Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Triphenylarsonium-functionalised gold nanoparticles: potential nanocarriers for intracellular therapeutics†
Nikhil
Lalwani
a,
Yu-Su
Chen
a,
Gemma
Brooke
a,
Neil A.
Cross
a,
David W.
Allen
a,
Alan
Reynolds
b,
Jesús
Ojeda
b,
Graham J.
Tizzard
c,
Simon J.
Coles
 c and
Neil
Bricklebank
*a
c and
Neil
Bricklebank
*a
aBiomedical Research Centre, Sheffield Hallam University, City Campus, Sheffield S1 1WB, UK. E-mail: n.bricklebank@shu.ac.uk
bExperimental Techniques Centre, Brunel University, Kingston Lane, Uxbridge, Middlesex UB8 3PH, UK
cEPSRC National Crystallography Service, School of Chemistry, University of Southampton, Southampton, SO17 1BJ, UK
First published on 4th February 2015
Abstract
Two new triphenylarsonium alkylthiolate precursors, a thiosulfate zwitterion and a thioacetate salt, have been structurally characterised and their cytotoxicity evaluated against PC3 cells. The arsonium compounds have been used to prepare gold nanoparticles decorated with triphenylarsonium groups.
Arsenic has attracted the attention of scientists for centuries and its compounds have a variety of applications ranging from electronic and semiconductor materials1 to organic reagents, arsonium ylides finding utility in the Wittig reaction.2 Historically, arsenic compounds have been widely investigated for their medicinal properties although interest declined as greater understanding of their toxicity became apparent.3,4 Generally inorganic As(III) and As(V) species are highly toxic, whereas organic arsenic compounds are significantly less toxic.1,5 More recently there has been a resurgence of interest in the medicinal properties of arsenic compounds, including the use of arsenic trioxide and organic arsenic derivatives as treatments for leukaemia and other cancers,1,3,4 the characterisation of an arsenic trioxide analogue of cisplatin,6 and the observation that 72As and 74As radiopharmaceuticals could be useful in positron emission tomography (PET).1
The chemistry of arsenic is broadly similar to that of phosphorus, and organic phosphonium salts are known to act as lipophilic cations that are preferentially accumulated in the mitochondria of cells.7 Similarly, arsonium cations are also lipophilic and lipophosphoramidate derivatives containing arsonium head-groups (1)8 have been studied as gene delivery systems, and 64Cu-labelled complexes of 1,4,7,10-tetraazacyclododecane-4,7,10-triacetic acid-conjugated triphenylarsonium cations (2) have been found to act as tumour-selective PET imaging agents.9 Phosphonium species have been found to be extremely valuable for mitochondria-targeted therapeutics and diagnostics. Mitochondrial dysfunction is associated with a number of disorders and there is increasing awareness of the importance of targeting drugs to this organelle.7 Recent work has demonstrated the potential of combining phosphonium compounds with nanotechnological approaches to traffic pharmaceutical and diagnostic moieties into mitochondria.10 For example, incorporation of phosphonium groups into the lipid bilayer of liposomes,11 or onto the surface of dendrimers12 and polymer nanoparticles,13 facilitates their preferential uptake by mitochondria. We have prepared phosphonium-functionalised gold nanoparticles14–16 which are accumulated by cells and shown by TEM to be localised in the mitochondria.17 Inspired by the success of arsonium systems such as 1 and 2 we decided to broaden the scope of our studies to include triphenylarsonium compounds and herein report our preliminary results on the biological properties of triphenylarsonium alkylthiosulfate zwitterions (3) and salts (4) and their application in the synthesis of triphenylarsonium-functionalised gold nanoparticles (AuNPs).
