Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Non-covalent composites of antiaromatic isophlorin–fullerene†
Baddigam Kiran
Reddy
,
Santosh C.
Gadekar
and
Venkataramanarao G.
Anand
*
Department of Chemistry, Indian Institute of Science Education and Research (IISER), Pune – 411008, Maharashtra, India. E-mail: vg.anand@iiserpune.ac.in
First published on 15th April 2015
Abstract
Cocrystallates of fullerene C60 and antiaromatic planar tetraoxaisophlorins have been characterized by single crystal X-ray diffraction analysis. The ring-juncture bonds of C60 are found to be at a very close distance to the plane of the antiaromatic isophlorins. NMR measurements and MALDI-TOF mass spectrometry show that this interaction can persist in both solution and gaseous states, which can be attributed to van der Waals dispersion forces.
The curved π surface of fullerene has attracted significant attention in the synthesis of heterogeneous self-assembled architectures. It was discovered that fullerene and calixarene can form molecular complex through non-covalent interaction.1,2 Their binding was found to be dependent on the size of the spheroids interacting with the macrocyclic hosts. Later, it was also observed that molecules with a flat surface such as porphyrin or its metal derivatives possessing a π plane could harbor the curved π surface of fullerene.3 This intermolecular interaction between two dissimilar π systems is largely attributed to the van der Waals dispersion forces. Similarly, other planar systems also have the ability to form a non-covalent complex with the π surface of fullerene.4–8 Even though π–π interaction is common between fullerene and flat molecules, the shape of these assemblies can be either non-linear or in the form of columnar stacks. A variety of hosts have been designed, synthesized and explored to bind the C60 spheroid.9–16 In this context, the unique aromatic character of fullerene is a topic of intense discussion.17,18 Small aromatic sub-units such as benzene, pyrrole, and thiophene19 are a common feature of all such hosts that favor close contacts with C60. Therefore, is aromaticity the only driving force to attract fullerene towards a planar π surface? Unlike its interaction with aromatic molecules, it is uncommon to observe the binding of fullerene to antiaromatic hosts.
Isophlorin,201, is an unstable 20π antiaromatic tetrapyrrolic macrocycle with a structural resemblance to porphyrin, 2 (Chart 1). Nonetheless, its furan/thiophene derivatives are stable under ambient conditions and sustain a planar π surface.21,22 They represent ideal examples to explore the interaction of fullerene with a 4nπ macrocyclic host. In addition, isophlorins do not exhibit π stacking which can favour better interaction with guest molecules. In contrast to aromatic hosts, such studies are not well explored with anti-aromatic hosts. Herein we describe the discrete nature of fullerene–isophlorin interaction along with the structural characterization of these non-covalent complexes.
 | ||
| Chart 1 20π anti-aromatic isophlorins (1), 18π aromatic porphyrin (2) and 20π anti-aromatic tetraoxaisophlorin (3). | ||
It has been observed that the meso substituents on the porphyrin ring provide cooperative effects in the binding of fullerene. In this context we employed three different tetraoxaisophlorins, 3–5, by varying the substituents on the meso carbon atoms. Tetraoxaisophlorins 4 and 5 were synthesized by acid catalyzed reactions of suitable precursors, followed by oxidation (Scheme 1).
