Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Homoleptic low-valent polyazides of group 14 elements†
Benjamin
Peerless
,
Theo
Keane
,
Anthony J. H. M.
Meijer
 and
Peter
Portius
*
and
Peter
Portius
*
Department of Chemistry, University of Sheffield, Brook Hill, Sheffield, S3 7HF, UK. E-mail: p.portius@sheffield.ac.uk
First published on 23rd February 2015
Abstract
First examples of coordinatively unsaturated, homoleptic azido complexes of low-valent group 14 elements are reported. A simple strategy uses low-valent precursors, ionic azide transfer reagents and bulky cations to obtain salt-like compounds containing E(N3)3− of Ge(II)/Sn(II) which are fully characterised, including XRD. Remarkably, these compounds are kinetically stable at r.t. and isolable in sub-gram quantities.
Binary azides are known for all elements in group 14 and exist as covalent E(N3)4 compounds (E = C, Si),2 as hyper-coordinate E(N3)62− complexes (Si–Pb)1 and as E(N3)2 compounds (Sn, Pb).5c However, no low-valent, homoleptic group 14 complex has yet been reported. All known binary p-block azides are highly endothermic primary explosives most of which possess exceedingly high electrostatic and friction sensitivities and a propensity to release N2. As covalent, N-rich compounds, their isolation is generally challenging and experimental characterisation is limited.5 In contrast, stability-inducing effects of hyper-coordination and of bulky, weakly coordinating counter ions (WCC)6 allow many salt-like homoleptic polyazides to be synthesised in bulk and characterised fully, including via X-ray crystallography and IR spectroscopy,5a owing to azide groups (N3) giving rise to intense bands in the mid-IR region. It has been shown that azide anions (N3−) are able to coordinate to low-valent centres in compounds such as E(L)(N3) and E(L′)(N3)2, E = Ge, Sn.3,4 On the other hand, the stability of low valent molecules, e.g. carbenes, silylenes, germylenes, stannylenes,7 increases by saturating the electron deficient centre with sterically demanding, electron donating groups, such as N-based C(NiPr)2(NiPr2) and HC{(CMe)(2,6-iPr2C6H3N)}2 ligands.7a,d This insight has led to tri- and tetracoordinate complexes bearing uni-, bi- and terdentate ligands, e.g. E(NHC)X2, Ge(NHC)2Cl+ and Ge{HB(Me2pz)3}Cl, E = Si, Ge; X = Cl-I, N3; NHC = N-heterocyclic carbene.4a,8 Exploitation of these concepts has permitted the synthesis and characterisation of the first low-valent and homoleptic Ge and Sn azides described in this paper.
Compounds already containing WCC ions and low-valent germanium, AsPh4GeCl3, PPh4GeCl3,9 PPNGeCl3 (ref. 10) (1a–c), were prepared in high yield from the GeCl2(diox) adduct18 and WCC chlorides19 (Scheme 1, route A).‡11 These colourless, moderately air sensitive crystalline trichlorogermanates react readily with THF suspensions of NaN3. In situ IR spectra of the reaction (2b) show bands due to asymmetric NNN stretches, νas(N3), typical for coordinated N3 groups at ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) max/(cm−1) = 2092 and 2058, which have grown fully after a reaction time of 1 h. Exposure of the reaction solution to fresh NaN3 results in no further spectral change. From the solution, a highly air sensitive, colourless solid (3b) was precipitated, the IR spectrum of which exhibits the finger print of PPh4+ and the νas(N3) bands. The νas(N3) frequencies lie within the range of those reported previously for semi-covalent germanium(II) azides (2027–2077 cm−1, Fig. 1), below those of Ge(IV) azides (Ge(N3)4, PPN2Ge(N3)6, 5c)1b and above that of the N3− ion. While solution 1H, 13C and 31P NMR spectra of 3b show signals of the WCC cations only, two peaks are observed in the 14N NMR spectra at −263 and −207 ppm next to the solvent (−136 ppm) with FWHM line widths of 552, 147 and 24 Hz, respectively. These characteristics are typical for the Nα and Nγ nuclei of coordinated N3 groups while the signal for Nβ is obscured by solvent.5a
