Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Single molecule level plasmonic catalysis – a dilution study of p-nitrothiophenol on gold dimers†
Zhenglong
Zhang
a,
Tanja
Deckert-Gaudig
a,
Pushkar
Singh
a and
Volker
Deckert
*ab
aLeibniz Institute of Photonic Technology, Albert-Einstein-Str. 9, 07745 Jena, Germany. E-mail: volker.deckert@ipht-jena.de
bInstitute of Physical Chemistry and Abbe Center of Photonics, Friedrich-Schiller University Jena, Helmholtzweg 4, 07743 Jena, Germany
First published on 7th January 2015
Abstract
Surface plasmons on isolated gold dimers can initiate intermolecular reactions of adsorbed p-nitrothiophenol. At the single molecule level when dimerization is not possible an intramolecular reaction can be observed. Experimental evidence indicates that plasmon-induced hot electrons provide the required activation energy.
In the presence of surface plasmons molecular chemical reactions can be investigated at the nanoscale, as it has been demonstrated for a variety of catalyzed systems.1–19 Recently, plasmon catalyzed dimerization of p-nitrothiophenol (pNTP) to 4,4-dimercaptoazobenzene (DMAB) has been realized using surface-enhanced Raman spectroscopy (SERS) and tip-enhanced Raman spectroscopy (TERS).2–6 For any dimerization the distance between the reactants is decisive. If the distance between pNTP molecules gets too large a dimerization reaction cannot occur. In this case the question arises, whether surface plasmons can initiate a further intramolecular reaction like dissociation or rearrangement. To address this issue, a detection sensitivity of signals from a few or ultimately even a single educt molecule is required. In our experiments we employed SERS, which is widely used for noninvasive detection, biological and chemical sensing.20–25 In SERS laser irradiation of silver or gold nanoparticles induces localized surface plasmon resonances at the nanostructured surface generating enhanced electromagnetic fields.25 If molecules are brought in closest vicinity to such nanoparticles (e.g. by adsorption) a large enhancement of the Raman scattering signal can be observed. Using the junctions or gaps of nanoparticles, so-called “hot spots”, very high enhancement factors (∼108) can be achieved, providing sufficient signal enhancement for single molecule detection. Therefore such junctions are ideally suited for the intended reactivity detection.23–27
In previous studies, it was found that the plasmon catalyzed intermolecular reaction of pNTP (adsorbed on gold or silver nanoparticles) to DMAB can be controlled by laser intensity.3,4 A higher laser intensity generates a stronger plasmon resonance with higher kinetic energy yielding an increased reaction rate. If the concentration, however, is low enough to prevent clustering and self-assembly, the distance of pNTP molecules adsorbed on the nanoparticles should become too large for an intermolecular reaction and no reaction should occur even at high laser powers. Then, any detected SERS signal can be related to single or only a few isolated and non-interacting separated molecules.
In this communication, single gold nanoparticle dimers were employed for SERS detection of pNTP molecules. In the presented experiments a so far unknown behavior of pNTP could be monitored, which strongly differs from previous reports as separated pNTP molecules exclusively reacted to thiophenol (TP), and is a new finding in surface catalyzed experiments. In addition, a step-like signal intensity change during the process strongly indicates that the dissociation reaction of pNTP to TP occurs at or close to a single molecule level.
As shown in Fig. 1, SERS active gold nanoparticle dimers (GDs) were prepared using a wet chemistry method.28 Further experimental details can be found in the ESI.† In order to achieve SERS of separated molecules, three different concentrations of aqueous pNTP solutions (5 × 10−7, 10−8 and 10−9 M) were mixed with a gold nanoparticle dimer colloidal solution. The dimerization of pNTP to DMAB is characterized by the disappearance of the Raman band at 1332 cm−1 (νNO2), and the appearance of new Raman bands around 1140 (βC–H), 1387 (νNN + νCC + νC–N) and 1432 (νNN + νCC + βC–H) cm−1, which are related to the –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– unit of DMAB.2
N– unit of DMAB.2
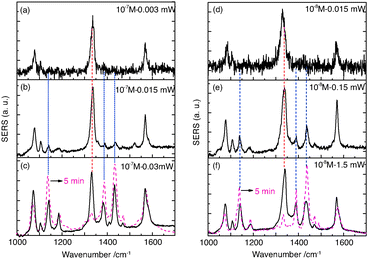
First, SERS experiments of the 10−7 M pNTP–GD mixture were made with increasing laser intensity. Starting with 3 μW (see Fig. 2(a)) only the three main peaks of pNTP were detected in the SERS spectrum. Increasing the laser power to 15 μW and 30 μW, respectively, dimerization of pNTP to DMAB was verified by the afore-mentioned bands at 1140, 1387 and 1432 cm−1 (Fig. 2(b)). Fig. 2(b) and (c) indicate also that an increased laser power yields a higher reaction rate. After continuous irradiation for 5 min almost all pNTP molecules in the laser spot have reacted to DMAB (see dotted red curve in Fig. 2(c)).
