Open Access Article
Open Access ArticleFunctional helquats: helical cationic dyes with marked, switchable chiroptical properties in the visible region†
Paul E.
Reyes-Gutiérrez
a,
Michael
Jirásek
a,
Lukáš
Severa
a,
Pavlína
Novotná
b,
Dušan
Koval
a,
Petra
Sázelová
a,
Jan
Vávra
a,
Andreas
Meyer
a,
Ivana
Císařová
c,
David
Šaman
a,
Radek
Pohl
a,
Petr
Štěpánek
a,
Petr
Slavíček
d,
Benjamin J.
Coe
e,
Miroslav
Hájek
a,
Václav
Kašička
a,
Marie
Urbanová
b and
Filip
Teplý
*a
aInstitute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, v.v.i., Flemingovo n. 2, 166 10 Prague 6, Czech Republic. E-mail: teply@uochb.cas.cz
bDepartment of Analytical Chemistry and Department of Physics and Measurements, Institute of Chemical Technology, Technická 5, 166 28 Prague 6, Czech Republic
cDepartment of Inorganic Chemistry, Charles University, Hlavova 2030/8, 128 43 Prague 2, Czech Republic
dDepartment of Physical Chemistry, Institute of Chemical Technology, Technická 5, 166 28 Prague 6, Czech Republic
eSchool of Chemistry, University of Manchester, Oxford Road, Manchester M13 9PL, UK
First published on 15th December 2014
Abstract
Helquat dyes are the first helicene-like cationic styryl dyes obtained as separate enantiomers. Their remarkable chiroptical properties are due to the unique combination of a cationic hemicyanine chromophore and a helicene-like motif. The magnitude of the ECD response and the pH switching along with their positioning in the visible region are unprecedented among helicenoids.
Cationic dyes receive great attention due to their widely useful photophysical properties.1 This field played a pivotal role in transforming organic chemistry from a purely academic effort into a highly profitable industrial endeavour.2 However, chiral cationic dyes are attractive yet surprisingly underdeveloped,3,4 compared to achiral dyes. Specifically, cationic dyes based on a helicene structure5 remain largely overlooked. Rare exceptions are helicenium systems from the groups of Lacour6 and Arai7 (e.g.1, 2, Scheme 1a). The seldom visited territory of helical cationic dyes promises compounds with significant chiral properties such as sizeable chiroptical response in the visible region.
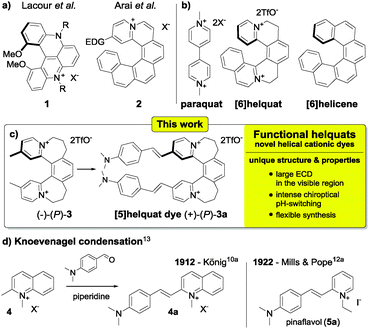 | ||
| Scheme 1 (a) Rare examples of helical cationic dyes;6,7 (b) structural relationship of [6]helquat with paraquat and [6]helicene; (c) one-step diversification strategy providing access to a range of helical dyes from a common methyl-substituted helquat precursor as presented in this paper; (d) synthesis of hemicyanine dye 4a as reported by Walter König in 191210a and structure of pinaflavol (5a) prepared analogously by Knoevenagel condensation.13 EDG = electron-donating group. | ||
We introduced recently helquats as a structural link between helicenes and viologens (Scheme 1b).8 The present work is motivated by an expectation that methyl-substituted helquats will lead to an original class of helically chiral cationic dyes (e.g. (+)-(P)-3a, Scheme 1c) with unique properties, opening up intriguing application opportunities for chiral sensing9 or chiroptical pH-switching25–27 (3k and 7k, Scheme 5). Here, we describe such dyes and demonstrate their remarkable chiroptical properties such as large electronic circular dichroism (ECD) in the visible region and prominent switching of the chiroptical response by pH. The magnitude of the ECD response and the pH-switching effect along with their positioning in the visible region are unprecedented among helicenoids.
