show good agreement between the calculated and experimental XRPD patterns in the final Rietveld refinement (Fig. S3, ESI†). Among them, one (Fig. 2b) showed the best agreement with the experimental data (Fig. 2a and b). Although the precise determination of disordered H2O and K+ positions was technically difficult, every refinement result showed flat 2D-sheets composed of K+TPHAP− dimer-like layers (Fig. 2c) forming 1.2 nm-thick H2O layers. These dimer-like layers are stabilized by the intermolecular interactions between the N atoms of the central HAP skeleton and the disordered K+ ions. These interactions are strong enough to be detected by CSI-MS.5b Some pyridine groups of TPHAP− are close to each other, but the short contact can be explained by severe disorder. The amount of H2O calculated from XRPD analysis is 31H2O molecules per K+TPHAP− unit, which is close to the maximum value obtained by direct weight measurement (12–28H2O molecules per K+TPHAP− unit, Table S1, ESI†). Notably, other disordered K+ ions were dispersed in the 1.2 nm water layer. Therefore, stabilization of this H2O layer is probably due to a large number of H-bonding interactions between H2O molecules and TPHAP skeletons at the boundary and K+ ions on the inner part of the H2O layer. In addition, the whole structure is constructed as alternating stacks of positively-charged large H2O layers and TPHAP− dimer-like layers, which are composed of a negatively-charged TPHAP sheet and a positively-charged K+ layer (Fig. 2d). This electrically neutral arrangement contributes to stabilization of the 2D layered stacking structure.
Pulsed-Field-Gradient 1H-NMR measurement of 2 strongly supported the hypothesized formation of an H2O layer with a liquid-like state (Fig. S4, ESI†). Solid state 1H-NMR measurement of 1 (as control) and 2 revealed only one peak corresponding to internal H2O at δ = 5.1 and 4.4 ppm, respectively. The peak corresponding to 1 was small and broad due to a much smaller amount of H2O in 1 than in 2. Furthermore, the large number of hydrogen bonds between H2O molecules and the TPHAP− skeleton in 1 reduced the mobility of H2O.10 In contrast, compared to 1, H2O in fully-hydrated powder 2 could move more freely to give a sharper and larger signal. The diffusion coefficient DH2O of water in 2 was obtained from the plot of spin-echo intensity I/I0 = exp[−Dγ2g2δ2(Δ − δ/3)] against the gradient strength g,11 where I and I0 are the signal intensities with each g and without g, respectively, and γ is the gyromagnetic ratio. Applied gradient strengths reached 800 G cm−1 while gradient δ and gradient delay Δ time values were 0.5 ms and 3.55 ms, respectively. The obtained DH2O value was 1.0 × 10−9 m2 s−1, which is close to the self-diffusion coefficient of pure H2O at rt (ca. 2 × 10−9 m2 s−1).12 This quite high DH2O value indicates that the diffusion speed of H2O molecules in 2 is similar to that in bulk H2O.
The structural effect of dehydration on the 2D layered structure was also revealed by the XRPD analysis of 1 day dried powder 3 which showed the formation of a porous structure having a monoclinic P2/a system with excellent agreement between the calculated and experimental XRPD patterns in the final Rietveld refinement (Fig. 3). In the crystal structure, the ordered TPHAP−s also maintain dimer-like structures connected by K+ ions and interact with each other by π–π stacking to form a 2D layer (Fig. 3b and c). The 2D layers stack with the shortest distance (Fig. 3d), followed by formation of 1D channels along the c axis with a size of ca. 12 Å × 16 Å (Fig. 3e). In the XRPD analysis, we could model only two H2O molecules per K+TPHAP− unit in the channel, although elemental analysis indicated five H2O molecules per K+TPHAP− unit. Notably, the 1D channel is surrounded by N atoms on a HAP skeleton as observed in a K+TPHAP− single crystal 1 in which K+ ions and H2O molecules are less mobile than in 2. Therefore, the significant mobility decrease of K+ ions and H2O was expected as a result of the structural transformation from a 2D-layered structure to a 1D-porous structure. This reduction is also suggested by humidity-dependent IR spectra (Fig. S5, ESI†). As the amount of absorbed H2O decreased, the peaks at ∼1500–1600 cm−1 attributable to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
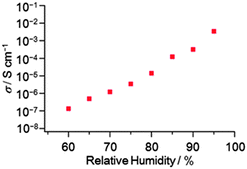
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching modes of pyridine and the central skeleton of TPHAP− showed clear red shifts: ∼10 cm−1 from the fully-hydrated powder 2 to single crystal 1; 4–5 cm−1 from 2 to 1 day dried powder 3. These peak shifts can be explained by enhancement of Coulombic interaction between K+ and the anionic HAP skeleton and by hydrogen bond formation between TPHAP− and H2O.13 This discussion indicates that this dynamic structure transformation can make a significant contribution to the ion conductivity of K+TPHAP− because the conductivity value has a strong relationship with charge carrier mobility.14 Therefore, we finally performed humidity-dependent ion conductivity measurement and tried to interpret the relationship between the structure and the conduction property.