 | ||
| Scheme 1 Syntheses of 20π tetraoxaisophlorins. (i) One equiv. of BF3·OEt2, five equiv. of FeCl3, dry DCM (100 ml), rt, N2, 2 h. (ii) Five equiv. of DDQ, N2H4·H2O, DCM, reflux.23 | ||
Isophlorins 4 and 5 were characterized by mass spectrometry, 1H NMR spectroscopy and single crystal X-ray diffraction analysis (ESI†). Their anti-aromatic character was confirmed by the upfield chemical shift values for the proton on the β-carbons of the furan in the 1H NMR spectra (ESI†). The estimated NICS(0)24 values of +38.13 and +39.64 for 4 and 5 are amongst the highest reported positive values for any anti-aromatic macrocycles.25–27 The solutions of these three anti-aromatic macrocycles were individually treated with a toluene solution of C60. The colour of the solutions displayed a distinct change from green to brown, upon the addition of fullerene, suggesting the formation of the π complex. Co-crystals of isophlorins (4 and 5) and fullerene were grown from a combination of toluene–acetone solvents by a slow evaporation method at room temperature, to yield black coloured crystals. The molecular complex of (4)3·C60 revealed a fullerene trigonally engulfed by three isophlorins (a, b and c in Fig. 1).18 The isophlorin surface was found to be extremely close to the π surface of the fullerene, compared to standard values for π–π interactions. These values are comparable to that observed between C60 and a free base porphyrin. C60 is centered over the isophlorin with electron-deficient 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 ring juncture C–C bonds at a close distance to the plane of the isophlorin core (C to mean 24-atom plane distances C with a, b and c are 2.61 Å, 2.68 Å, and 2.76 Å respectively). No close fullerene–fullerene contacts were observed in the packing diagram for this crystal. The short contacts of ortho-F atoms of the isophlorin “a” and “c” to the nearest carbon atom of fullerene were found to be 3.09 and 3.10 Å respectively. Curiously, isophlorin “c” was distorted from a regular planar structure in this non-covalent complex.
6 ring juncture C–C bonds at a close distance to the plane of the isophlorin core (C to mean 24-atom plane distances C with a, b and c are 2.61 Å, 2.68 Å, and 2.76 Å respectively). No close fullerene–fullerene contacts were observed in the packing diagram for this crystal. The short contacts of ortho-F atoms of the isophlorin “a” and “c” to the nearest carbon atom of fullerene were found to be 3.09 and 3.10 Å respectively. Curiously, isophlorin “c” was distorted from a regular planar structure in this non-covalent complex.
When crystals were grown upon addition of excess fullerene to a solution of 4, it was observed that the macrocycle was sandwiched by two fullerene units,284·(C60)2 (Fig. 2). The close contacts observed between both C60 and the macrocycle are 2.64 and 2.66 Å. These values are extremely short compared to conventional π–π stacking even in a homogeneous system.
 | ||
Fig. 2 (a) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 contact with a single isophlorin in 4·(C60)2. Pentafluorophenyl rings are omitted for clarity. (b) Packing structure of 4·(C60)2. 2 contact with a single isophlorin in 4·(C60)2. Pentafluorophenyl rings are omitted for clarity. (b) Packing structure of 4·(C60)2. | ||
In contrast to the above structures, the molecular complex of 5·C60 revealed a zig-zag assembly of alternating isophlorin–C60 interactions (Fig. 3). The orientation of C60 is very similar to that found in 4·(C60)2 with the 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 ring junction carbon atoms lying over the centre of the isophlorin ring (Fig. 3) at distances of 2.64 and 2.66 Å from the mean 24-atom plane of isophlorins (ESI†). The closest atom to atom contacts are from the two 5
6 ring junction carbon atoms lying over the centre of the isophlorin ring (Fig. 3) at distances of 2.64 and 2.66 Å from the mean 24-atom plane of isophlorins (ESI†). The closest atom to atom contacts are from the two 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
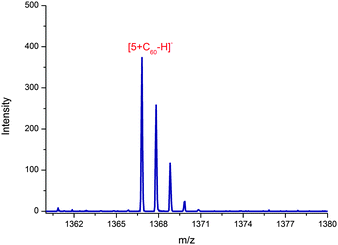