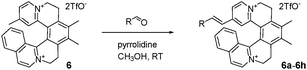
Reacting arylaldehydes with methyl-substituted cationic heteroaromatics to produce hemicyanine dyes10,11 (e.g.4 → 4a, Scheme 1d) has been since the early 20th century the enabling technology that led to sensitizers widely used in photography (e.g. pinaflavol).12 This transformation (Knoevenagel condensation,13Scheme 1d) is generally reliable, selective, experimentally simple, and versatile.14 At the outset of the present study, we proposed novel methyl-substituted helquats 3, 6, and 7 (Scheme 2) as dye precursors suitable for Knoevenagel condensation.
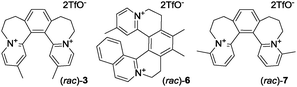 | ||
| Scheme 2 Structures of novel racemic methyl-substituted helquats used in this work. For synthetic details, see the ESI,† Section S3. | ||
Our approach relies on a convenient three-step synthesis of the methyl-substituted helquats,8a,c involving [2+2+2] cycloaddition.15 This strategy affords racemic helquat dye precursors 3, 6, and 7 in gram quantities (Scheme 2, for synthetic details, see the ESI,† Sections S3A–S3C).
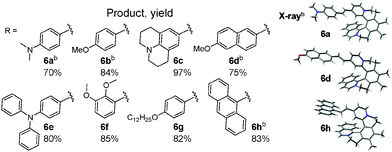
Using [6]helquat 6 demonstrates the potential of our approach, reacting with various arylaldehydes to give a series of dyes 6a–h (Table 1). Their triflate salts are typically easy to purify by precipitation. From the three methyl groups in helquat 6, only the one attached to a pyridinium moiety proved to be reactive in the Knoevenagel condensations. Precursors 3 and 7 show that helquats with two active methyl groups can be used for double Knoevenagel condensations (e.g.3 → 3a, 7 → 7a, Scheme 3 and Sections S3G and S3H, ESI†).
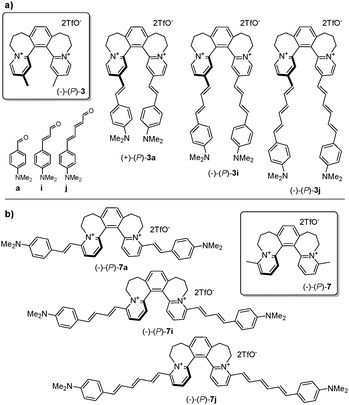 | ||
| Scheme 3 (a) Three representative (P)-configured helquat dyes 3a, 3i, and 3j obtained from the precursor (−)-(P)-3via Knoevenagel condensation with respective aldehydes; (b) the isomeric (P)-configured dyes 7a, 7i, and 7j derived from (−)-(P)-7. For (M)-configured dyes and further details, see the ESI,† Sections S3G and S3H. | ||
A further key favorable feature of our strategy is that a single non-racemic methyl-helquat such as (−)-(P)-3 (Scheme 3a) provides access to a whole range of non-racemic (P)-configured dyes. To this end, racemic helquat 3 is resolved via a dibenzoyltartrate method16 into (−)-(P)-3 and (+)-(M)-3 (Section S3D, ESI†). The former is transformed into (P)-configured dyes 3a, 3i, and 3j (Scheme 3a). The absolute configuration of both enantiomeric series of dyes derived from 3 is established unambiguously by helicity assignment of (M)-3 and (M)-3a by X-ray crystallography (Section S7, ESI†). Similarly, a series of dyes 7a, 7i, and 7j (Scheme 3b and Section S3H, ESI†) are synthesized as both enantiomers from the respective precursors (−)-(P)-7 and (+)-(M)-7.
