 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Photocatalytic benzylic C–H bond oxidation with a flavin scandium complex†
Bernd
Mühldorf
and
Robert
Wolf
*
University of Regensburg, Institute of Inorganic Chemistry, D-93040 Regensburg, Germany. E-mail: robert.wolf@ur.de
First published on 5th February 2015
Abstract
The enhanced reduction potential of riboflavin tetraacetate coordinating to scandium triflate enables the challenging photocatalytic C–H oxidation of electron-deficient alkylbenzenes and benzyl alcohols.
The important role of flavins as photoreceptors and redox cofactors in nature has inspired the use of synthetic flavin analogues as bioinspired photocatalysts.1 The most prominent example, riboflavin tetraacetate (RFT), catalyses the aerobic photooxidation of benzyl alcohols,2 benzyl amines,3 and sulfoxides (Scheme 1).4,5 A particularly intriguing application of RFT is the photocatalytic C–H bond oxidation of alkyl benzenes to the corresponding aldehydes.6,7 Spectroscopic studies revealed an initial electron transfer from the aromatic substrate to the singlet excited state 1RFT* as the basis of this process.8 However, the limited reduction potential E0(1RFT*/2RFT−) = 1.67 V vs. SCE exclusively allows the oxidation of very few selected substrates which feature strongly electron-donating arene substituents. Most other substrates are unsuccessful, because their oxidation potential is too positive.
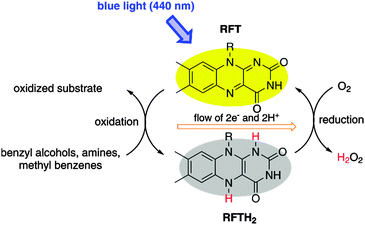 | ||
| Scheme 1 Photocatalytic cycle for the aerobic oxidation of various organic substrates with riboflavin tetraacetate (RFT) and blue light.5 | ||
Fukuzumi et al. found that the redox potential of RFT can be modified by metal ion coordination.9 As shown in Fig. 1, complexes of RFT with Mg2+, Zn2+, Yb3+ and Sc3+ ions have a significantly more positive reduction potential E0(1RFT*/2RFT−) in the excited singlet state. In particular, the Sc3+ system‡ appears promising as it features high fluorescence quenching rate constants of (1RFT–2Sc3+)* in the presence of alkyl- and methoxy-substituted benzenes.8 This indicates an efficient single electron transfer from the substrate to (1RFT–2Sc3+)*, which is a prerequisite for photocatalytic activity.
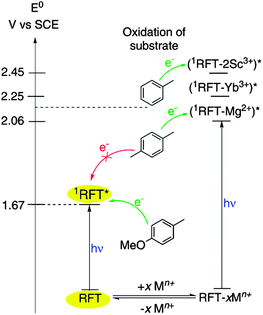 | ||
| Fig. 1 Enhanced reduction potentials E0(1RFT*/2RFT−) of RFT–metal ion complexes (RFT−xMn+).8,9 | ||
Motivated by these insights, we sought to explore the catalytic properties of RFT/metal salt combinations for challenging benzylic C–H bond oxidations. The reaction of ethylbenzene (1) to acetophenone (2) was chosen as a benchmark (Scheme 2), because 1 shows a high oxidation peak potential (E0p(1+˙/1) = 2.14 vs. SCE) and therefore cannot be oxidised by 1RFT* alone.8 A screening of various Lewis acids (Table S1, ESI†) and solvents (Table S2†) indicated Sc(OTf)3 in acetonitrile to be the best choice. Irradiation of 1 (0.02 mmol) in CH3CN for 2.5 h with blue light (440 nm) in the presence of RFT (10 mol%) and Sc(OTf)3 (20 mol%) afforded acetophenone 2 in 58% yield. Substrate 1 was completely consumed, and the formation of H2O2 was confirmed by UV-vis spectroscopy (Fig. S1†). Note that 2 is formed in <10% yield in the absence of Sc3+-ions, while Mg(OTf)2 and Zn(OTf)2 gave only very low yields of 2. The reaction is significantly accelerated by higher Sc3+ concentrations (Fig. S2†). In order to reduce the amount of Sc(OTf)3 required, the effect of acids and other additives was investigated (Table S3†). Importantly, 1 is converted nearly four times as fast in the presence of HCl (30 mol%) with the same Sc(OTf)3 concentration (Fig. S3†).
Using this optimized system, we subsequently assessed the substrate scope (Table 1). Toluene is converted to benzaldehyde in 71% yield, while p-tert-butylbenzaldehyde and p-chlorobenzaldehyde are obtained in 68% and 84% yield, respectively (entry 1). Benzylethers do not give the corresponding esters, but benzaldehydes (entry 3). Diarylmethylene derivatives (entry 4) and benzyl alcohols (entries 5 and 6) are oxidised with good to excellent yields as well. Triphenylmethane and diphenylacetic acid both yield benzophenone via oxidative C–C cleavage.10 Note that the oxidations of p-trifluorobenzyl alcohol and p-nitrobenzyl alcohol proceed selectively, but the reaction speed is slow, resulting in an incomplete conversion.
| Entry | Substrate | Product | No Sc3+ yieldb [%] | R | Conv.b [%] | Yieldb [%] |
|---|---|---|---|---|---|---|
| a All reactions were performed with substrate (0.02 mmol), RFT (10 mol%), HCl (37%, 0.8 μL) and Sc(OTf)3 (4.6 mM) in 1 mL MeCN and irradiated with blue light (440 nm, 3 W) for 2.5 h when not indicated otherwise (see footnote d). b Conversion and yield determined by GC-FID integration. c No HCl added; n.d. = not determined. d Irradiation time: 0.5 h (R = Me), 1 h (R = Cl) and 7 h (R = NO2). | ||||||
| 1 |
|