max/(cm−1) = 2092 and 2058, which have grown fully after a reaction time of 1 h. Exposure of the reaction solution to fresh NaN3 results in no further spectral change. From the solution, a highly air sensitive, colourless solid (3b) was precipitated, the IR spectrum of which exhibits the finger print of PPh4+ and the νas(N3) bands. The νas(N3) frequencies lie within the range of those reported previously for semi-covalent germanium(II) azides (2027–2077 cm−1, Fig. 1), below those of Ge(IV) azides (Ge(N3)4, PPN2Ge(N3)6, 5c)1b and above that of the N3− ion. While solution 1H, 13C and 31P NMR spectra of 3b show signals of the WCC cations only, two peaks are observed in the 14N NMR spectra at −263 and −207 ppm next to the solvent (−136 ppm) with FWHM line widths of 552, 147 and 24 Hz, respectively. These characteristics are typical for the Nα and Nγ nuclei of coordinated N3 groups while the signal for Nβ is obscured by solvent.5a
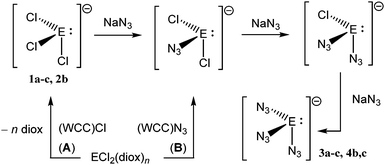 | ||
| Scheme 1 Synthesis of azido(chloro) germanates (1−) and stannates (1−), E = Ge, n = 1 (1, 3); E = Sn, n = 0 (2, 4); WCC = AsPh4 (a), PPh4 (b), N(PPh3)2 (PPN, c). | ||
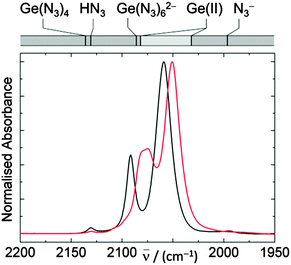 | ||
| Fig. 1 IR spectra of Ge(N3)3− (black), Sn(N3)3− (red) in THF; νas(N3) frequency ranges of related azides, Ge(N3)4,12 HN3,10 Ge(N3)62−,1b LnGe(II) azides L1 = {Me2(tBuO)Si}2N;4c L2 = nPr2ATI, Mes2DAP,4f,g (NHC)2;3 L3 = HB(R2pz)3, (C5R5)Co{P(O)(OEt)2}3,4a,e,10 (2,6-iPr2C6H3)2C2H2N2CGe(N3)2 (ref. 8d) and N3−,4e are indicated in the top bar; see Table S1 (ESI†) for exact values. | ||
Alternative routes to 3 and 4 use WCC azides as azide transfer reagents. GeCl2(diox) reacts directly also with (PPN)N3 in MeCN solution (Scheme 1, route B). Intriguingly, equimolar reactant mixtures produce only one νas(N3) band (2078 cm−1); increasing the stoichiometric ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) results in two additional bands (2088, 2066 cm−1), while at ratios of 1
2) results in two additional bands (2088, 2066 cm−1), while at ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 and above only bands at 2095, 2064 cm−1 and that of N3− were detected. These observations are interpreted tentatively in terms of the formation of mono, di- and triazido complexes.
9 and above only bands at 2095, 2064 cm−1 and that of N3− were detected. These observations are interpreted tentatively in terms of the formation of mono, di- and triazido complexes.
Tin dichloride was subjected to a similar treatment as GeCl2(diox) using WCC(N3) and NaN3; however, complete Cl/N3 exchange requires a larger excess of azide transfer reagent. Similar observations as with 3b were made, including the intermediate rise and decay of a νas(N3) band (2064 cm−1) and the ultimate rise of bands of the final product 4b (2081, 2050 cm−1) in the expected region between Sn(N3)62− and charge-neutral Sn(II) monoazides (Table S1, ESI†), and 14N resonances at −218.5 ppm (FWHM ≈ 32 Hz) and −260.0 ppm (166 Hz). The 14N NMR signals of 3b and 4b, in particular those assigned to Nα, are deshielded in comparison to those of E(N3)62− dianions.1b,5a,173b and 4b are soluble in MeCN, THF and CH2Cl2.