C stretching modes of pyridine and the central skeleton of TPHAP− showed clear red shifts: ∼10 cm−1 from the fully-hydrated powder 2 to single crystal 1; 4–5 cm−1 from 2 to 1 day dried powder 3. These peak shifts can be explained by enhancement of Coulombic interaction between K+ and the anionic HAP skeleton and by hydrogen bond formation between TPHAP− and H2O.13 This discussion indicates that this dynamic structure transformation can make a significant contribution to the ion conductivity of K+TPHAP− because the conductivity value has a strong relationship with charge carrier mobility.14 Therefore, we finally performed humidity-dependent ion conductivity measurement and tried to interpret the relationship between the structure and the conduction property.
The ion conductivity of K+TPHAP− single crystalline powder 1 was significantly correlated with the outer humidity. We measured the ion conductivity of a compressed pellet of 1 (ϕ 13 mm, thickness ∼0.2 mm) by ac impedance spectroscopy under various conditions of humidity and temperature (ESI†). The conductivity drastically changed from insulator at 20% RH to 3.4 × 10−3 S cm−1 at 95% RH (25 °C, Fig. 4). Although a large amount of H2O adsorption in the highly conductive state was observed (Table S1, ESI†), the pellet of 1 remained soft and solid after conductivity measurement (Fig. S6, ESI†). We unambiguously confirmed that the highly-conductive state corresponds to 2 having a 2D layered structure by XRPD measurement of the pellet sample (Fig. S7, ESI†). In contrast, drying of the fully-hydrated pellet even for several seconds reduced its conductivity value significantly. This phenomenon reminded us of the quick structural transformation of 2 to the dried state 3. We confirmed by XRPD analysis that the dried pellet corresponds to 3 which possesses a porous structure (Fig. S6, ESI†). Because the system contains no mobile proton (H+),15 the main charge carrier is very likely K+. In fact, the K+-exchanged proton conducting polymer shows σ = ∼3.0 × 10−2 S cm−1 (fully-hydrated at 25 °C) which gives the diffusion coefficient of K+ rather than of H+.16 These facts indicate that the main carrier that contributes to ion conductivity is a metal cation. From the above discussion, this drastic conductivity change can be explained based on the changes of the structure and the amount of water in the system. In the hydrated state of 2, K+ can readily diffuse by weak van der Waals interaction with H2O within the 1.2 nm H2O layer because the diffusion rate of H2O is almost the same as that of bulk H2O. This wide H2O layer also contributes to the smooth migration of K+ by keeping away K+ from the surface of the TPHAP− layer. This effective K+ migration supported by the fast diffusion of bulk-like H2O realized the high conductivity under humid conditions. Oppositely, the structure transformation of 2 to porous 3 by drying causes a significant decrease in the mobility of K+ ions and H2O; the large amount of H2O removal involving this structure transformation increases the contribution of strong H-bonds between N atoms on the TPHAP skeleton and K+ or H2O compared to that in 2. Therefore, the significant conductivity decrease by dehydration is attributed to the decreased mobility of K+ and H2O due to structural transformation from the 2D-layered structure to the interactive 1D-porous structure.
We revealed formation of a nanometer-thick H2O layer on a multi-interactive ligand, K+TPHAP−, by XRPD analysis. We also found reversible shrinkage/expansion of the hydrated powder by dehydration/hydration. This structural transformation corresponded to a drastic change of ion conductivity from insulator to 3.4 × 10−3 S cm−1. Our material may provide better understanding of the correlation between the structure and the physical properties of H2O- or humidity-triggered functional materials.
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2014R1A2A1A11049978) and Basic Science Research Institute Grant from POSTECH. This work has been approved by the Photon Factory Advisory Committee (Proposal No. 2014G008, beamline NW2A). X-ray powder diffraction studies with synchrotron radiation were performed at the Pohang Accelerator Laboratory (Beamline 2D and 9B) and at the BL15XU, SPring-8 with the approval of the National Institute for Materials Science (NIMS) (Proposal No. 2009A4800).
Notes and references
- Supramolecular Chemistry, ed. J. W. Steed and J. L. Atwood, Wiley, Chichester, UK, 2nd edn, 2009 Search PubMed.
- (a) D. Stock, A. G. W. Leslie and J. E. Walker, Science, 1999, 286, 1700–1705 CrossRef CAS; (b) N. Ban, P. Nissen, J. Hansen, P. B. Moore and T. A. Steitz, Science, 2000, 289, 905–920 CrossRef CAS; (c) N. H. Joh, A. Min, S. Faham, J. P. Whitelegge, D. Yang, V. L. Woods, Jr. and J. U. Bowie, Nature, 2008, 453, 1266–1270 CrossRef CAS PubMed; (d) D. M. Rosenbaum, S. G. F. Rasmussen and B. K. Kobilka, Nature, 2009, 459, 356–363 CrossRef CAS PubMed.