In the next experiment the 10−8 M pNTP–GD mixture were investigated, all other experimental parameters except the laser power as stated below remained unchanged. The corresponding SERS spectra are shown in Fig. 2(d)–(f). Similar to the previous experiment, a dimerization of pNTP to DMAB occurred, however, higher laser intensities (150 μW) were needed to initiate the reaction. The results of both experiments demonstrate that with 10−7 M and 10−8 M pNTP solutions the gold surface is still densely covered with pNTP molecules enabling a dimerization.
For the final experiment a 10−9 M pNTP solution was used. The resulting SERS spectra are shown in Fig. 3(a)–(c). During these measurements no dimerization of pNTP to DMAB was observed. Even at highest laser power (3 mW) for one hour (see Fig. 3(d)–(h) and for the full trace ESI,† Fig. S1) no DMAB signals could be detected. It was concluded that the distance between pNTP molecules in the plasmonic hot-spot of the GD was indeed too large to allow intermolecular reactions under these conditions. That the reaction of pNTP to DMAB could not be initiated even after 1 hour of continuous irradiation, indicates that SERS signals from only a few separated molecules in the hot-spot were acquired. This finding is in accordance with the estimated molecular coverage n of pNTP on a single gold dimer that was estimated as follows:
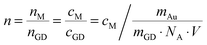 | (1) |
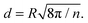 | (2) |
 | ||
| Fig. 3 (a)–(c) Laser intensity dependent SERS spectra excited at 0.03, 0.15 and 3 mW laser power, respectively. (d)–(h) Time dependent SERS spectra at 3 mW laser at the sample. | ||
Probably the most striking observation in the SERS experiments of pNTP at 10−9 M was an intensity decrease of the dominating band at 1332 cm−1 (νNO2) over time. This peak disappeared after 10 min, simultaneously a new peak at 1017 cm−1 was detected after 5 min, as shown in Fig. 3(d)–(h). Since the NO2 mode of pNTP disappeared, we hypothesize that the nitro group was cleaved from the benzene ring in the hot-spot of the gold dimer resulting in the formation of thiophenol (TP). Comparing SERS spectra of neat TP (Fig. 4(b)) with “pNTP on GDs” spectra from late stages (see Fig. 4(a) and also Fig. 3(h)) it is obvious that the new bands at 995 (βCCC) and 1017 (βCH) cm−1 as well as the bands at 420 and 685 cm−1 in the low wavenumber region match those of TP strikingly well (see also ESI,† Fig. S3). The necessary hydrogen atom for the formation of TP was most probably abstracted from the surrounding water layer that was ubiquitously present since pNTP was adsorbed from aqueous solution and the experiment was performed under ambient conditions.
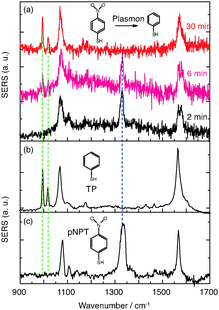 | ||
| Fig. 4 (a) Time dependent SERS spectra of reacting pNTP (c = 10−9 M) at 3 mW laser at 2, 6 and 30 min. (b) and (c) normal SERS spectra of TP and pNTP, respectively. | ||
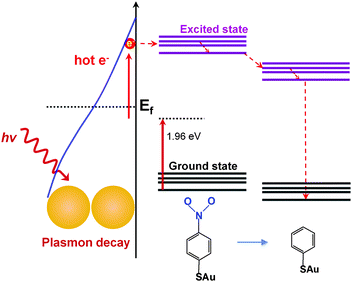
The experimental data demonstrate that a dissociation of pNTP to TP proceeds under our special SERS conditions at a concentration of 10−9 M (corresponding to a surface coverage of ∼10 pNTP molecules per dimer). These results clearly differ from the dimerization reaction of pNTP to DMAB. The light induced dissociation of nitro substituted aromatic molecules is not new but usually requires drastic conditions. For example, the –NO2 group in nitrobenzene and nitrophenol can be cleaved using UV excitation.29–31 The required dissociation energy of the C–N bond is ∼3.03 eV,29 which is higher than the energy provided by the incident photon of 632.8 nm (1.96 eV). The power dependent experiments in Fig. 3 show no nonlinear increase of the products, so nonlinear optical effects including two/multi-photon absorption can be ruled out. Hence, the activation energy is too large to be simply overcome by the excitation laser. However, localized surface plasmons excited on silver or gold nanoparticle surfaces can decay non-radiatively into hot electrons with an energy between the vacuum energy and Fermi level (∼−5 eV).32–34 Hot electrons can scatter into an excited state of the absorbed molecules, triggering a chemical reaction by reducing the activation energy.34 Applied to the present reaction our hypothesis is that plasmon-induced hot electrons initiate –NO2 cleavage from the phenyl ring. A scheme of the proposed mechanism is shown in Fig. 5. Initially, hot electrons generated from plasmon decay on the nanoparticle surface, soon lose coherence and form a nonequilibrium Fermi–Dirac type distribution.8 The hot electrons in the high energy level have sufficient energy to transfer to the excited state of pNTP molecule, creating a transient negative ion of pNTP. This negative ion “travels” to the excited state of TP and transfers the electron back to the GD surface, where it returns to the ground state of TP and dissociates.