|
0 | H | 96 | 71 |
| 5 | t Bu | 100 | 68 | |||
| 8 | Med | 100 | 62 | |||
| 0 | Cld | 100 | 84 | |||
| 0 | CN | 56 | 29 | |||
| 0 | CO2Me | 44 | 15 | |||
| 2 |
|

|
3 | Me | 100 | 60 |
| 0 | CO2Me | 92 | 49 | |||
| 3c |

|

|
0 | H | 93 | 90 |
| 5 | OMe | 100 | 63 | |||
| 4 |

|

|
4 | H | 100 | 93 |
| 6 | Ph | 89 | 52 | |||
| 23 | COOH | n.d. | 80 | |||
| 5 |

|

|
17 | H | 100 | 95 |
| 0 | Me | 100 | 81 | |||
| 6c |

|
|
7 | F | 100 | 88 |
| 12 | Cl | 100 | 73 | |||
| 14 | Br | 100 | 84 | |||
| 0 | CF3 | 63 | 53 | |||
| 0 | NO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d d |
66 | 44 | |||
Control experiments confirmed that the reaction does not proceed in the dark, in the absence of RFT or under anaerobic conditions (Table S4,† entries 1–5). When the reaction was carried out in an atmosphere of pure dioxygen, slower bleaching of RFT was observed (Fig. S4†), but the yield of 2 did not improve (Table S4, entry 6). Moreover, a very similar yield (44%) was obtained in deuterated acetonitrile, therefore, a singlet oxygen pathway seems unlikely (Table S4,† entries 7 and 8).11,12
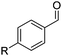
The reaction mechanism was probed by UV-vis spectroscopy. Before starting to irradiate a mixture of 1, RFT, Sc(OTf)3 and HCl in acetonitrile with blue light (440 nm), an absorption band can be identified at λmax = 390 nm both under aerobic conditions (Fig. S5†) and under argon (Fig. 2). This band may be assigned to RFTH+–2Sc3+ by comparison with the characteristic spectrum of uncoordinated RFTH+.13 The IR spectrum of the mixture shows that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching bands are shifted to lower frequency compared to those of RFTH+ in the absence of metal ions (Table S5†). This indicates that the scandium(III) ions coordinate to the carbonyl groups in RFTH+–2Sc3+.‡8
O stretching bands are shifted to lower frequency compared to those of RFTH+ in the absence of metal ions (Table S5†). This indicates that the scandium(III) ions coordinate to the carbonyl groups in RFTH+–2Sc3+.‡8
 | ||
| Fig. 2 UV-vis absorption spectra of ethylbenzene (5.8 mM) and RFT (0.14 mM) in the presence of Sc(OTf)3 (0.68 mM) and HCl (2.7 mM) during irradiation with blue light in deaerated MeCN at 298 K under nitrogen (straight: 0 s, 60 s, 120 s, 180 s, 360 s; dashed: 2 h). Inset: ESR-spectrum of 2RFTH2˙+–2Sc3+ generated in the photocatalytic reaction RFT (3.0 mM) with ethylbenzene (20 mM), Sc(OTf)3 (10 mM) and 10 mM HClO4 in deaerated MeCN at 298 K. Parameters obtained by computer simulation: g = 2.0033, a(N5) = 6.7 G, a(N10) = 4.6 G, a(H5) = 10.6 G, a(3H8) = 2.9 G, a(N10–CH2) = 4.3 G; see the ESI† for the labelling scheme. | ||