Capillary electrophoresis (CE) with sulfated cyclodextrin chiral selectors17 allows direct enantiocomposition analysis of the new dyes (e.g.7a, Fig. 1 and Section S4, ESI†). This analysis confirms that the stereointegrity of the helix persists during the Knoevenagel condensations forming dyes shown in Scheme 3 and Section S3G and S3H (ESI†).18
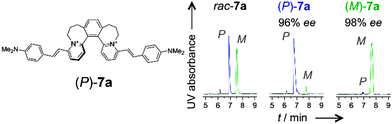 | ||
| Fig. 1 Results from chiral analysis of the racemic and non-racemic samples of helquat dye 7a by CE with a sulfated γ-cyclodextrin selector. Analysis established the enantiomeric purity of the non-racemic dyes to be greater than 95% ee in (P)- as well as (M)-series. See Section S4 (ESI†) for details. | ||
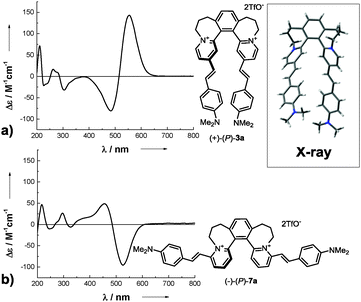
The non-racemic dyes show notable chiroptical properties. Compound (+)-(P)-3a exhibits a large molar rotation ([ϕ]20D = +223![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 830 deg cm2 dmol−1), and exciton coupling19 leads to significantly intense Cotton effects in ECD spectra in the visible region (Scheme 4a and Section S5, ESI†). Specifically, the dye with right-handed helicity (+)-(P)-3a shows a strong positive ECD band at 555 nm (Δε = +143 M−1 cm−1). While the prominent chiroptical response of many helicenoids in the UV region is documented amply in the literature,5,20 systems with substantial ECD in the visible are very rare.21,22 Thus, in this spectral region, the title dyes exhibit the most intense ECD bands among the helicenoids known.
830 deg cm2 dmol−1), and exciton coupling19 leads to significantly intense Cotton effects in ECD spectra in the visible region (Scheme 4a and Section S5, ESI†). Specifically, the dye with right-handed helicity (+)-(P)-3a shows a strong positive ECD band at 555 nm (Δε = +143 M−1 cm−1). While the prominent chiroptical response of many helicenoids in the UV region is documented amply in the literature,5,20 systems with substantial ECD in the visible are very rare.21,22 Thus, in this spectral region, the title dyes exhibit the most intense ECD bands among the helicenoids known.
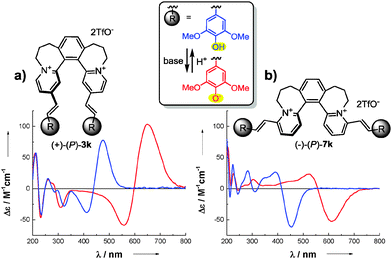 | ||
| Scheme 5 ECD switching between protonated (blue line) and deprotonated form (red line) of (a) (+)-(P)-3k and (b) (−)-(P)-7k. | ||
The dye (−)-(P)-7a is a positional isomer of (+)-(P)-3a. The former also shows significant molar rotation ([ϕ]20D = −100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 880 deg cm2 dmol−1) and a markedly strong visible Cotton effect (Δε = −96 M−1 cm−1 at 527 nm, Scheme 4b). However, it is noteworthy that, despite their same absolute configuration, (+)-(P)-3a and (−)-(P)-7a display opposite visible Cotton effects (compare Scheme 4a and b). Also, this phenomenon is observed consistently for the other two isomeric pairs, (−)-(P)-3i/(−)-(P)-7i and (−)-(P)-3j/(−)-(P)-7j (Section S5, ESI†).23 This effect is verified by first-principles calculations at several levels of theory (Section S6, ESI†). The ECD spectra simulated for (P)-3a and (P)-7a reproduce the experimental results in all major features (Fig. S35 and S37, ESI†).24 Transitions between orbitals of the opposite symmetry with respect to the C2 axis of the molecule give rise to positive ECD in (P)-3a and negative ECD in (P)-7a. On the other hand, transitions between orbitals of the same symmetry give rise to negative ECD in (P)-3a and positive ECD in (P)-7a (see Fig. S39 and S41 and Tables S13 and S15, ESI†).
880 deg cm2 dmol−1) and a markedly strong visible Cotton effect (Δε = −96 M−1 cm−1 at 527 nm, Scheme 4b). However, it is noteworthy that, despite their same absolute configuration, (+)-(P)-3a and (−)-(P)-7a display opposite visible Cotton effects (compare Scheme 4a and b). Also, this phenomenon is observed consistently for the other two isomeric pairs, (−)-(P)-3i/(−)-(P)-7i and (−)-(P)-3j/(−)-(P)-7j (Section S5, ESI†).23 This effect is verified by first-principles calculations at several levels of theory (Section S6, ESI†). The ECD spectra simulated for (P)-3a and (P)-7a reproduce the experimental results in all major features (Fig. S35 and S37, ESI†).24 Transitions between orbitals of the opposite symmetry with respect to the C2 axis of the molecule give rise to positive ECD in (P)-3a and negative ECD in (P)-7a. On the other hand, transitions between orbitals of the same symmetry give rise to negative ECD in (P)-3a and positive ECD in (P)-7a (see Fig. S39 and S41 and Tables S13 and S15, ESI†).