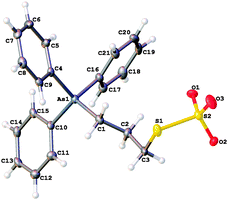
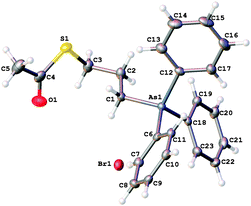
The synthesis of 3, 4 and the triphenylarsoniumalkylthiolate-functionalised AuNPs are described in the ESI.† The structures of 3 and 4 were confirmed by X-ray crystallography. Perhaps surprisingly, the structures of few organic thiosulfate zwitterions have been reported.18 Both compounds display the expected tetrahedral geometry around the arsenic atoms with mean C–As–C bond angles of 109.5(2)° in 3 and 109.46(11)° in 4. In zwitterion 3 (Fig. 1), the S–O bonds in the thiosulfate group are similar with a mean length of 1.447(4) Å, indicative of multiple bond character, and that the negative charge is delocalised over the entire sulfate group. The mean O–S–O angle [113.8(2)°] is consistent with those in other thiosulfate ions.18 The S–S bond length [2.1081(18) Å], is slightly shorter than that of the corresponding phosphonium compound [2.1117(9) Å],15 but longer than the S–S bond in ionic thiosulfate ions such as Me2HN(CH2S2O3)2−Na+.18 Within the crystal lattice the zwitterions pack fairly loosely, held together by hydrogen-bonding interactions between the sulfate oxygens and the phenyl hydrogens, but there is no close interaction between the arsonium and the thiosulfate moieties. The bond lengths and angles of the thioacetate group in salt 4 (Fig. 2), are as expected. The molecular packing shows no significant interactions between the arsonium centre and the bromide anion or between the bromide and the carbonyl group.
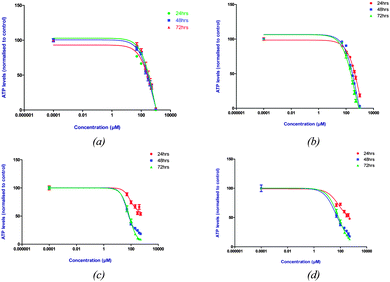
In order to evaluate the efficacy of the triphenylarsonium-functionalised AuNPs as cellular transport systems we first screened the parent arsonium compounds 3 and 4 against the PC3 prostate cancer cell line. Cell viability was assessed using MTT and CellTitre-Glo® assays (Fig. 3). MTT measures mitochondrial activity to determine the in vitro cytotoxic effects of chemical entities. The results showed 3 and 4 to have IC50 values of 75 μM and 72 μM, respectively, after 72 hours. These values compare very favourably with those of phosphonium compounds, a large number of which have been screened using MTT against PC3 cells, and which displayed IC50 values in the range 0.4–5 μM.19 These results are also in accordance with cellular toxicity data for lipophosphoramidate derivatives, where phosphonium compounds show greater cytotoxicity than the corresponding arsonium compounds.8 We also used the CellTitre-Glo® assay to confirm the MTT cytotoxicity data. This uses luminescence to determine the number of viable cells based on a quantification of ATP levels. The data showed a similar trend to that determined using MTT.
The cytotoxicity of arsenic compounds is crucially dependent on the nature and oxidation state of the species. Inorganic compounds, such as arsenite and arsenate, show acute toxicity; for example, the IC50 of sodium arsenate was reported as 6 μM.20 In contrast, the organic derivative arsenobetaine, Me3As+CH2CO2−, an important metabolite of arsenic which is widely distributed in marine ecosystems and found in comparatively high levels in seafood, is reported to have no toxic effects and was found to significantly enhance the cell viability of bone marrow cells in vitro in a concentration-dependent manner.20
Phosphonioalkylthiosulfate zwitterions,15 and phosphonium alkylthioacetate salts,16 are known to act as ‘masked thiolates’ and under reductive conditions cleavage of the thiosulfate S–S or thioacetate S–C bonds, respectively, takes place, generating phosphonioalkylthiolate zwitterions that can coordinate to the surface of gold films.