A possible catalytic cycle is displayed in Scheme 3. In line with previous fluorescence quenching experiments by Fukuzumi et al., we propose that the electron transfer occurs between the substrate and the photoexcited flavin metal complex (1RFTH+–2Sc3+)* in its singlet state (step i).8 This electron transfer produces the ethylbenzene radical cation 4 and the protonated flavin radical complex 2RFTH˙–2Sc3+. It seems likely that the 2RFTH˙–2Sc3+ complex is then protonated to yield 2RFTH2˙+–2Sc3+, while the strongly acidic ethylbenzene radical cation 4 is deprotonated to the benzyl radical 5 (step ii).§142RFTH2˙+–2Sc3+ should give rise to broad absorptions at λmax = 400–550 nm similar to those of the uncoordinated dihydroflavin radical cation 2RFTH2˙+.15 Such a broad band is indeed observed in the UV-vis spectrum of the reaction mixture under argon (Fig. 2). In addition, the ESR spectrum of the reaction mixture of 1, RFT, Sc(OTf)3 and HClO4 obtained while irradiating at 440 nm exhibits a signal at g = 2.0033 (Fig. 2, inset), which is in line with the expected spectrum for RFTH2˙+–2Sc3+.8 The presence of the scandium(III) ions appears to have only a slight effect on the shape of the ESR spectrum. The hyperfine coupling constants obtained by computer simulation are similar to the values reported for free RFTH2˙+.15 The hyperfine coupling constants obtained by computer simulation are similar to those reported for free 2RFTH2˙+.16 The ESR spectrum of a mixture of 1, RFT, Sc(OTf)3 and HCl (instead of HClO4, Fig. S6†) is more complicated and thus defied a satisfactory simulation so far. This is presumably due to the formation of an equilibrium between RFTH2˙+–2Sc3+ and RFTH˙–2Sc3+ with the weaker acid HCl.
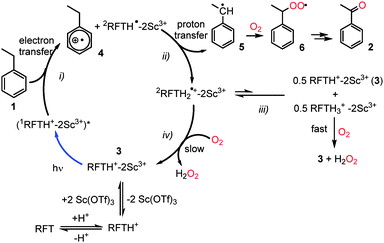 | ||
| Scheme 3 Proposed mechanism for the photocatalytic aerobic oxidation of ethylbenzene (1) to acetophenone (2) with RFT in presence of Sc3+-ions and HCl. | ||
There are at least two conceivable pathways that connect the benzyl radical 5 with the final product 2 (Scheme 3). One possibility is that 2RFTH2˙+–2Sc3+ recombines with 5 to form a covalent RFT-benzyl radical adduct (not shown in Scheme 3), which rapidly collapses under irradiation in air to product 2 and RFTH+–2Sc3+ (3).17 However, this pathway seems less likely based on the UV-vis spectra of the reaction mixture, where characteristic broad absorptions are expected for such an adduct at λmax = 600–630 nm. An alternative pathway is the conversion of 5 into the benzylperoxyl radical 6, which subsequently transforms into 2via the benzyl hydroperoxide.18 As observed for 2RFTH2˙+, 2RFTH2˙+–2Sc3+ may disproportionate into oxidized RFTH+–2Sc3+ and the reduced dihydroflavin RFTH3+–2Sc3+ (step iii).15 The formation of the latter species is supported by the observation of an absorption band at 295 nm that increases over time (see Fig. 2).15 RFTH3+–2Sc3+ can react with dioxygen, regenerating 3 while producing H2O2 (Fig. S8†).16 In addition, RFTH+–2Sc3+ (3) may also be regenerated by the direct reaction of 2RFTH2˙+–2Sc3+ with O2 (step iv, Fig. S7†). This process may conceivably be facilitated by Lewis acid coordination.19