Incorporating two pH-active phenol units into our helically chiral bischromophoric dyes can engender an outstanding chiroptical pH-switchability. To this end, we have synthesized two dyes with phenol moieties, (+)-(P)-3k and (−)-(P)-7k (Scheme 5, Sections S3I and S3J, ESI†). For both of these compounds, pH changes trigger sizeable and reversible modulation of ECD response.25,26 Notably, (+)-(P)-3k shows particularly intense chiroptical pH-switching (Δ(Δε) = 100 M−1 cm−1 at 650 nm). The magnitude of this pH-switching effect and its positioning in the visible region are significant in general, and exceptional among helicenoids in particular.
In summary, this study introduces an original class of dicationic helical dyes with prominent chiroptical properties. The results are significant on multiple counts: (1) many non-racemic dyes are prepared easily from common precursors via a single-step Knoevenagel condensation; (2) the syntheses are convenient to perform and the products are typically easy to purify; (3) the new dyes show very intense ECD responses beyond the UV region due to a unique combination of a cationic hemicyanine chromophore with a helicene-like structural motif; (4) opposite Cotton effects in isomeric bis-chromophoric dyes of the same scaffold helicity are observed; (5) efficient pH-switching of the chiroptical responses is achieved (Δ(Δε) = 100 M−1 cm−1 at 650 nm) and the magnitude of this pH-effect as well as its positioning in the visible region are unprecedented among helicenoids.27 As the field of helical cationic dyes is extremely underdeveloped, helquat derivatives are attractive for many potential applications such as chiral environment-sensitive probes.
Financial support from the Czech Science Foundation (13-19213S to F.T., 13-32974S to D.K., P206/12/0453 to V.K., 13-34168S to P.Sl., 13-03978S to P.Št., and P208/11/0105), ASCR (RVO: 61388963, M200551208), Specific University Research MŠMT No. 20/2014 to P.N. (A1_FCHI_2014_003), and InterBioMed LO1302 from Ministry of Education of the Czech Republic is gratefully acknowledged. B.J.C. thanks the EPSRC for support (grants EP/G020299/1 and EP/J018635/1). Computational resources were provided by the MetaCentrum under the program LM2010005 and the CERIT-SC under the program Centre CERIT Scientific Cloud, part of the Operational Program Research and Development for Innovations, Reg. no. CZ.1.05/3.2.00/08.0144.
Notes and references
- Selected reviews on cationic dyes: (a) H. Berneth, Methine Dyes and Pigments, Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2008 Search PubMed; (b) N. Tyutyulkov, J. Fabian, A. Melhorn, F. Dietz and A. Tadjer, Polymethine Dyes, St. Kliment Ohridski University Press, Sofia, 1991 Search PubMed; (c) D. M. Sturmer, in The Chemistry of Heterocyclic Compounds, ed. A. Weissberger and E. C. Taylor, Wiley, New York, 1977, vol. 30, pp. 441–587 Search PubMed; (d) F. M. Hamer, in The Chemistry of Heterocyclic Compounds, ed. A. Weissberger, Wiley-Interscience, New York, 1964, vol. 18, pp. 398–510 Search PubMed; (e) Heterocyclic Polymethine Dyes, Topics in Heterocyclic Chemistry, ed. L. Strekowski, 2008, vol. 14 Search PubMed; (f) A. Mishra, R. K. Behera, P. K. Behera, B. K. Mishra and G. B. Behera, Chem. Rev., 2000, 100, 1973 CrossRef CAS PubMed; (g) T. Deligeorgiev, A. Vasilev, S. Kaloyanova and J. J. Vaquero, Color. Technol., 2010, 126, 55 CrossRef CAS PubMed.
- (a) K. C. Nicolaou and T. Montagnon, Molecules that Changed the World, Wiley-VCH, Weinheim, 2008, p. 53 Search PubMed; (b) A. S. Travis, Technology and Culture, 1990, 31, 51 CrossRef.