The synthetic strategy was extended to AsPh4+ and PPN+ counter ions affording compounds 3a,c and 4c (Scheme 1A) which all have spectroscopic properties analogous to those of 3b and 4b described already. The combined analytical evidence, including the absence of chlorine in 3b and the 119Sn NMR signal of 4b (δ = −220 ppm, see ESI†) point to the formation of anionic complexes in compounds of the type (WCC)E(N3)3 as the final products of Cl/N3 exchange.
Further insight into the nature of intermediates and products of the exchange reactions was obtained from quantum chemical calculations20 on the ECl(3−n)(N3)n− species, which were performed at the B3LYP/cc-pVTZ level21 with effective core potentials22 for Ge and Sn. Solvent (THF) was described using PCM.23 The calculations found conformational isomerism resulting in several minima for n = 1, 2, 3, that were close in energy. These conformers are related by rotation of ligands. Since rotational barriers of sterically unhindered N3 groups are small (cf. GeH3N3, ∼1 kJ mol−1),13 fast interconversion involves all significantly thermally populated rotamers above the minimum energy conformation (Erel < 5.8 kJ mol−1). This process is likely to result in averaged absorption bands weighted by the rotamer population (rotamers may have more than one degenerate, absolute spatial configuration, and inter-rotamer vibrational energy transfer is unaccounted for). Taking account of the theoretical equilibrium mole fractions, absorption intensities and scaled vibrational frequencies,24 approximate average frequencies of the in-phase and out-of-phase νas(N3) stretches and the qualitative intensity ratios could be determined (Table S1, ESI†), which match those observed (e.g. Ge(N3)3−, 2059, 2091 vs. 2060, 2093; Sn(N3)3−, 2051, 2080 vs. 2050, 2078 cm−1). This approach leads to the assignment of the observed bands of intermediates to GeCl2(N3)−, GeCl(N3)2−, SnCl2(N3)− and SnCl(N3)2−. Calculations using the Gauge-Independent Atomic Orbital method25 verify the assignment of 14N NMR data (see ESI†).
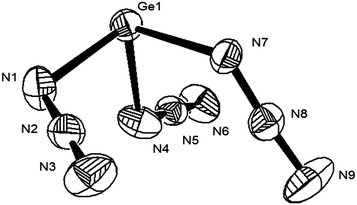
Crystals of azido germanates were grown from THF–Et2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) solutions at −18 °C (3b, needles) or by diffusion of Et2O into concentrated THF solutions (3c).§¶ According to single crystal X-ray diffraction studies, 3b consists of PPh4+ and Ge(N3)3− ions (Fig. 2). The shortest interionic Ge⋯N and N⋯N distances were found to be 4.13 and 5.07 Å, respectively (Fig. 4, left), hence, covalent {[Ge(N3)3]−}⋯{[Ge(N3)3]−} interactions are absent (Fig. 3). Germanium is coordinated by three, essentially linear N3 ligands and occupies the apical position in a trigonal-pyramidal Ge[N]3 framework. The ligands are bound in the fashion typical of covalent azides and adopt Ge–Nα–Nβ angles between 116° and 121°. All inter-ligand angles are close to 90° which indicates stereochemical inactivity of the lone electron pair at germanium.14
10) solutions at −18 °C (3b, needles) or by diffusion of Et2O into concentrated THF solutions (3c).§¶ According to single crystal X-ray diffraction studies, 3b consists of PPh4+ and Ge(N3)3− ions (Fig. 2). The shortest interionic Ge⋯N and N⋯N distances were found to be 4.13 and 5.07 Å, respectively (Fig. 4, left), hence, covalent {[Ge(N3)3]−}⋯{[Ge(N3)3]−} interactions are absent (Fig. 3). Germanium is coordinated by three, essentially linear N3 ligands and occupies the apical position in a trigonal-pyramidal Ge[N]3 framework. The ligands are bound in the fashion typical of covalent azides and adopt Ge–Nα–Nβ angles between 116° and 121°. All inter-ligand angles are close to 90° which indicates stereochemical inactivity of the lone electron pair at germanium.14