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 fullerene carbon atoms to the isophlorin oxygen atoms, and they range from 2.96 to 4.31 Å. The ortho-F atoms of the meso pentafluorophenyl ring were found to be at a distance of 2.98, 3.12 and 3.13 Å to the nearest carbon atom of fullerene suggesting their significant contribution in the complexation. The angle between the isophlorin planes was found to be 32.7°. The closest carbon-to-carbon atom distance between fullerenes in adjacent chains is 3.16–3.26 Å. In addition to close fullerene–isophlorin π–π interactions, weak C–F⋯H–C bonds29 were also observed between the C6F5 groups and the furan β hydrogens of neighbouring macrocycles. Soft ionization MALDI mass spectrometry also confirmed the complex formation between 5 and C60. Using dihydroxybenzoic acid as the matrix, an m/z value of 1366.96 was observed, corresponding to a 1
6 fullerene carbon atoms to the isophlorin oxygen atoms, and they range from 2.96 to 4.31 Å. The ortho-F atoms of the meso pentafluorophenyl ring were found to be at a distance of 2.98, 3.12 and 3.13 Å to the nearest carbon atom of fullerene suggesting their significant contribution in the complexation. The angle between the isophlorin planes was found to be 32.7°. The closest carbon-to-carbon atom distance between fullerenes in adjacent chains is 3.16–3.26 Å. In addition to close fullerene–isophlorin π–π interactions, weak C–F⋯H–C bonds29 were also observed between the C6F5 groups and the furan β hydrogens of neighbouring macrocycles. Soft ionization MALDI mass spectrometry also confirmed the complex formation between 5 and C60. Using dihydroxybenzoic acid as the matrix, an m/z value of 1366.96 was observed, corresponding to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of the 5·C60 complex (Fig. 4).
1 ratio of the 5·C60 complex (Fig. 4).
 | ||
| Fig. 3 (a) Molecular structure of the 5 and C60 complex. (b) The packing diagram in the crystal lattice of 5·C60 reveals a zigzag chain of alternating isophlorin and C60. | ||
The crystal-packing diagram of 3·C60 displayed a columnar stacking of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 alternative C60–isophlorin in benzene (Fig. 5). However, the zig-zag assembly was not displayed and the isophlorin planes are parallel, leading to a linear chain kind of assembly. The C60 is centered over the porphyrin with electron-rich 6
1 alternative C60–isophlorin in benzene (Fig. 5). However, the zig-zag assembly was not displayed and the isophlorin planes are parallel, leading to a linear chain kind of assembly. The C60 is centered over the porphyrin with electron-rich 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 ring-juncture C–C bonds at a close distance to the plane of the porphyrin core (C to mean 24-atom plane distance), 2.62 Å. The closest atom-to-atom contacts are from the two 6
6 ring-juncture C–C bonds at a close distance to the plane of the porphyrin core (C to mean 24-atom plane distance), 2.62 Å. The closest atom-to-atom contacts are from the two 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 fullerene carbon atoms to the isophlorin oxygen atoms, which are in the range 2.94 to 3.76 Å. Short contacts of ortho-F atoms of the isophlorin to the nearest carbon atom of fullerene are found at 3.16 and 3.18 Å, indicating that the ortho C–F bonds have a relatively weak contribution to the association compared to that in 5·C60. Interestingly, there are no close fullerene–fullerene contacts. Benzene molecules occupied channels between columns, which suggest that aromatic solvents stabilize the assembly. Here we also found weak C–F⋯H–C type H-bonding interactions like 5·C60. Our attempts at co-crystallization with different compositions of C60–isophlorin in various solvents selectively yielded a 1