- An excellent review with the provocative title Chiral polymethine dyes: a remarkable but forgotten conjugated π system: C. Reichardt, J. Phys. Org. Chem., 1995, 8, 761 CrossRef CAS . Although the underdeveloped status of the field of chiral cationic dyes was highlighted 19 years ago, the situation has not changed considerably since then.
- Pioneering reports on chiral cationic dyes: (a) W. König, Angew. Chem., 1928, 41, 615 Search PubMed; (b) J. Götze, Ber. Dtsch. Chem. Ges., 1938, 71, 2289 CrossRef; (c) C. Reichardt, et al. , Chem. Ber., 1990, 123, 565 CrossRef CAS; (d) C. Reichardt, et al. , Liebigs Ann. Chem., 1995, 329 CrossRef CAS ; further notable reports on chiral cationic dyes: ; (e) P. Yan, A. C. Millard, M. Wei and L. M. Loew, J. Am. Chem. Soc., 2006, 128, 11030 CrossRef CAS PubMed; (f) T. Bříza, Z. Kejík, P. Vašek, J. Králová, P. Martásek, I. Císařová and V. Král, Org. Lett., 2005, 7, 3661 CrossRef PubMed; (g) N. Berova, D. Gargiulo, F. Derguini, K. Nakanishi and N. Harada, J. Am. Chem. Soc., 1993, 115, 4769 CrossRef CAS; (h) V. Buss and C. Reichardt, Chem. Commun., 1992, 1636 RSC; (i) V. Buss, S. Falzewski and K. Kolster, J. Org. Chem., 1999, 64, 1071 CrossRef CAS; (j) B. R. Renikuntla and B. A. Armitage, Langmuir, 2005, 21, 5362 CrossRef CAS PubMed; (k) S. Sergeyev, et al. , Chem. – Eur. J., 2010, 16, 8181 CrossRef CAS PubMed; (l) C.-X. Yuan, et al. , J. Phys. Chem. C, 2007, 111, 12811 CrossRef CAS; (m) J.-D. Compain, et al. , Inorg. Chem., 2009, 48, 6222 CrossRef CAS PubMed; (n) B. J. Coe, E. C. Harper, K. Clays and E. Franz, Dyes Pigm., 2010, 87, 22 CrossRef CAS PubMed.
- Recent reviews on the synthesis and applications of helicenes and their congeners: (a) M. Gingras, Chem. Soc. Rev., 2013, 42, 1051 RSC; (b) Y. Shen and C.-F. Chen, Chem. Rev., 2012, 112, 1463 CrossRef CAS PubMed; (c) I. G. Stará and I. Starý, in Science of Synthesis, ed. J. S. Siegel and Y. Tobe, Thieme, Stuttgart, 2010, vol. 45b, pp. 885–953 Search PubMed; (d) A. Rajca and M. Miyasaka, in Functional Organic Materials, ed. T. J. J. Müller and U. H. F. Bunz, Wiley-VCH, Weinheim, 2007, p. 547 Search PubMed; (e) A. Urbano, Angew. Chem., Int. Ed., 2003, 42, 3986 CrossRef CAS PubMed; (f) A. Urbano and M. C. Carreno, Org. Biomol. Chem., 2013, 11, 699 RSC; (g) N. Saleh, C. Shen and J. Crassous, Chem. Sci., 2014, 5, 3680 RSC; (h) J. Bosson, J. Gouin and J. Lacour, Chem. Soc. Rev., 2014, 43, 2824 RSC ; recent remarkable report: ; (i) Y. Yang, R. C. da Costa, M. J. Fuchter and A. J. Campbell, Nat. Photonics, 2013, 7, 634 CrossRef CAS.
- (a) F. Torricelli, J. Bosson, C. Besnard, M. Chekini, T. Bürgi and J. Lacour, Angew. Chem., Int. Ed., 2013, 52, 1796 CrossRef CAS PubMed; (b) C. Herse, D. Bas, F. C. Krebs, T. Bürgi, J. Weber, T. Wesolowski, B. W. Laursen and J. Lacour, Angew. Chem., Int. Ed., 2003, 42, 3162 CrossRef CAS PubMed; (c) J. Gouin, T. Bürgi, L. Guénée and J. Lacour, Org. Lett., 2014, 16, 3800 CrossRef CAS PubMed.