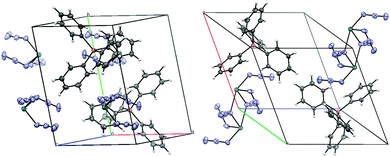 | ||
| Fig. 4 Packing diagrams of PPh4Ge(N3)3 (3b, left) and PPh4Sn(N3)3 (4b, right); H (bright grey), C (dark grey), N (light blue), P (orange), Ge and Sn (turquoise). | ||
This structural feature has been found in the valence isoelectronic complexes of Ge2(μ-pz*)3+ GeCl3− (ref. 11f) and pilocarpine-trichlorogermanate hemihydrate.11g The Ge–Nα bonds of 3b are shorter than those of tetracoordinate Ge(II) azides 2.088(6)–2.094(7) Å (Fig. 1), longer than those of the homoleptic Ge(IV) azide PPN2Ge(N3)6 (5c, Table S1, ESI†) and rather within the range of previously investigated, tricoordinate Ge(II) azides (1.969–2.047(2) Å, Table 1). All other bond lengths and angles are close to those of 5c (Table 1). The crystallographic structure of Ge(N3)3− is consistent with one of the geometries predicted by DFT (vide supra).
| E, n | E–Nα | Nα–Nβ | Nβ–Nγ | ΔNNav![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
|
|---|---|---|---|---|---|
a
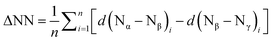 , , 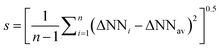 in parentheses.
b
s ≪ σ, error estimated by in parentheses.
b
s ≪ σ, error estimated by 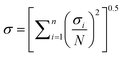 .
c This work.
d
Ref. 1b.
e
Ref. 15. .
c This work.
d
Ref. 1b.
e
Ref. 15.
|
|||||
| Ge, 1 | 1.984(3)–2.011(2) | 1.206(4)–1.213(4) | 1.140(4)–1.148(4) | 6.6(5)b | |
| Ge, 2 | 1.969(2)–1.980(3) | 1.210(4)–1.214(3) | 1.143(3)–1.151(3) | 6.5(4) | |
| Sn, 1 | 2.193(3)–2.262(3) | 1.189(6)–1.203(4) | 1.143(5)–1.163(6) | 4.6(17) | |
| Sn, 2 | 2.117(3)–2.134(2) | 1.182(3)–1.213(3) | 1.111(4)–1.148(3) | 5.7(10) | |
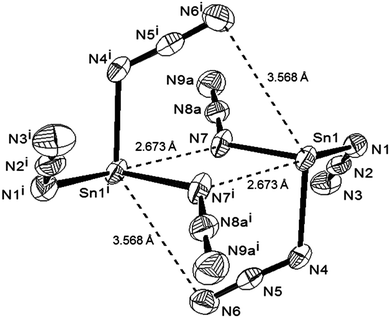
Single crystalline needles of 4b were obtained and investigated with the methods used for 3b.¶ The asymmetric unit of 4b also contains a E(N3)3− moiety; however, the packing is at variance with 3b, which allows Sn(N3)3− to interact via two long E⋯Nα bonds and form {Sn(N3)3}22− dimers (Fig. 3). The interaction involves asymmetric μ1,1-N3 bridges with short (2.207(3) Å) and long (2.674(3) Å) Sn–Nα bonds, the latter being considerably shorter than the sum of the van der Waals radii (3.72 Å).16 Weak intermolecular interactions have been observed previously between neutral Sn(nPr2ATI)N3 complexes (vide supra),4b where a slightly longer Sn⋯Nα bond (2.87 Å) was found. The sum of bond angles involving the bridging Nα indicates planarity and effective sp2 hybridisation. As in the crystal of 3b, the primary E(II)–Nα bonds are significantly longer (2.193(3)–2.262(3) Å) than those found in the homoleptic E(IV) azide 6 (2.125 Å).15 The potential for dimerisation was studied by DFT using the geometry of {E(N3)2(μ1,1-N3)}22− in crystalline 4b as a starting point. Optimisation results in separate anions devoid of covalent interionic interactions in the case of Ge(N3)3−, whereas a dimeric structure was found for Sn(N3)3− that resembles the molecular structure in the crystal. Estimates of the basis set superposition error for the solution phase were obtained from BSSE calculations26 in the gas phase. After BSSE correction, {Sn(N3)2(μ1,1-N3)}22− was found to be at least 4 kcal mol−1 less stable than two monomers, rendering the existence of a dimer in solution highly unlikely.