We presume that the mechanism of the catalytic oxidation of benzyl alcohols (Table S1 (ESI†), entries 5 and 6) is analogous to the one previously suggested by Fukuzumi et al. for the oxidation p-chlorobenzyl alcohol.8 The proposed catalytic cycle involves an initial electron transfer from the substrate to (1RFT–2Sc3+)*, followed by proton transfer forming the hydroxybenzyl radical (p-R-C6H4CHOH˙) and the protonated RFT radical anion (2RFTH˙–2Sc3+)*. Subsequent H atom transfer between these species yields the aldehyde and RFTH2–2Sc3+.
In summary, RFT/scandium triflate is an efficient photocatalytic system for the aerobic oxidation of alkylbenzenes and electron deficient benzyl alcohols. The results show that the well-known effect of Lewis acid coordination on the redox potential of flavins8,9 can be exploited to improve their photocatalytic properties. An extension of this principle, and an exploration of the effects of other metal ions including redox-active ones, is hand.
We thank Dr Michael Spörner and Helmut Schüller for assistance with ESR measurements and Prof. Burkhard König for stimulating discussions. Support by the DFG Graduate Program “Chemical Photocatalysis” (GRK 1626) is gratefully acknowledged.
Note added after first publication: This article replaces the version published on 03 Feb 2015, which contained errors in the text introduced by the Editorial Office.
Notes and references
- (a) S. Ghisla and V. Massey, Eur. J. Biochem., 1989, 181, 1–17 CrossRef CAS PubMed; (b) Flavins: Photochemistry and Photobiology, ed. E. Silva and A. M. Edwards, Royal Society of Chemistry, Cambridge, 2006, vol. 6 Search PubMed.
- (a) H. Schmaderer, P. Hilgers, R. Lechner and B. König, Adv. Synth. Catal., 2009, 351, 163–174 CrossRef CAS; (b) J. Svoboda, H. Schmaderer and B. König, Chem. – Eur. J., 2008, 14, 1854–1865 CrossRef CAS PubMed; (c) R. Cibulka, R. Vasold and B. König, Chem. – Eur. J., 2004, 10, 6223–6231 CrossRef CAS PubMed; (d) Chemical Photocatalysis, ed. B. König, S. Kümmel, R. Cibulka and B. König, de Gruyter, Berlin, 2013, pp. 44–61 Search PubMed.
- R. Lechner and B. König, Synthesis, 2010, 1712–1718 CAS.
- J. Dad’ová, E. Svobodová, M. Sikorski, B. König and R. Cibulka, ChemCatChem, 2012, 4, 620–623 CrossRef.
- U. Megerle, M. Wenninger, R.-J. Kutta, R. Lechner, B. König, B. Dick and E. Riedle, Phys. Chem. Chem. Phys., 2011, 13, 8869 RSC.
- (a) J. Rosenthal, T. D. Luckett, J. M. Hodgkiss and D. G. Nocera, J. Am. Chem. Soc., 2006, 128, 6546–6547 CrossRef CAS PubMed; (b) K. Ohkubo and S. Fukuzumi, Org. Lett., 2000, 2, 3647–3650 CrossRef CAS PubMed; (c) K. Ohkubo, K. Suga, K. Morikawa and S. Fukuzumi, J. Am. Chem. Soc., 2003, 125, 12850–12859 CrossRef CAS PubMed.