- Racemic Arai's dyes have been mentioned only in a review and synthetic procedures and other details have not been published. See: K. Sato and S. Arai, in Cyclophane Chemistry for the 21st Century, ed. H. Takemura, Research Signpost, Trivandrum, 2002, p. 173 (see p. 192) Search PubMed.
- (a) L. Adriaenssens, et al. , Chem. – Eur. J., 2009, 15, 1072 CrossRef CAS PubMed; (b) L. Pospíšil, et al. , J. Am. Chem. Soc., 2014, 136, 10826 CrossRef PubMed; (c) J. Vávra, et al. , Eur. J. Org. Chem., 2012, 489 CrossRef.
- For an illustrative application example of a parent, non-functionalized [7]helquat, see: D. Balogh, et al. , Nano Lett., 2012, 12, 5835 CrossRef CAS PubMed.
- Initial reports: (a) W. König, J. Prakt. Chem., 1912, 86, 166 CrossRef; (b) H. Barbier, Bull. Soc. Chim. Fr., 1920, 27, 427 CAS; (c) W. König and O. Treichel, J. Prakt. Chem., 1921, 102, 63 CrossRef ; for reviews, see: ; (d) G. Jones, Org. React., 1967, 15, 204–699 CAS ; and ref. 1c,d; recent report: ; (e) B. J. Coe, et al. , J. Am. Chem. Soc., 2010, 132, 10498 CrossRef CAS PubMed.
- All dyes in our report can be generally classified as cationic styryl dyes sometimes also called stilbazolium dyes when a single vinyl group links the phenyl and pyridinium rings. Helquat dyes 6a, 6c, 6e, 3a, 3i, 3j, 7a, 7i, and 7j also belong to hemicyanines (specifically phenylogous hemicyanines). Detailed classification of cationic dyes: ref. 1a.
- (a) W. H. Mills and W. J. Pope, J. Chem. Soc., 1922, 121, 946 RSC; (b) T. Tani, Photographic Science, OUP, Oxford, 2011 Search PubMed.
- November 2012 marks the centennial of the pioneering hemicyanine dye synthesis by Knoevenagel condensation reported by a famous dye chemist Walter König from Dresden Technical University (compound 4a, Scheme 1d).10a It is of note that this landmark report is rarely acknowledged in the literature and synthesis of pinaflavol12a (5a, Scheme 1d) is frequently cited instead.1d,10d.
- Despite the excellent track record of this transformation, only the Arai group has used it for diversification of cationic helicene systems. However, synthetic details for Arai’s monocationic azonia[6]helicene dyes have not been reported7 and potential of these compounds in enantiopure series has never been realized.
- [2+2+2] Cycloaddition to access helical scaffolds was originally developed by Starý and Stará for non-ionic helical systems. For the pioneering report, see: I. G. Stará, I. Starý, A. Kollárovič, F. Teplý, D. Šaman and M. Tichý, J. Org. Chem., 1998, 63, 4046 CrossRef.
- J. Vávra, et al. , J. Org. Chem., 2013, 78, 1329 CrossRef PubMed.
- Enantiocomposition analysis of non-functionalized helquats by CE: (a) ref. 8c,16, and ; (b) D. Koval, et al. , Electrophoresis, 2011, 32, 2683 CrossRef CAS PubMed; (c) L. Severa, et al. , New J. Chem., 2010, 34, 1063 RSC.
- Minimal loss of stereointegrity of enantiopure dye samples under study occurred when handling the dye solutions at 25 °C over a few hours. For example, racemization of (−)-(P)-7a has a free energy barrier (ΔG≠) of 116 kJ mol−1 at 66 °C corresponding to a racemization half-life of 9 h. The olefin moieties in the dyes in this study were found to be (E)-configured as evidenced by X-ray crystal structures (see the structures in Table 1, inset in Scheme 4, and Section S7, ESI†). See Section S4 (ESI†) for details.
- Reviews on the use of exciton coupled circular dichroism (ECCD): (a) Comprehensive Chiroptical Spectroscopy, ed. N. Berova, P. L. Polavarapu, K. Nakanishi and R. W. Woody, Wiley, Hoboken, NJ, 2012, vol. 1 and 2 Search PubMed; (b) G. Pescitelli, L. Di Bari and N. Berova, Chem. Soc. Rev., 2011, 40, 4603 RSC and references therein.