According to differential scanning calorimetry measurements (Fig. S11 and S12, ESI†), compound 3b decomposes in two exothermic processes with (ΔH = −270 and −467 J g−1).|| Remarkably, step 1 occurs at temperatures (Tex1on = 99 °C) that are drastically below the decomposition onset of the related, hypercoordinate azide 5c (Tex1on = 256 °C),1b whereas step 2 sets in at Tex2p = 310 °C, which is nearly identical with the temperature found in 5c (312 °C).** Furthermore, the molar enthalpies of step 2 (−251 vs. −482 kJ mol−1) scale approximately with the complex charge; however, step 1 releases much less energy than expected (145 kJ mol−1, 3bvs. 705 kJ mol−1, 5c). This phenomenon is still under investigation. Further experiments show that heating of 3b at 150 °C produces PPh4N3, which suggests that the release of N3− initiates the decomposition of Ge(N3)3−. The tin homologue 4b melts at Ton = 115 °C and decays at 215 °C and 308 °C and thus behaves as expected relative to 6c.15 No sensitivity was noted during preparation and analysis of compounds 3b and 4b on the stated reaction scale. The material remains unchanged when struck by a hammer. Upon lighting up, it burns rapidly with an orange flame leaving black residues.
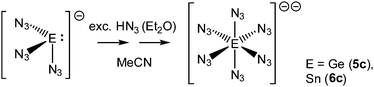
In solution, Ge(N3)3− and Sn(N3)3− react with hydrazoic acid leading to a precipitate with the IR νas(N3) absorptions characteristic for Ge(N3)62− and Sn(N3)62− complexes, respectively, which can be verified by comparison with spectra of the fully characterized salts 5c, 6c (Scheme 2, Fig. S13 and S14, ESI†).
The first low-valent homoleptic azido complexes of group 14 have been synthesized and fully characterised. The preparative approach to salt-like compounds containing these complexes has been demonstrated on a 0.2–0.7 g scale for a range of weakly coordinating cations and involves chloro(azido) species, EClx(N3)y−. Unlike their hypercoordinate E(N3)62− analogues and despite the presence of innocent cations, the new class of compounds is highly reactive, exhibiting low thermal stability and a propensity to oxidation. Crystallographic analysis revealed that in the solid state, E(N3)3− complexes of group 14 may dimerise via azido ligand bridges. Neither of the low-valent coordination centres exhibits a stereochemically active lone electron pair. DFT calculations correctly predict the dimerisation and suggest furthermore that the dimers are unstable in polar solvents.
The authors thank the EPSRC (EP/E054978/1), the University of Sheffield and Humboldt-Universität zu Berlin for support and Prof. A. C. Filippou for advice.