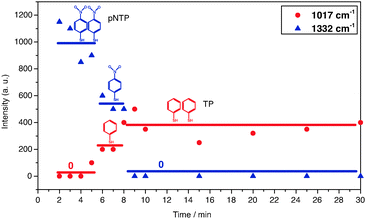
It should be noted that the simultaneous detection of pNTP and TP in Fig. 4(a) – spectrum at 6 min – actually points to the presence of at least two molecules in the hot spot. Although the experiment was performed at a very low concentration it cannot be regarded as “single molecule” SERS in the classical sense. According to the time dependent SERS spectra, the peak intensity change vs. time is shown for the pNTP band at 1332 cm−1, and the TP band at 1017 cm−1 in Fig. 6, respectively. It was found that three clear steps can be discerned: first, some molecules of pNTP were detected in the first 5 min of the measurement; after 5 min, half of the pNTP molecules reacted to TP; the remaining pNTP molecules reacted after 8 min. After that no significant change occurred in the remaining experiment (60 min). Consequently, it can be assumed that two distant, non-interacting molecules were detected in the hotspot of a single GD because both pNTP and TP were detected between 5–8 min. Apparently, the two molecules were too far apart from each other for an intermolecular reaction, and instead an intramolecular reaction was initiated. Because of the step-like reaction process, the other possibilities of having 4-2-0, 6-3-0, or 8-4-0 molecules synchronously reacting seem very unlikely. The involvement of even more molecules can be ruled out, as at 10 times higher concentration dimerization already starts.
In summary, we present the detection of a new plasmon catalyzed reaction of pNTP near the single molecule level. A dissociation of pNTP to TP was detected at low concentration where the distance between pNTP molecules adsorbed on single isolated gold dimer became too large for a dimerization reaction. In combination with the results with higher concentrations this further confirms a dimerization reaction of pNTP to DMAB. In accordance with previous reports we assume that plasmon-induced hot electrons provide the activation energy necessary for such a dissociation of pNTP. The tracked step-like process of the dissociation of pNTP to TP strongly indicates a single molecule behavior. The results demonstrate that irradiation of an adsorbate in a gold nanoparticle gap can decrease the activation energy barrier such, that a reaction usually requiring UV light can be triggered in the visible.
We gratefully acknowledge support from the Deutsche Forschungsgemeinschaft (DEP4TERS, FR 1348/19-1) and the Thüringer Aufbau Bank (No. 2011SE9048). Z. Zhang acknowledges financial support from the Alexander von Humboldt foundation.
Notes and references
- P. Christopher, H. L. Xin and S. Linic, Nat. Chem., 2011, 3, 467–472 CAS.
- B. Dong, Y. R. Fang, X. W. Chen, H. X. Xu and M. T. Sun, Langmuir, 2011, 27, 10677–10682 CrossRef CAS PubMed.
- E. M. van Schrojenstein Lantman, T. Deckert-Gaudig, A. J. G. Mank, V. Deckert and B. M. Weckhuysen, Nat. Nanotechnol., 2012, 7, 583–586 CrossRef CAS PubMed.
- M. T. Sun, Z. L. Zhang, H. R. Zheng and H. X. Xu, Sci. Rep., 2012, 2, 647 Search PubMed.
- V. Joseph, C. Engelbrekt, J. D. Zhang, U. Gernert, J. Ulstrup and J. Kneipp, Angew. Chem., Int. Ed., 2012, 51, 7592–7596 CrossRef CAS PubMed.
- M. T. Sun and H. X. Xu, Small, 2012, 8, 2777–2786 CrossRef CAS PubMed.
- J. Lee, S. Mubeen, X. L. Ji, G. D. Stucky and M. Moskovits, Nano Lett., 2012, 12, 5014–5019 CrossRef CAS PubMed.
- S. Mukherjee, F. Libisch, N. Large, O. Neumann, L. V. Brown, J. Cheng, J. B. Lassiter, E. A. Carter, P. Nordlander and N. J. Halas, Nano Lett., 2013, 13, 240–247 CrossRef CAS PubMed.
