Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
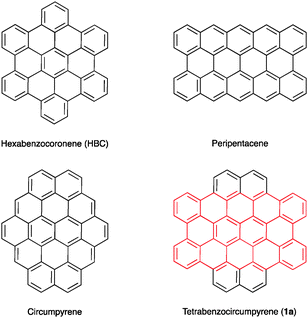
Tetrabenzocircumpyrene: a nanographene fragment with an embedded peripentacene core†
Ruth
Dorel
a,
Carlos
Manzano
b,
Maricarmen
Grisolia
c,
We-Hyo
Soe
b,
Christian
Joachim
c and
Antonio M.
Echavarren
*ad
aInstitute of Chemical Research of Catalonia (ICIQ), Av. Països Catalans 16, 43007 Tarragona, Spain. E-mail: aechavarren@iciq.es
bIMRE, A*STAR (Agency for Science, Technology and Research), 3 Research Link, 117602, Singapore
cGNS & MANA Satellite, CEMES-CNRS, 29 rue J. Marvig, 31055 Toulouse Cedex, France
dDepartament de Química Analítica i Química Orgànica, Universitat Rovira i Virgili, C/Marcel li Domingo s/n, 43007 Tarragona, Spain
First published on 12th March 2015
Abstract
A new disc-shaped highly symmetric C54H20 nanographene fragment, tetrabenzocircumpyrene, has been synthesized and characterized by scanning tunnelling microscopy, demonstrating the potential of this technique for identifying highly insoluble graphenic molecules.
Large polycyclic aromatic hydrocarbons (PAHs) have recently attracted much attention due to their unique electronic and self-assembly properties, which provide a wide range of opportunities for the fabrication of novel devices.1 One of the challenges in this area is the development of efficient synthetic methodologies for the preparation of well-defined sections of graphene (nanographenes). In this regard, novel organic protocols have been developed, although they are always limited by the low solubility of planar aromatic molecules and the number of side reactions that may take place, which increase with the molecular weight.2 Disc-shaped molecules are of particular interest since they are able to self-organize into columnar phases and therefore can be used as conducting layers in organic molecular electronics.3 One of the most extensively studied discotic PAH is hexa-peri-benzocoronene (HBC), also known as ‘superbenzene’, owing to its intrinsic charge-carrier ability.4 It is predictable that enlarging the aromatic framework would enhance the columnar order due to more intense π–π interactions and, consequently, charge mobility. The stacking of PAHs as prototypes for graphene multilayers has been theoretically studied, finding that the binding energies strongly depend on the size of the PAHs.5 On the other hand, there is growing interest in acene-based molecules such as periacenes, which are conjugated systems with two rows of peri-fused acenes.6 These compounds might serve as biradical materials, and are believed to be suitable materials for spintronic, electronic, optical, and magnetic devices. Small periacenes have been known for a long time, and recently larger analogues such as teranthene and quarteranthene have been described.7 However, the synthesis of large periacenes such as peritetracene or peripentacene has not been achieved yet,8 although peripentacene has been detected by sublimation of pentacene by mass spectrometry.9
Motivated by its promising properties, we targeted tetrabenzocircumpyrene 1a, a yet unreported highly symmetric disc-shaped nanographene fragment containing 54 carbon atoms. Polyarene 1a includes a peripentacene core and can be considered as a ‘expanded HBC’ (Fig. 1). According to that expected,10,11 DFT calculations predicted a ca. 30% decrease in the HOMO–LUMO gap in going from HBC (3.59 eV) to tetrabenzocircumpyrene 1a (2.45 eV).