6 fullerene carbon atoms to the isophlorin oxygen atoms, which are in the range 2.94 to 3.76 Å. Short contacts of ortho-F atoms of the isophlorin to the nearest carbon atom of fullerene are found at 3.16 and 3.18 Å, indicating that the ortho C–F bonds have a relatively weak contribution to the association compared to that in 5·C60. Interestingly, there are no close fullerene–fullerene contacts. Benzene molecules occupied channels between columns, which suggest that aromatic solvents stabilize the assembly. Here we also found weak C–F⋯H–C type H-bonding interactions like 5·C60. Our attempts at co-crystallization with different compositions of C60–isophlorin in various solvents selectively yielded a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of 3·C60 and 5·C60 complexes. These results are consistent with NMR results in solution. We also investigated the attraction of fullerene towards the isophlorin in solution by using variable-temperature NMR experiments. Toluene-d8 was chosen as solvent because of good solubility for both the molecules. This solution shows remarkable shifts in both 13C as well as 1H NMR spectra. A 1
1 ratio of 3·C60 and 5·C60 complexes. These results are consistent with NMR results in solution. We also investigated the attraction of fullerene towards the isophlorin in solution by using variable-temperature NMR experiments. Toluene-d8 was chosen as solvent because of good solubility for both the molecules. This solution shows remarkable shifts in both 13C as well as 1H NMR spectra. A 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 solution of 4 and C60 displayed 0.03 ppm upfield shift for the β hydrogens of furan and 0.03 ppm downfield shift for the meso phenyl hydrogens. Simultaneously, a 0.10 ppm shift was observed for the ortho fluorine atoms in 19F NMR at room temperature. In the 13C NMR, C60 displayed a single resonance for 4·C60 at 298 K with a marginal downfield shift of 0.26 ppm relative to free C60. Upon cooling to 203 K, this signal was downfield shifted by 0.98 ppm. A large downfield shift of 1.33 ppm was observed upon cooling to 183 K but with significant signal broadening. Similarly, a 1
1 solution of 4 and C60 displayed 0.03 ppm upfield shift for the β hydrogens of furan and 0.03 ppm downfield shift for the meso phenyl hydrogens. Simultaneously, a 0.10 ppm shift was observed for the ortho fluorine atoms in 19F NMR at room temperature. In the 13C NMR, C60 displayed a single resonance for 4·C60 at 298 K with a marginal downfield shift of 0.26 ppm relative to free C60. Upon cooling to 203 K, this signal was downfield shifted by 0.98 ppm. A large downfield shift of 1.33 ppm was observed upon cooling to 183 K but with significant signal broadening. Similarly, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 solution of the 5·C60 complex exhibited a 0.18 ppm upfield shift for the C60 carbon in 13C NMR at 183 K. In the case of a 1
1 solution of the 5·C60 complex exhibited a 0.18 ppm upfield shift for the C60 carbon in 13C NMR at 183 K. In the case of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex of 3·C60 we observed a 0.16 ppm upfield shift in 1H NMR at 183 K. The larger shift suggests significant interaction between C60 and isophlorin. However, UV-Visible experiments did not display any salient changes for all the complexes in solution. This can be attributed to the purely non-covalent interaction. The absence of any charge-transfer bands in the electronic absorption spectra further supported the lack of donor–acceptor complexes. The isophlorins are poor emitters compared to porphyrin. However, it was observed that even the negligible fluorescence intensity reduced upon the addition of fullerene, which can be attributed to cluster formation between the two π conjugated molecules (see ESI†).
1 complex of 3·C60 we observed a 0.16 ppm upfield shift in 1H NMR at 183 K. The larger shift suggests significant interaction between C60 and isophlorin. However, UV-Visible experiments did not display any salient changes for all the complexes in solution. This can be attributed to the purely non-covalent interaction. The absence of any charge-transfer bands in the electronic absorption spectra further supported the lack of donor–acceptor complexes. The isophlorins are poor emitters compared to porphyrin. However, it was observed that even the negligible fluorescence intensity reduced upon the addition of fullerene, which can be attributed to cluster formation between the two π conjugated molecules (see ESI†).