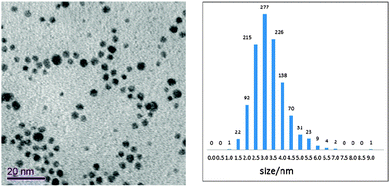
We have exploited this chemistry to generate water-soluble cationic gold nanoparticles functionalised with alkylthiolate ligands bearing phosphonium head-groups. We have now extended this approach to the analogous arsonium compounds. Reduction of tetrachloroaurate salts in situ with sodium borohydride in the presence of 3 or 4 in a biphasic water/dichloromethane mixture yields the triphenylarsonium-capped AuNPs. Recent work has reported rare examples of the coordination chemistry of tertiary arsine ligands bearing pendant thiolate groups towards Ni(II), Pt(II) and Pd(II).21 However, to the best of our knowledge, there are no reports of the coordination chemistry of triorganoarsonium thiolate species or of the use of tertiary arsines or arsonium compounds as capping ligands in the formation of functionalised nanoparticles, although nanoscale liposomes or nanobins, composed of lipids and metal salts, have been used to encapsulate and stabilise arsenic trioxide in order to extend the clinical utility of this compound.22 The triphenylarsonium-AuNPs can be purified by extraction with dichloromethane followed by freeze drying. The UV-Vis spectrum of a typical sample of arsonium-capped nanoparticles shows an absorption band with λmax of 520 nm. High resolution TEM analysis of the AuNPs derived from the thiosulfate zwitterions is shown in Fig. 4, and revealed the AuNPs to have spherical shapes. Size distribution analysis of 1000 particles using Abel imaging software revealed a mean diameter of 2.7 ± 0.9 nm. This compares favourably with the corresponding phosphonium-capped AuNPs which have an average particle size of 3.0 ± 1.2 nm. Wide scan XPS spectra of the arsonium-AuNPs contained signals due to Au, S, As and C, with the gold displaying the characteristic doublet for Au (4f7/2) and Au (4f5/2) with binding energies of ca. 83.9 and 87.5 eV respectively, indicative of the presence of Au(0).14 Research is now ongoing to understand the cellular uptake of the triphenylarsonium-AuNPs and confirm their presence inside the cells using TEM to realise their potential intracellular transport properties.
We are grateful to Sheffield Hallam University and Indian Institute of Science (NL) for financial support.
Notes and references
- A. Mudhoo, S. K. Sharma, V. K. Garg and C.-H. Tseng, Crit. Rev. Environ. Sci. Technol., 2011, 41, 435–519 CrossRef CAS.
- H. S. He, C. W. Y. Chung, T. Y. S. But and P. H. Toy, Tetrahedron, 2005, 61, 1385–1405 CrossRef CAS PubMed.
- S.-J. Chen, G.-B. Zhou, X.-W. Zhang, J.-H. Mao, H. de Thé and Z. Chen, Blood, 2011, 117, 6425–6437 CrossRef CAS PubMed.
- E. P. Swindell, P. L. Hankins, H. Chen, Đ. U. Miodragović and T. V. O'Halloran, Inorg. Chem., 2013, 52, 12292–12304 CrossRef CAS PubMed.
- K. Jomova, Z. Jenisova, M. Festerova, S. Baros, J. Liska, D. Hudecova, C. J. Rhodes and M. Valko, J. Appl. Toxicol., 2011, 31, 95–107 CAS.
- Đ. U. Miodragović, J. A. Quentzel, J. W. Kurutz, C. L. Stern, R. W. Ahn, I. Kandela, A. Mazar and T. V. O'Halloran, Angew. Chem., Int. Ed., 2013, 52, 10749–10752 CrossRef PubMed.
- (a) A. T. Hoyle, J. E. Davoren, P. Wipf, M. P. Fink and V. E. Kagan, Acc. Chem. Res., 2008, 41, 87–97 CrossRef PubMed; (b) V. Weissig, Pharm. Res., 2011, 28, 2657–2668 CrossRef CAS PubMed.
- (a) E. Picquet, K. Le Ny, P. Delépine, T. Montier, J.-J. Yaouanc, D. Cartier, H. des Abbayes, C. Férec and J.-C. Clément, Bioconjugate Chem., 2005, 16, 1051–1053 CrossRef CAS PubMed; (b) T. Le Gall, D. Loizeau, E. Picquet, N. Carmoy, J.-J. Yaouanc, L. Burel-Deschamps, P. Delépine, P. Giamarchi, P.-A. Jaffrès, P. Lehn and T. Montier, J. Med. Chem., 2010, 53, 1496–1508 CrossRef CAS PubMed; (c) M. Berchel, T. Le Gall, H. Couthon-Gourvès, J.-P. Haelters, T. Montier, P. Midoux, P. Lehn and P.-A. Jaffrès, Biochimie, 2012, 94, 33–41 CrossRef CAS PubMed.