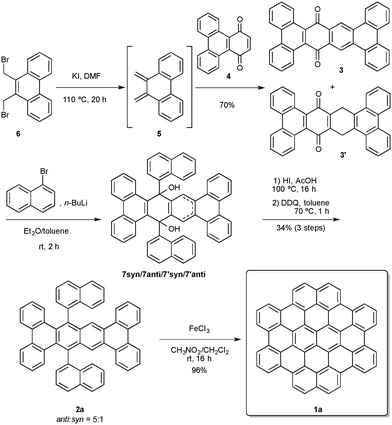
Our retrosynthetic approach for the synthesis of 1a considers the formation of the final nanographene fragment by means of Scholl oxidation from 9,20-di(naphthalen-1-yl)tetrabenzo[a,c,j,l]tetracene (2a) in which six C–C bonds would be formed in a single step (Scheme 1). Polyarene 2a could be obtained by double nucleophilic addition of the naphthyl moieties to quinone 3, which could result from Diels–Alder cycloaddition between quinone 4 and o-quinodimethane 5. Quinone 4, the starting point of our synthesis, has been prepared by oxidation of triphenylene with excess H2O2, albeit in low yield.12 However, when we tried to scale up this reaction, the yield dropped significantly to 1–6%. After some optimization, we found that by using CrO3 as the oxidant in the presence of 18-crown-6,13 multigram amounts of quinone 4 could routinely be obtained in up to 41% yield.
We chose 6 as a precursor of diene 5, which was generated in situ14 and subjected to Diels–Alder cycloaddition with 4 to form a non-isolable adduct which spontaneously underwent oxidation to give a mixture of quinones 3 and 3′ in 70% yield (Scheme 2). Treatment of this mixture with DDQ did not result in the complete oxidation of 3′ to 3, presumably due to the low solubility of these compounds in standard organic solvents. For this reason we decided to continue the synthesis using a mixture of both quinones. Double nucleophilic addition of 1-naphthyllithium to 3/3′ afforded the crude mixture of diols 7syn, 7anti, 7′syn and 7′anti, which was directly reduced by treatment with HI in acetic acid. Without isolation, this mixture was treated with DDQ in toluene to give key intermediate 2a as a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of anti
1 mixture of anti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) syn rotational isomers (Fig. 2).15 Final Scholl cyclodehydrogenation of 2a in the presence of excess of FeCl3 gave the target nanographene fragment 1a in 96% yield. The synthesis of tetrabenzocircumpyrene (1a) proceeds in just six steps, requires only two chromatographic purifications, and every step can be conducted on a gram scale (1.02 g of 1a were prepared in a single round from 6 g of triphenylene).
syn rotational isomers (Fig. 2).15 Final Scholl cyclodehydrogenation of 2a in the presence of excess of FeCl3 gave the target nanographene fragment 1a in 96% yield. The synthesis of tetrabenzocircumpyrene (1a) proceeds in just six steps, requires only two chromatographic purifications, and every step can be conducted on a gram scale (1.02 g of 1a were prepared in a single round from 6 g of triphenylene).
 | ||
| Fig. 2 ORTEP plot (50% thermal ellipsoids) of the crystal structure of 2a-anti (left) and 2a-syn (right). | ||
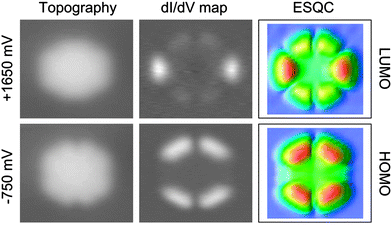
The extreme insolubility of this compound precluded its characterization by NMR spectroscopy. However, we were able to obtain a clean MALDI+ mass spectrum in which we could only identify one peak corresponding to the mass and isotopic pattern of 1a. Furthermore, we attempted the characterization of 1a by means of scanning tunnelling microscopy (STM). STM and differential conductance (dI/dV) imaging in conjunction with tunnelling spectroscopy were used respectively to obtain topographic images of 1a molecules deposited on an Au(111) surface and to characterize their molecular electronic states. dI/dV mapping of single 1a molecules at resonances captured in the spectra allowed us to imagine the spatial distribution of molecular electronic states. Electron Scattering Quantum Chemical (ESQC) calculations including all the elements of the experimental method, i.e. the substrate, 1a molecule and STM tip, were done in order to assign the experimental dI/dV images to the corresponding molecular orbitals.
The topographic, dI/dV and computed images are shown in Fig. 3. The experimental dI/dV images were taken at voltages corresponding to two resonances captured in the dI/dV spectra of a single 1a molecule, namely at −0.75 eV and 1.65 eV (see ESI†). The maps computed at the energies corresponding to HOMO and LUMO orbitals showed identical features to those from the experimental dI/dV images, clearly confirming the structure assigned to tetrabenzocircumpyrene 1a. The experimental 2.40 eV HOMO–LUMO gap determined on 1a deposited on Au(111) is fully consistent with the calculated value of 2.45 eV in the gas phase (DFT, B3LYP/6-31G(d)).