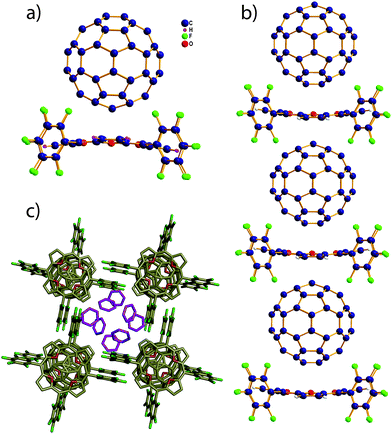 | ||
| Fig. 5 (a) Molecular structure of the complex formed by 3 and C60. (b) Columnar stacking of alternating isophlorin–C60 in the 3·C60 complex. (c) The solvent molecules are trapped in the voids. | ||
The complex formation in the solution state between 4 or 5 and C60 was studied using isothermal titration calorimetric (ITC) experiments30 and fluorescence (see ESI†). The estimated association constant (Ka) was found to be 9.91 × 102 M−1 and 7.16 × 103 M−1 for 4·C60 and 5·C60 respectively. The thermodynamic parameters, ΔG (−5.24 kcal mol−1), ΔH (−0.146 kcal mol−1), and TΔS (−5.24 kcal mol−1), for 4·C60 and ΔG (−4.09 kcal mol−1), ΔH (−0.434 kcal mol−1), and TΔS (−3.66 kcal mol−1), for 5·C60 further confirmed the binding between isophlorins and C60. The value of the binding constant estimated from fluorescence spectroscopy was also found to be of the same order (see ESI†).
In conclusion, the first molecular complexes of fullerene with antiaromatic isophlorin were obtained and successfully characterized by single-crystal X-ray diffraction studies. The close contacts between fullerenes and isophlorin arise from a favourable van der Waals attraction of the curved π surface of a fullerene to the anti-aromatic π surface of isophlorin. These studies suggest that anti-aromatic π surfaces are as good as aromatic surfaces for binding fullerenes. The estimated binding constants also confirmed the interaction between the isophlorins and C60 in the solution state.
The authors thank IISER Pune and Department of Science and Technology New Delhi, India, for the financial support. BKR thanks UGC, New Delhi, India, for senior research fellowship. The authors thank Dr Amitava Das, NCL Pune, for ITC measurements.
Notes and references
- J. L. Atwood, G. A. Koutsantonis and C. L. Raston, Nature, 1994, 368, 229–231 CrossRef CAS.
- T. Suzuki, K. Nakashima and S. Shinkai, Chem. Lett., 1994, 699–702 CrossRef CAS.
- Y. Sun, T. Drovetskaya, R. D. Bolskar, R. Bau, P. D. W. Boyd and C. A. Reed, J. Org. Chem., 1997, 62, 3642–3649 CrossRef.
- P. D. W. Boyd, M. C. Hodgson, C. E. F. Rickard, A. G. Oliver, L. Chaker, P. J. Brothers, R. D. Bolskar, F. S. Tham and C. A. Reed, J. Am. Chem. Soc., 1999, 121, 10487–10495 CrossRef.
- P. D. Boyd and C. A. Reed, Acc. Chem. Res., 2005, 38, 235–242 CrossRef PubMed.
- A. Hosseini, M. C. Hodgson, F. S. Tham, C. A. Reed and P. D. W. Boyd, Cryst. Growth Des., 2006, 6, 397–403 CAS.
- J. Song, N. Aratani, H. Shinokubo and A. Osuka, J. Am. Chem. Soc., 2010, 132, 16356–16357 CrossRef PubMed.
- Z. Wang, F. Dotz, V. Enkelmann and K. Mullen, Angew. Chem., Int. Ed., 2005, 44, 1247–1250 CrossRef PubMed.
- A. Ikeda, M. Yoshimura, H. Udzu, C. Fukuhara and S. Shinkai, J. Am. Chem. Soc., 1999, 121, 4296–4297 CrossRef.
- N. Kishi, M. Akita, M. Kamiya, S. Hayashi, H. F. Hsu and M. Yoshizawa, J. Am. Chem. Soc., 2013, 135, 12976–12979 CrossRef PubMed.
- O. D. Fox, M. G. Drew and P. D. Beer, Angew. Chem., Int. Ed., 2000, 39, 135–140 CrossRef.
- O. D. Fox, E. J. S. Wilkinson, P. D. Beer and M. G. B. Drew, Chem. Commun., 2000, 391–392 RSC.