Notes and references
- (a) A. C. Filippou, P. Portius and G. Schnakenburg, J. Am. Chem. Soc., 2002, 124, 12396 CrossRef CAS PubMed; (b) A. C. Filippou, P. Portius, D. U. Neumann and K.-D. Wehrstedt, Angew. Chem., Int. Ed., 2000, 39, 4333 CrossRef CAS; (c) D. Fenske, H. D. Dörner and K. Dehnicke, Z. Naturforsch., B: J. Chem. Sci., 1983, 38, 1301 Search PubMed; (d) K. Polborn, E. Leidl and W. Beck, Z. Naturforsch., B: J. Chem. Sci., 1988, 43, 1206 CAS.
- (a) K. Banert, Y.-H. Joo, T. Rüffer, B. Walfort and H. Lang, Angew. Chem., Int. Ed., 2007, 46, 1168 CrossRef CAS PubMed; (b) P. Portius, A. C. Filippou, G. Schnakenburg, M. Davis and K.-D. Wehrstedt, Angew. Chem., Int. Ed., 2010, 49, 8013 CrossRef CAS PubMed.
- Y. Xiong, S. Yao and M. Driess, Chem. Commun., 2014, 50, 418 RSC.
- (a) A. C. Filippou, P. Portius and G. Kociok-Köhn, Chem. Commun., 1998, 2327 RSC; (b) H. V. R. Dias and A. E. Ayers, Polyhedron, 2002, 21, 611 CrossRef CAS; (c) M. Veith and A. Rammo, Z. Anorg. Allg. Chem., 2001, 627, 662 CrossRef CAS; (d) V. N. Khrustalev, I. A. Portnyagin, N. N. Zemlyansky, I. V. Borisova, Y. A. Ustynyuk and M. Y. Antipin, J. Organomet. Chem., 2005, 690, 1056 CrossRef CAS PubMed; (e) A. C. Filippou, P. Portius, G. Kociok-Köhn and V. Albrecht, Dalton Trans., 2000, 1759 RSC; (f) A. E. Ayers, D. S. Marynick and H. V. R. Dias, Inorg. Chem., 2000, 39, 4147 CrossRef CAS; (g) A. E. Ayers, T. M. Klapötke and H. V. R. Dias, Inorg. Chem., 2001, 40, 1000 CrossRef CAS.
- (a) P. Portius and M. Davis, Coord. Chem. Rev., 2013, 257, 1011 CrossRef CAS PubMed; (b) W. P. Fehlhammer and W. Beck, Z. Anorg. Allg. Chem., 2013, 639, 1053 CrossRef CAS , ref. cited; (c) T. Müller, F. Karau, W. Schnick and F. Kraus, Angew. Chem., Int. Ed., 2014, 53, 13695 CrossRef PubMed.
- (a) C. J. Price, H.-Y. Chen, L. M. Launer and S. A. Miller, Angew. Chem., Int. Ed., 2009, 48, 956 CrossRef CAS PubMed; (b) I. Krossing and A. Reisinger, Coord. Chem. Rev., 2006, 250, 2721 CrossRef CAS PubMed.
- (a) M. Asay, C. Jones and M. Driess, Chem. Rev., 2011, 111, 354 CrossRef CAS PubMed; (b) S. Nagendran and H. W. Roesky, Organometallics, 2008, 27, 457 CrossRef CAS; (c) Y. Mizuhata, T. Sasamori and N. Tokitoh, Chem. Rev., 2009, 109, 3479 CrossRef CAS PubMed; (d) B. Blom, M. Stoelzel and M. Driess, Chem. – Eur. J., 2013, 19, 40 CrossRef CAS PubMed; (e) M. Mück, K. Junold, J. A. Baus, C. Burschka and R. Tacke, Eur. J. Inorg. Chem., 2013, 5821 CrossRef.
- (a) R. S. Ghadwal, H. W. Roesky, S. Merkel, J. Henn and D. Stalke, Angew. Chem., Int. Ed., 2009, 48, 5683 CrossRef CAS PubMed; (b) A. C. Filippou, O. Chernov and G. Schnakenburg, Angew. Chem., Int. Ed., 2009, 48, 5687 CrossRef CAS PubMed; (c) Y. Xiong, S. Yao and M. Driess, Chem. Commun., 2014, 50, 418 RSC; (d) B. Lyhs, D. Bläser, C. Wölper, S. Schulz, R. Haack and G. Jansen, Inorg. Chem., 2013, 52, 7236 CrossRef CAS PubMed.