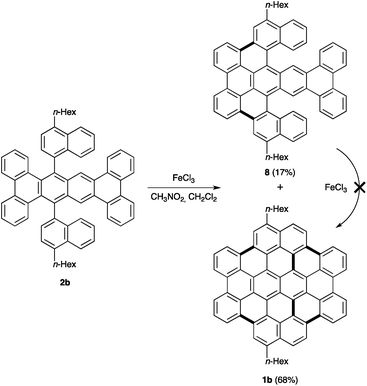
We also considered the extension of this synthetic route to prepare tetrabenzocircumpyrene derivatives by using substituted 1-bromonaphthalenes as the starting materials for the double nucleophilic addition to the mixture of 3 and 3′ (Scheme 3). Thus, we prepared dialkyltetrabenzocircumpyrenes 1b and 1c, as well as the higher analogue hexabenzocircumpyrene 1d. The structure of the direct precursors of nanographene fragments 2b–d could be confirmed by X-ray diffraction (Fig. 4).15
 | ||
| Fig. 4 ORTEP plot (50% thermal ellipsoids) of the crystal structures of 2b (top), 2c (center) and 2d (bottom). | ||
We observed that when we treated alkyl-substituted precursors of nanographene fragments with FeCl3 two products were formed. We decided to use 2b as a model substrate to examine this transformation in detail (Scheme 4). The major product was the expected dialkyltetrabenzocircumpyrene 1b, which could be easily separated by filtration. After meticulous purification, we were able to isolate from the filtrate a brown solid slightly more soluble in organic solvents at temperatures over 100 °C. This solid could be crystallized and the structure was confirmed to be 8 by X-ray diffraction (Fig. 5).15 This partially fused polyarene shows a contorted aromatic surface, which might also be of interest in material science.16 To prove if partially cyclized 8 was an intermediate in the formation of 1b, we resubjected 8 to the oxidative cyclization conditions. However, after 24 h only unchanged 8 could be detected by mass spectrometry, demonstrating that this polyarene is not an intermediate in the formation of 1b, but rather a product resulting from a competitive pathway. This result further demonstrates that not all the conceivable pathways in Scholl cyclodehydrogenation processes are equally productive and underscores that the factors controlling the successive formation of C–C bonds under the conditions of the Scholl reaction are still not well understood.17
In conclusion, tetrabenzocircumpyrene 1a, a new disc-shaped nanographene cutout containing 54 carbon atoms, has been efficiently prepared in a simple and practical manner (6 steps from commercially available starting materials), and fully characterized by means of STM. The potential of the route to obtain other related nanographene fragments has also been demonstrated. Application of this strategy for the synthesis of larger analogues and other polyrenes with contorted aromatic surfaces is underway.
We thank ATMOL (Contract No. FP7-270028), the European Research Council (Advanced Grant No. 321066), the MINECO (Severo Ochoa Excellence Accreditation 2014-2018 (SEV-2013-0319) and project CTQ2013-42106-P), the AGAUR (2014 SGR 818), and the ICIQ Foundation. We gratefully acknowledge the ICIQ X-ray diffraction, NMR, and Mass Spectrometry units, and SGIker of UPV/EHU for technical support.