- J. Wang, C. T. Yang, Y. S. Kim, S. G. Sreerama, Q. Cao, Z. Li, Z. He, X. Chen and S. Liu, J. Med. Chem., 2007, 50, 5057–5069 CrossRef CAS PubMed.
- S. S. Malhi and R. S. R. Murthy, Expert Opin. Drug Delivery, 2012, 9, 909–935 CrossRef CAS PubMed.
- (a) S. V. Boddapati, G. G. M. D'Souza, S. Erdogan, V. P. Torchilin and V. Weissig, Nano Lett., 2008, 8, 2559–2563 CrossRef CAS PubMed; (b) S. Biswas, N. S. Dodwadkar, P. P. Deshpande and V. P. Torchilin, J. Controlled Release, 2012, 159, 393–402 CrossRef CAS PubMed; (c) S. V. Boddapati, P. Tongcharoensirikul, R. N. Hanson, G. G. M. D'Souza, V. P. Torchilin and V. Weissig, J. Liposome Res., 2005, 15, 49–58 CrossRef CAS PubMed.
- S. Biswas, N. S. Dodwadkar, A. Piroyan and V. P. Torchilin, Biomaterials, 2012, 33, 4773–4782 CrossRef CAS PubMed.
- X.-H. Wang, H.-S. Peng, L. Yang, F.-T. You, F. Teng, A.-W. Tang, F.-J. Zhang and X.-H. Li, J. Mater. Chem. B, 2013, 1, 5143–5152 RSC.
- Y. Ju-Nam, Y.-S. Chen, J. J. Ojeda, D. W. Allen, N. A. Cross, P. H. E. Gardiner and N. Bricklebank, RSC Adv., 2012, 2, 10345–10351 RSC.
- Y. Ju-Nam, N. Bricklebank, D. W. Allen, P. H. E. Gardiner, M. E. Light and M. B. Hursthouse, Org. Biomol. Chem., 2006, 4, 4345–4351 CAS.
- Y. Ju-Nam, D. W. Allen, P. H. E. Gardiner and N. Bricklebank, J. Organomet. Chem., 2008, 693, 3504–3508 CrossRef CAS PubMed.
- Y.-S. Chen, PhD thesis, Sheffield Hallam University, 2014.
- J.-X. Chen, Q.-F. Xu, Y. Zhang, S. M. Zain, S. W. Ng and P.-P. Lang, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2004, 60, 0572–0574 Search PubMed.
- M. Millard, D. Pathania, Y. Shabaik, L. Taheri, J. Deng and N. Neamati, PLoS One, 2010, 5, e13131 Search PubMed.
- T. Sakurai and K. Fujiwara, Br. J. Pharmacol., 2001, 132, 143–150 CrossRef CAS PubMed.
- (a) A. Hildebrand, I. Sárosi, P. Lönnecke, L. Silaghi-Dumitrescu, M. B. Sárosi, I. Silaghi-Dumitrescu and E. Hey-Hawkins, Inorg. Chem., 2012, 51, 7125–7133 CrossRef CAS PubMed; (b) A.-M. Valean, S. Gomex-Ruiz, L. Silaghi-Dumitrescu and E. Hey-Hawkins, Z. Anorg. Chem., 2013, 639, 1220–1226 CrossRef CAS.
- (a) R. W. Ahn, S. L. Barrett, M. R. Raja, J. K. Jozefik, L. Spaho, H. Chen, A. P. Mazar, M. J. Avram, J. N. Winter, L. I. Gordon, L. D. Shea, T. V. O'Halloran and T. K. Woodruff, PLoS One, 2013, 8, e58491 CAS; (b) S.-M. Lee, O.-S. Lee, T. V. O'Halloran, G. C. Schatz and S. T. Nguyen, ACS Nano, 2011, 3961–3969 CrossRef CAS PubMed; (c) H. Chen, S. Pazicni, N. L. Krett, R. W. Ahn, J. Penner-Hahn, S. T. Rosen and T. V. O'Halloran, Angew. Chem., Int. Ed., 2009, 121, 9459–9463 CrossRef; (d) H. Chen, R. C. MacDonald, S. Li, N. L. Krett, S. T. Rosen and T. V. O'Halloran, J. Am. Chem. Soc., 2006, 128, 13348–13349 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1020083 and 1020084. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4cc09304f |
| This journal is © The Royal Society of Chemistry 2015 |