- U. M. Tripathi, G. L. Wegner, A. Schier, A. Jockisch and H. Schmidbaur, Z. Naturforsch., B: J. Chem. Sci., 1998, 53, 939 CAS.
- P. Portius, PhD thesis, Humboldt-Universität zu Berlin Weißensee Verlag, Berlin, 2002, ISBN 3-934479-63-4.
- (a) X. Tian, T. Pape and N. W. Mitzel, Z. Naturforsch., B: J. Chem. Sci., 2004, 59, 1524 CAS; (b) S. Nogai, A. Schriewer and H. Schmidbaur, Dalton Trans., 2003, 3165 RSC; (c) G. Kociok-Köhn, J. G. Winter and A. C. Filippou, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1999, 55, 351 Search PubMed; (d) G. L. Wegner, A. Jockisch and H. Schmidbaur, Z. Naturforsch., B: J. Chem. Sci., 1998, 53, 430 CAS; (e) M. Karnop, W. W. du Mont, P. G. Jones and J. Jeske, Chem. Ber., 1997, 130, 1611 CrossRef CAS; (f) A. Steiner and D. Stalke, Inorg. Chem., 1995, 34, 4846 CrossRef CAS; (g) S. Fregerslev and S. E. Rasmussen, Acta Chem. Scand., 1968, 22, 2541 CrossRef CAS PubMed.
- J. E. Drake and R. T. Hemmings, Can. J. Chem., 1973, 51, 302 CrossRef CAS.
- D. T. Durig, M. S. Durig and J. R. Durig, Spectrochim. Acta, Part A, 2005, 61, 1287 CrossRef PubMed.
- (a) D.-K. Seo, N. Gupta, M.-H. Whangbo, H. Hillebrecht and G. Thiele, Inorg. Chem., 1998, 37, 407 CrossRef CAS PubMed; (b) U. Schwarz, H. Hillebrecht, M. Kaupp, K. Syassen and H.-G. v. Schnering, J. Solid State Chem., 1995, 118, 20 CrossRef CAS.
- R. Campbell, B. Peerless and P. Portius, unpublished results.
- A. Bondi, J. Phys. Chem., 1964, 68, 441 CrossRef CAS.
- (a) W. Beck, W. Becker, K. F. Chew, W. Derbyshire, N. Logan, D. M. Revitt and D. B. Sowerby, Dalton Trans., 1972, 245 RSC; (b) W. Beck, W. P. Fehlhammer, P. Pöllmann, E. Schuierer and K. Feldl, Chem. Ber., 1967, 100, 2335 CrossRef CAS.
- (a) J. Kouvetakis, A. Haaland, D. J. Shorokhov, H. V. Volden, G. V. Girichev, V. I. Sokolov and P. Matsunaga, J. Am. Chem. Soc., 1998, 120, 6738 CrossRef CAS; (b) S. P. Kolesnikov, I. S. Rogozhin and O. M. Nefedov, Izv. Akad. Nauk SSSR, Ser. Khim., 1974, 23, 2379 Search PubMed.
- (a) V. Y. Kukushkin and A. I. Moiseev, Inorg. Chim. Acta, 1990, 176, 79 CrossRef CAS; (b) A. Martinsen and J. Songstad, Acta Chem. Scand., Ser. A, 1977, 31, 645 CrossRef CAS PubMed.
- M. J. Frisch, et al., Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford, CT, 2013 Search PubMed.
- (a) A. D. Becke, J. Chem. Phys., 1993, 98, 5648 CrossRef CAS PubMed; (b) C. Lee, W. Yang and R. Parr, Phys. Rev. B, 1988, 37, 785 CrossRef CAS; (c) T. H. Dunning Jr., J. Chem. Phys., 1989, 90, 1007 Search PubMed.