Notes and references
- (a) J. Wu, W. Pisula and K. Müllen, Chem. Rev., 2007, 107, 718–747 CrossRef CAS PubMed; (b) M. D. Watson, A. Fhtecenkötter and K. Müllen, Chem. Rev., 2001, 101, 1267–1300 CrossRef CAS PubMed; (c) H. Seyler, B. Purushothaman, D. J. Jones, A. B. Holmes and W. W. H. Wong, Pure Appl. Chem., 2012, 84, 1047–1067 CrossRef CAS; (d) X. Feng, W. Pisula and K. Müllen, Pure Appl. Chem., 2009, 81, 2203–2224 CrossRef CAS; (e) K. Kawasumi, Q. Zhang, Y. Segawa and L. T. Scott, Nat. Chem., 2013, 5, 739–744 CrossRef CAS PubMed; (f) L. Chen, Y. Hernandez, X. Feng and K. Müllen, Angew. Chem., Int. Ed., 2012, 51, 7640–7654 CrossRef CAS PubMed; (g) W. Jin, T. Fukushima, A. Kosaka, M. Niki, N. Ishii and T. Aida, J. Am. Chem. Soc., 2005, 127, 8284–8285 CrossRef CAS PubMed; (h) K. Müllen and J. P. Rabe, Acc. Chem. Res., 2008, 41, 511–520 CrossRef PubMed; (i) S. Fujii and T. Enoki, Acc. Chem. Res., 2013, 46, 2202–2210 CrossRef CAS; (j) K. Müllen, ACS Nano, 2014, 8, 6531–6541 CrossRef PubMed.
- (a) L. T. Scott, Angew. Chem., Int. Ed., 2004, 43, 4994–5007 CrossRef CAS PubMed; (b) K. Tahara and Y. Tobe, Chem. Rev., 2006, 106, 5274–5290 CrossRef CAS PubMed; (c) B. Schuler, S. Collazos, L. Gross, G. Meyer, D. Pérez, E. Guitián and D. Peña, Angew. Chem., Int. Ed., 2014, 53, 9004–9006 CrossRef CAS PubMed; (d) S. J. Hein, H. Arslan, I. Keresztes and W. R. Dichtel, Org. Lett., 2014, 16, 4416–4419 CrossRef CAS PubMed; (e) S. Xiao, S. J. Kang, Y. Wu, S. Ahn, J. B. Kim, Y. L. Loo, T. Siegrist, M. L. Steigerwald, H. Li and C. Nuckolls, Chem. Sci., 2013, 4, 2018–2023 RSC; (f) Q. Zhang, H. Peng, G. Zhang, Q. Lu, J. Chang, Y. Dong, X. Shi and J. Wei, J. Am. Chem. Soc., 2014, 136, 5057–5064 CrossRef CAS PubMed.
- (a) W. Pisula, X. Feng and K. Müllen, Adv. Mater., 2010, 22, 3634–3649 CrossRef CAS PubMed; (b) S. Sergeyev, W. Pisula and Y. H. Geerts, Chem. Soc. Rev., 2007, 36, 1902–1929 RSC; (c) W. Pisula, X. Feng and K. Müllen, Chem. Meter., 2011, 23, 554–567 CrossRef CAS; (d) M. Kivala, W. Pisula, S. Wang, A. Mavrinskiy, J.-P. Gisselbrecht, X. Feng and K. Müllen, Chem. – Eur. J., 2013, 19, 8117–8128 CrossRef CAS PubMed; (e) L. Chen, X. Dou, W. Pisula, X. Yang, D. Wu, G. Floudas, X. Feng and K. Müllen, Chem. Commun., 2012, 48, 702–704 RSC; (f) W. Jin, Y. Yamamoto, T. Fukushima, N. Ishii, J. Kim, K. Kato, M. Takata and T. Aida, J. Am. Chem. Soc., 2008, 130, 9434–9440 CrossRef CAS PubMed; (g) F. Hinkel, D. Cho, W. Pisula, M. Baumgarten and K. Müllen, Chem. – Eur. J., 2015, 21, 86–90 CrossRef CAS PubMed.
- Lead references on the synthesis of substituted hexabenzocoronenes: (a) R. Rathore and C. L. Burns, J. Org. Chem., 2003, 68, 4071–4074 CrossRef CAS PubMed; (b) X. Feng, J. Wu, V. Enkelmann and K. Müllen, Org. Lett., 2006, 8, 1145–1148 CrossRef CAS PubMed; (c) X. Dou, X. Yang, G. J. Bodwell, M. Wagner, V. Enkelmann and K. Müllen, Org. Lett., 2007, 9, 2485–2488 CrossRef CAS PubMed; (d) S. H. Wadumethrige and R. Rathore, Org. Lett., 2008, 10, 5139–5142 CrossRef CAS PubMed.