- W. Xie, B. Walkenfort and S. Schlucker, J. Am. Chem. Soc., 2013, 135, 1657–1660 CrossRef CAS PubMed.
- M. T. Sun, Z. L. Zhang, Z. H. Kim, H. R. Zheng and H. X. Xu, Chem. – Eur. J., 2013, 19, 14958–14962 CrossRef CAS PubMed.
- K. Ueno and H. Misawa, J. Photochem. Photobiol., C, 2013, 15, 31–52 CrossRef CAS PubMed.
- Z. L. Zhang, M. T. Sun, P. P. Ruan, H. R. Zheng and H. X. Xu, Nanoscale, 2013, 5, 4151–4155 RSC.
- S. Mukherjee, L. A. Zhou, A. M. Goodman, N. Large, C. Ayala-Orozco, Y. Zhang, P. Nordlander and N. J. Halas, J. Am. Chem. Soc., 2014, 136, 64–67 CrossRef CAS PubMed.
- E. M. v. S. Lantman, O. L. J. Gijzeman, A. J. G. Mank and B. M. Weckhuysen, ChemCatChem, 2014, 6, 3342–3346 Search PubMed.
- Z. L. Zhang, S. X. Sheng, H. R. Zheng, H. X. Xu and M. T. Sun, Nanoscale, 2014, 6, 4903–4908 RSC.
- P. Singh and V. Deckert, Chem. Commun., 2014, 50, 11204–11207 RSC.
- C. L. Wang and D. Astruc, Chem. Soc. Rev., 2014, 43, 7188–7216 RSC.
- M. J. Kale, T. Avanesian and P. Christopher, ACS Catal., 2014, 4, 116–128 CrossRef CAS.
- X. J. Lang, X. D. Chen and J. C. Zhao, Chem. Soc. Rev., 2014, 43, 473–486 RSC.
- D. L. Jeanmaire and R. P. Vanduyne, J. Electroanal. Chem., 1977, 84, 1–20 CrossRef CAS.
- P. L. Stiles, J. A. Dieringer, N. C. Shah and R. R. Van Duyne, Annu. Rev. Anal. Chem., 2008, 1, 601–626 CrossRef CAS PubMed.
- L. Li, T. Hutter, A. S. Finnemore, F. M. Huang, J. J. Baumberg, S. R. Elliott, U. Steiner and S. Mahajan, Nano Lett., 2012, 12, 4242–4246 CrossRef CAS PubMed.
- S. M. Nie and S. R. Emery, Science, 1997, 275, 1102–1106 CrossRef CAS PubMed.
- K. Kneipp, Y. Wang, H. Kneipp, L. T. Perelman, I. Itzkan, R. Dasari and M. S. Feld, Phys. Rev. Lett., 1997, 78, 1667–1670 CrossRef CAS.
- H. X. Xu, J. Aizpurua, M. Kall and P. Apell, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 2000, 62, 4318–4324 CrossRef CAS.
- T. Deckert-Gaudig, E. Kammer and V. Deckert, J. Biophotonics, 2012, 5, 215–219 CrossRef CAS PubMed.
- R. Zhang, Y. Zhang, Z. C. Dong, S. Jiang, C. Zhang, L. G. Chen, L. Zhang, Y. Liao, J. Aizpurua, Y. Luo, J. L. Yang and J. G. Hou, Nature, 2013, 498, 82–86 CrossRef CAS PubMed.
- Z. L. Zhang, P. F. Yang, H. X. Xu and H. R. Zheng, J. Appl. Phys., 2013, 113, 033102 CrossRef PubMed.
- M. L. Hause, N. Herath, R. S. Zhu, M. C. Lin and A. G. Suits, Nat. Chem., 2011, 3, 932–937 CrossRef CAS PubMed.
- B. Chen, C. Yang and N. K. Goh, J. Environ. Sci., 2005, 17, 598–604 CAS.
- Y. M. Li, J. L. Sun, H. M. Yin, K. L. Han and G. Z. He, J. Chem. Phys., 2003, 118, 6244–6249 CrossRef CAS PubMed.
- M. W. Knight, H. Sobhani, P. Nordlander and N. J. Halas, Science, 2011, 332, 702–704 CrossRef CAS PubMed.
- I. Goykhman, B. Desiatov, J. Khurgin, J. Shappir and U. Levy, Nano Lett., 2011, 11, 2219–2224 CrossRef CAS PubMed.
- A. O. Govorov, H. Zhang and Y. K. Gun'ko, J. Phys. Chem. C, 2013, 117, 16616–16631 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and Fig. S1–S3. See DOI: 10.1039/c4cc09008j |
| This journal is © The Royal Society of Chemistry 2015 |