- Recent selected examples: (a) D. Mendola, et al. , Angew. Chem., Int. Ed., 2014, 53, 5786 CrossRef CAS PubMed; (b) J. Roose, et al. , Eur. J. Org. Chem., 2013, 3223 CrossRef CAS; (c) M. Miyasaka, et al. , Angew. Chem., Int. Ed., 2009, 48, 5954 CrossRef CAS PubMed ; theoretical study: ; (d) Y. Nakai, et al. , J. Phys. Chem. A, 2012, 116, 7372 CrossRef CAS PubMed; (e) Y. Kimura, et al. , Angew. Chem., Int. Ed., 2014, 53, 8480 CrossRef CAS PubMed ; chiroptical properties of non-ionic vinylcarbo[6]helicene derivatives: ; (f) E. Anger, et al. , J. Am. Chem. Soc., 2012, 134, 15628 CrossRef CAS PubMed , and ; (g) M. El Sayed Moussa, et al. , Chirality, 2013, 25, 455 CrossRef PubMed.
- (a) For rare examples of helicenoids with significant ECD response in the visible region, see: ref. 20f; (b) ref. 6a, 8b ; (c) A. Latorre, A. Urbano and M. C. Carreno, Chem. Commun., 2011, 47, 8103 RSC.
- Related porphyrinoid systems: (a) A. Werner, et al. , Angew. Chem., Int. Ed., 1999, 38, 3650 CrossRef CAS; (b) H. S. Rzepa, Org. Lett., 2009, 11, 3088 CrossRef CAS PubMed; (c) S. Yamaguchi, et al. , J. Am. Chem. Soc., 2008, 130, 14440 CrossRef CAS PubMed ; related (hemi)cyanine systems: ; (d) ref. 4e–g .
- For comparison of (M)-configured dye pairs (−)-(M)-3a/(+)-(M)-7a, (+)-(M)-3i/(+)-(M)-7i, and (+)-(M)-3j/(+)-(M)-7j, see Section S5 (ESI†).
- ECD spectra simulations at various levels of theory: Section S6 (ESI†).
- For a related non-cationic pH-responsive system, see: (a) S. E. Boiadjiev and D. A. Lightner, Tetrahedron: Asymmetry, 1996, 7, 2825 CrossRef CAS ; further examples with pH–switchable ECD response: ; (b) W. Lu, et al. , J. Phys. Chem. A, 2014, 118, 283 CrossRef CAS PubMed; (c) R. Lin, et al. , Chem. – Eur. J., 2011, 17, 2420 CrossRef CAS PubMed ; for recent example of acid–base chiroptical switching in an osmium complex with a [6]helicene ligand, see: ; (d) E. Anger, M. Srebro, N. Vanthuyne, C. Roussel, L. Toupet, J. Autschbach, R. Réau and J. Crassous, Chem. Commun., 2014, 50, 2854 RSC ; see also references added in proof: ; (e) N. Saleh, B. Moore II, M. Srebro, N. Vanthuyne, L. Toupet, J. A. G. Williams, C. Roussel, K. K. Deol, G. Muller, J. Autschbach and J. Crassous, Chem. – Eur. J. DOI:10.1002/chem.201405176; (f) A. Wallabregue, P. Sherin, J. Guin, C. Besnard, E. Vauthey and J. Lacour, Eur. J. Org. Chem., 2014, 6431 CrossRef CAS.
- Reviews on chiroptical switches: (a) J. W. Canary, Chem. Soc. Rev., 2009, 38, 747 RSC; (b) Z. Dai, J. Lee and W. Zhang, Molecules, 2012, 17, 1247 CrossRef CAS PubMed; (c) ref. 19a and references therein.
- To the best of our knowledge, the only pH-triggered ECD switching in a helicenoid has been reported in a recent work on an organometallic osmium-helicene complex,25d having the main pH-switchable band located in the UV region. See also ref. 25e added in proof.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental and theoretical procedures, synthesis, spectroscopic, and crystallographic data. CCDC 925828–925831, 1004339–1004345, 1004883, 1004884, 1008162. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4cc08967g |
| This journal is © The Royal Society of Chemistry 2015 |