- C. Feng, C. S. Lin, W. Fan, R. Q. Zhang and M. A. Van Hove, J. Chem. Phys., 2009, 131, 194702 CrossRef CAS PubMed.
- Q. Ye and C. Chi, Chem. Mater., 2014, 26, 4046–4056 CrossRef CAS.
- (a) A. Konishi, Y. Hirao, M. Nakano, A. Shimizu, E. Botek, B. Champagne, D. Shiomi, K. Sato, T. Takui, K. Matsumoto, H. Kurata and T. Kubo, J. Am. Chem. Soc., 2010, 132, 11021–11023 CrossRef CAS PubMed; (b) A. Konishi, Y. Hirao, K. Matsumoto, H. Kurata, R. Kishi, Y. Shigeta, M. Nakano, K. Tokunaga, K. Kamada and T. Kubo, J. Am. Chem. Soc., 2013, 135, 1430–1437 CrossRef CAS PubMed; (c) L. Zöphel, R. Berger, P. Gao, V. Enkelmann, M. Baumgarten, M. Wagner and K. Müllen, Chem. – Eur. J., 2013, 19, 17821–17826 CrossRef; (d) A. Sun, Z. Zeng and J. Wu, Acc. Chem. Res., 2014, 47, 2582–2591 CrossRef PubMed.
- A. Narita, X. Feng, Y. Hernandez, S. A. Jensen, M. Bonn, H. Yang, I. A. Verzhbitskiy, C. Casiraghi, M. R. Hansen, A. H. R. Koch, G. Fytas, O. Ivasenko, B. Li, K. S. Mali, T. Balandina, S. Mahesh, S. De Feyter and K. Müllen, Nat. Chem., 2013, 6, 126–132 CrossRef PubMed.
- (a) L. B. Roberson, J. Kowalik, L. M. Tolbert, C. Kloc, R. Zeis, X. L. Chi, R. Fleming and C. Wilkins, J. Am. Chem. Soc., 2005, 127, 3069–3075 CrossRef CAS PubMed; (b) B. H. Northrop, J. E. Norton and K. N. Houk, J. Am. Chem. Soc., 2007, 129, 6536–6546 CrossRef CAS PubMed.
- (a) J. Aihara, J. Phys. Chem. A, 1999, 103, 7487–7495 CrossRef CAS; (b) Y. Ruiz-Morales, J. Phys. Chem. A, 2002, 106, 11283–11308 CrossRef CAS; (c) D. Moran, F. Stahl, H. F. Bettinger, H. F. Schaefer III and P. V. R. Schleyer, J. Am. Chem. Soc., 2003, 125, 6746–6752 CrossRef CAS PubMed; (d) X. L. Fan, X. Q. Wang, J. T. Wang and H. D. Li, Phys. Lett. A, 2014, 378, 1379–1382 CrossRef CAS PubMed.
- (a) N. Tyutyulkov, G. Madjarova, F. Dietz and K. Müllen, J. Phys. Chem. B, 1998, 102, 10183–10189 CrossRef CAS; (b) F. Dietz, N. Tyutyulkov, G. Madjarova and K. Müllen, J. Phys. Chem. B, 2000, 104, 1746–1761 CrossRef CAS.
- L. H. Klemm, E. Hall, L. Cousins and C. E. Klopfenstein, J. Heterocycl. Chem., 1987, 24, 1749–1755 CrossRef CAS.
- M. Juaristi, J. M. Aizpurua, B. Lecea and C. Palomo, Can. J. Chem., 1984, 62, 2941–2944 CrossRef CAS.
- M. P. Cava and D. R. Napier, J. Am. Chem. Soc., 1957, 79, 1701–1705 CrossRef CAS.
- CCDC 1035605 (4), 1035606 (2a), 1035608 (2b), 1035607 (2c), 1035609 (2d) and 1035610 (8).
- H. Arslan, F. J. Uribe-Romo, B. J. Smith and W. R. Dichtel, Chem. Sci., 2013, 4, 3973–3978 RSC.
- D. Lorbach, M. Wagner, M. Baumgarten and K. Müllen, Chem. Commun., 2013, 49, 10578–10580 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1035605–1035610. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5cc00693g |
| This journal is © The Royal Society of Chemistry 2015 |