- K. A. Peterson, J. Chem. Phys., 2003, 119, 11099 CrossRef CAS PubMed.
- (a) B. Mennucci and J. Tomasi, J. Chem. Phys., 1997, 106, 5151 CrossRef CAS PubMed; (b) M. Cossi, V. Barone, B. Mennucci and J. Tomasi, Chem. Phys. Lett., 1998, 286, 253 CrossRef CAS and references therein.
- K. K. Irikura, R. D. Johnson and R. N. Kacker, J. Phys. Chem. A, 2005, 109, 8430 CrossRef CAS PubMed.
- R. Ditchfield, Mol. Phys., 1974, 27, 789 CrossRef CAS.
- S. F. Boys and F. Bernardi, Mol. Phys., 1970, 19, 553 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Spectra, thermograms, full crystallographic data and computational details. CCDC 1030031 and 1030032. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5cc00259a |
| ‡ GeCl3− (ref. 9 and 11) and SnCl3− salts (ref. 11c and e–g) with various organic counter ions have been reported previously. |
| § All attempts to crystallize compound 3a have been futile. |
¶ Crystallographic data: 3b, CCDC 1030032, C24H20GeN9P, 538.07 g mol−1, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 7.7712(2) Å, b = 11.4711(4) Å, c = 14.2003(4) Å, α = 93.278 (2)°, β = 99.357(2)°, γ = 100.865(2)°, Z = 2, V = 1221.59(6) Å3, Dc = 1.463 g cm−3, T = 120(2) K, F(000) = 548, R1 = 0.0376 (316 param.), wR2 = 0.0807, GOOF = 1.090. 3c, P21, a = 10.7640(11) Å, b = 12.732(2) Å, c = 25.713(3) Å, α = γ = 90°, β = 100.682(12)°, Dc = 1.414 g cm−3, T = 180(2) K, extensive disorder of Ge(N3)3− part (see ESI† and ref. 10). 4b, CCDC 1030031, C24H20N9PSn, M = 584.15 g mol−1, P , a = 7.7712(2) Å, b = 11.4711(4) Å, c = 14.2003(4) Å, α = 93.278 (2)°, β = 99.357(2)°, γ = 100.865(2)°, Z = 2, V = 1221.59(6) Å3, Dc = 1.463 g cm−3, T = 120(2) K, F(000) = 548, R1 = 0.0376 (316 param.), wR2 = 0.0807, GOOF = 1.090. 3c, P21, a = 10.7640(11) Å, b = 12.732(2) Å, c = 25.713(3) Å, α = γ = 90°, β = 100.682(12)°, Dc = 1.414 g cm−3, T = 180(2) K, extensive disorder of Ge(N3)3− part (see ESI† and ref. 10). 4b, CCDC 1030031, C24H20N9PSn, M = 584.15 g mol−1, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 10.7560(6) Å, b = 11.0605(6) Å, c = 12.3540(7) Å, α = 91.668(4)°, β = 108.414(4)°, γ = 116.545(3)°, Z = 2, V = 1222.11(12) Å3, Dc = 1.587 g cm−3, T = 100(2) K, F(000) = 584, R1 = 0.0521 (334 param.), wR2 = 0.1020, GOOF = 1.053. , a = 10.7560(6) Å, b = 11.0605(6) Å, c = 12.3540(7) Å, α = 91.668(4)°, β = 108.414(4)°, γ = 116.545(3)°, Z = 2, V = 1222.11(12) Å3, Dc = 1.587 g cm−3, T = 100(2) K, F(000) = 584, R1 = 0.0521 (334 param.), wR2 = 0.1020, GOOF = 1.053. |
| || Estimated error approx. ±10%. |
| ** This step is assigned tentatively to the thermolysis of PPN(N3) and PPh4 (N3) since enthalpies and onset temperatures are comparable with those of genuine samples of these salts: PPh4N3, mp = 250 °C, Texon = 291 °C, ref. 15. |
| This journal is © The Royal Society of Chemistry 2015 |