- R. Lechner, S. Kümmel and B. König, Photochem. Photobiol. Sci., 2010, 9, 1367 CAS.
- S. Fukuzumi, K. Yasui, T. Suenobu, K. Ohkubo, M. Fujitsuka and O. Ito, J. Phys. Chem. A, 2001, 105, 10501–10510 CrossRef CAS.
- S. Fukuzumi, S. Kuroda and T. Tanaka, J. Am. Chem. Soc., 1985, 107, 3020 CrossRef CAS.
- R. Akaba, M. Kamata, H. Itoh, A. Nakao, S. Goto, K. Saito, A. Negishi, H. Sakuragi and K. Tokumaru, Tetrahedron Lett., 1992, 33, 7011–7014 CrossRef CAS.
- (a) E. Sikorska, M. Sikorski, R. P. Steer, F. Wilkinson and D. R. Worrall, J. Chem. Soc., Faraday Trans., 1998, 94, 2347–2353 RSC; (b) E. Sikorska, I. Khmelinskii, A. Komasa, J. Koput, L. F. V. Ferreira, J. R. Herance, J. L. Bourdelande, S. L. Williams, D. R. Worrall, M. Insińska-Rak and M. Sikorski, Chem. Phys., 2005, 314, 239–247 CrossRef CAS PubMed.
- Related reactions proceed via a singlet oxygen pathway: see ref. 4; S. Fukuzumi, K. Tanii and T. Tanaka, J. Chem. Soc., Perkin Trans. 2, 1989, 2103–2108 RSC.
- P. Hemmerich, C. Veeger and H. C. S. Wood, Angew. Chem., Int. Ed. Engl., 1965, 4, 671–687 CrossRef CAS.
- P. F. Heelis, Chem. Soc. Rev., 1982, 11, 15–39 RSC.
- S. Fukuzumi and S. Kuroda, Res. Chem. Intermed., 1999, 25, 789 CrossRef CAS PubMed.
- S. Fukuzumi and T. Kojima, JBIC, J. Biol. Inorg. Chem., 2008, 13, 321–333 CrossRef CAS PubMed.
- (a) W. H. Walker, P. Hemmerich and V. Massey, Eur. J. Biochem., 1970, 13, 258–266 CrossRef CAS PubMed; (b) W. H. Walker, P. Hemmerich and V. Massey, Helv. Chim. Acta, 1967, 50, 2269–2279 CrossRef CAS PubMed ; see ref. 7, and references therein.
- S. Fukuzumi, S. Kuroda, T. Goto, K. Ishikawa and T. Tanaka, J. Chem. Soc., Perkin Trans., 1989, 22, 1047 RSC.
- K. Ohkubo, S. C. Menon, A. Orita, J. Otera and S. Fukuzumi, J. Org. Chem., 2003, 68, 4720–4726 CrossRef CAS PubMed.
- (a) M. M. Green and S. L. Mielke, J. Org. Chem., 1984, 49, 1276–1278 CrossRef CAS; (b) A. M. de P. Nicholas and D. R. Arnold, Can. J. Chem., 1982, 60, 2165–2179 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Full experimental details, additional catalytic results, and GC-FID and UV-vis monitoring studies. See DOI: 10.1039/c5cc00178a |
| ‡ No structural information is presently available on the scandium(III) complexes in solution, though it seems likely that these correspond to neutral or cationic complexes or clusters with the general composition [ScxXy(RFTHn)z]m+ (X = OTf or Cl). We choose to designate the species involved in the catalytic mechanism as RFTHnm+–2Sc3+ for simplicity. |
| § The pKa of the closely-related RFTH2+˙ radical is approximately 2, while the pKa of a toluene radical cation in MeCN is estimated to −12 to −13.20 |
| This journal is © The Royal Society of Chemistry 2015 |