- O. D. Fox, J. Cookson, E. J. S. Wilkinson, M. G. B. Drew, E. J. MacLean, S. J. Teat and P. D. Beer, J. Am. Chem. Soc., 2006, 128, 6990–7002 CrossRef PubMed.
- K. Suzuki, K. Takao, S. Sato and M. Fujita, J. Am. Chem. Soc., 2010, 132, 2544–2545 CrossRef PubMed.
- W. Meng, B. Breiner, K. Rissanen, J. D. Thoburn, J. K. Clegg and J. R. Nitschke, Angew. Chem., Int. Ed., 2011, 50, 3479–3483 CrossRef PubMed.
- N. Martìn and J.-F. Nierengarten, Supramolecular Chemistry of Fullerenes and Carbon Nanotubes, Germany, 2012 Search PubMed.
- Z. Chen, J. I. Wu, C. Corminboeuf, J. Bohmann, X. Lu, A. Hirsch and P. Schleyer, Phys. Chem. Chem. Phys., 2012, 14, 14886–14891 RSC.
- M. M. Olmstead and D. J. Nurco, Cryst. Growth Des., 2006, 6, 109–113 Search PubMed.
- J. Zhang, J. Tan, Z. Ma, W. Xu, G. Zhao, H. Geng, C. A. Di, W. Hu, Z. Shuai, K. Singh and D. Zhu, J. Am. Chem. Soc., 2013, 135, 558–561 CrossRef PubMed.
- R. B. Woodward, Angew. Chem., 1960, 72, 651–662 CrossRef PubMed.
- M. Pohl, H. Schmickler, J. Lex and E. Vogel, Angew. Chem., Int. Ed. Engl., 1991, 30, 1693–1697 CrossRef PubMed.
- J. S. Reddy and V. G. Anand, J. Am. Chem. Soc., 2008, 130, 3718–3719 CrossRef PubMed.
- Z. Hu, J. L. Atwood and M. P. Cava, J. Org. Chem., 1994, 59, 8071–8075 CrossRef.
- P. V. Schleyer, C. Maerker, A. Dransfeld, H. J. Jiao and N. J. R. V. Hommes, J. Am. Chem. Soc., 1996, 118, 6317–6318 CrossRef CAS.
- R. R. Valiev, H. Fliegl and D. Sundholm, J. Phys. Chem. A, 2013, 117, 9062–9068 CrossRef PubMed.
- T. Ito, Y. Hayashi, S. Shimizu, J. Y. Shin, N. Kobayashi and H. Shinokubo, Angew. Chem., Int. Ed., 2012, 51, 8542–8545 CrossRef PubMed.
- T. Y. Gopalakrishna, J. S. Reddy and V. G. Anand, Angew. Chem., Int. Ed., 2014, 53, 10984–10987 CrossRef PubMed.
- D. V. Konarev, I. S. Neretin, Y. L. Slovokhotov, E. I. Yudanova, N. y. V. Drichko, Y. M. Shul'ga, B. P. Tarasov, L. L. Gumanov, A. S. Batsanov, J. A. K. Howard and R. N. Lyubovskaya, Chem. – Eur. J., 2001, 7, 2605–2616 CrossRef.
- G. R. Desiraju and T. Steiner, The Weak Hydrogen Bond in Structural Chemistry and Biology, OUP, Oxford, 1999, ch. 3 Search PubMed.
- F. P. Schmidtchen, Isothermal Titration Calorimetry in Supramolecular Chemistry, ed. C. A. Schalley, Wiley-VCH, 2006, pp. 55–78 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, synthesis, characterization coordinates for the DFT optimized structures and the X-ray crystal data of complexes (CIF). CCDC 1043384 (3·C60), 1043385 (4), 10433864·(C60)2, 1043387 (4)3·C60, 1043388 (5) and 1043389 (5·C60). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5cc00771b |
| This journal is © The Royal Society of Chemistry 2015 |