- (a) M. M. Gromiha and S. Selvaraj, Prog. Biophys. Mol. Biol., 2004, 86, 235–277 CrossRef CAS PubMed; (b) M. Chaplin, Nat. Rev. Mol. Cell Biol., 2006, 7, 861–866 CrossRef CAS PubMed.
- (a) M. Kawano, T. Haneda, D. Hashizume, F. Izumi and M. Fujita, Angew. Chem., Int. Ed., 2008, 47, 1269–1271 CrossRef CAS PubMed; (b) J. Martí-Rujas, Y. Matsushita, F. Izumi, M. Fujita and M. Kawano, Chem. Commun., 2010, 46, 6515–6517 RSC; (c) H. Kitagawa, H. Ohtsu and M. Kawano, Angew. Chem., Int. Ed., 2013, 52, 12395–12399 CrossRef CAS PubMed.
- (a) Y. Yakiyama, A. Ueda, Y. Morita and M. Kawano, Chem. Commun., 2012, 48, 10651–10653 RSC; (b) T. Kojima, T. Yamada, Y. Yakiyama, E. Ishikawa, Y. Morita, M. Ebihara and M. Kawano, CrystEngComm, 2014, 16, 6335–6344 RSC.
- (a) M. Takata, B. Umeda, E. Nishibori, M. Sakata, Y. Saito, M. Ohno and H. Shinohara, Nature, 1995, 377, 46–49 CrossRef CAS; (b) S. Pagola, P. W. Stephens, D. S. Bohle, A. D. Kosar and S. K. Madsen, Nature, 2000, 404, 307–310 CrossRef CAS PubMed; (c) IUCr Monographs on Crystallography 13, ed. W. I. F. David, D. Shankland, L. B. McCusker and C. Baerlocher, Oxford University Press, Oxford, UK, 2002 Search PubMed; (d) K. D. M. Harris and E. Y. Cheung, Chem. Soc. Rev., 2004, 33, 526–538 RSC; (e) J. Martí-Rujas and M. Kawano, Acc. Chem. Res., 2013, 46, 493–505 CrossRef PubMed.
- (a) S. Suzuki, Y. Morita, K. Fukui, K. Sato, D. Shiomi, T. Takui and K. Nakasuji, Inorg. Chem., 2005, 44, 8197–8199 CrossRef CAS PubMed; (b) S. Suzuki, K. Fukui, A. Fuyuhiro, K. Sato, T. Takui, K. Nakasuji and Y. Morita, Org. Lett., 2010, 12, 5036–5039 CrossRef CAS PubMed.
- W. I. F. David, K. Shankland and N. Shankland, Chem. Commun., 1998, 931–932 RSC.
- F. Izumi and K. Momma, Solid State Phenom., 2007, 130, 15–20 CrossRef CAS.
- S. Hietala, S. L. Maunu, F. Sundholm, T. Lehtinen and G. Sundholm, J. Polym. Sci., Part B: Polym. Phys., 1999, 37, 2893–2900 CrossRef CAS.
- (a) J. E. Tanner, J. Chem. Phys., 1970, 52, 2523–2526 CrossRef CAS PubMed; (b) T. A. Zawodzinski, Jr., M. Neeman, L. O. Sillerud and S. Gottesfeld, J. Phys. Chem., 1991, 95, 6040–6044 CrossRef.
- M. Holz, S. R. Heil and A. Sacco, Phys. Chem. Chem. Phys., 2000, 2, 4740–4742 RSC.
- M. Vilkman, A. Lankinen, N. Volk, P. Kostamo and O. Ikkala, Polymer, 2010, 51, 4095–4102 CrossRef CAS PubMed.
- Modern Electrochemistry vol 1: Ionics, ed. J. O. Bockris and A. K. N. Reddy, Springer, 2nd edn, 1998 Search PubMed.
- Examples of organic molecule-based water-mediated proton conductors which keep rigid structures in humid condition see; (a) M. Sadakiyo, T. Yamada and H. Kitagawa, J. Am. Chem. Soc., 2009, 131, 9906–9907 CrossRef CAS PubMed; (b) L. Jiménez-García, A. Kaltbeitzel, W. Pisula, J. S. Gutmann, M. Klapper and K. Müllen, Angew. Chem., Int. Ed., 2009, 48, 9951–9953 CrossRef PubMed; (c) M. Yoon, K. Suh, H. Kim, Y. Kim, N. Selvapalam and K. Kim, Angew. Chem., Int. Ed., 2011, 50, 7870–7873 CrossRef CAS PubMed; (d) J. M. Taylor, K. W. Dawson and G. K. H. Shimizu, J. Am. Chem. Soc., 2013, 135, 1193–1196 CrossRef CAS PubMed.
- T. Okada, H. Satou, M. Okuno and M. Yuasa, J. Phys. Chem. B, 2002, 106, 1267–1273 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Details of the experimental procedures, syntheses of powder crystals, water adsorption experiments, Pulsed-Field-Gradient 1H-NMR experiments, IR data and ion conductivity data. CCDC 952977 and 1031002–1031004. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5cc01568e |
| ‡ Y.Y. and G.L. share the first authorship. |
| This journal is © The Royal Society of Chemistry 2015 |