Wide-range thermochromic luminescence of organoboronium complexes†
Xiaofeng
Chen
,
Xuepeng
Zhang
and
Guoqing
Zhang
*
CAS Key Laboratory of Soft Matter Chemistry, Department of Polymer Science and Engineering, University of Science and Technology of China, 96 Jinzhai Road, Hefei, 230026, China. E-mail: gzhang@ustc.edu.cn; Fax: +86 551 3606607; Tel: +86 551 63606607
First published on 5th November 2014
Abstract
Alkyl-substituted tetra-coordinate organoboronium bisdiketone complexes exhibit dramatic luminescence thermochromism in organic solvents. In glass-forming alcohols, these complexes exhibit a reversible aqua blue to orange-red to greenish yellow luminescence emission colour change upon cooling.
Many luminescent materials possess thermochromic emissions, i.e., quantitative intensity, lifetime, and/or colour changes within specified temperature ranges.1 Most commonly, fluorescence intensity or lifetime changes vs. temperature are caused by the competition between radiative (i.e., fluorescence) and non-radiative (i.e., collisional or thermal) decay pathways. Therefore, luminescent materials usually exhibit reduced intensity and shortened lifetimes at high temperatures. Meanwhile, since the dielectric properties of media, which play an important role in dictating dipole–dipole interactions, may also be temperature-dependent, polarity-sensitive fluorophores (“solvatochromic” dyes2,3) could also exhibit luminescence thermochromism.4 For example, upon cooling, red-shifted emission is typically expected due to better stabilization of excited-state dyes by polar media at lower temperatures.5 Apart from dielectric changes, solvent-viscosity effects induced by temperature can also exert an influence on emitting characteristics of fluorophores, leading to colour and/or intensity changes.6 This well-studied process is often referred to as the “rigidochromic” effect,7 where blue-shifted emissions are typically observed in viscous media due to the reduced mobility of solvent molecules (diminished stabilization of ground and/or excited states). Such dyes are most desirable in the polymer industry to monitor polymerization processes in situ.8 Irrespective of the mechanism, thermochromic luminescent materials can be widely exploited as temperature sensors owing to their intrinsic advantages such as non-invasiveness, high sensitivity, and real-time capability.9 In recent years, along with advances in synthetic and materials chemistry, luminescent temperature sensors have also evolved from mere “optical thermometers” earlier on to multifunctional, stimuli-responsive nano-devices which can also carry out sophisticated tasks in the biological context.10
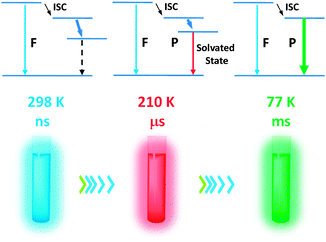
Given the importance of luminescence temperature sensing, here we report an unusual mechanism for luminescence thermochromism of organoboron complexes,11–22 which relies on the emergence and rigidochromism of environment-sensitive phosphorescence to show a wild colour change at different temperatures. Tetra-coordinate organoboronium β-diketonates (OBDKs), which were previously found to exhibit hydrochromic fluorescence,23 also show distinct luminescence emission colour changes over a wide range of temperatures (25 to −196 °C). It was also found that the emission colour changes are highly solvent dependent: in strongly polar solvents such as a methanol/ethanol mixture, the luminescence first becomes orange-red and then green when the temperature drops; in moderately polar solvents such as CH2Cl2, the blue luminescence becomes yellow and then red upon cooling. Spectroscopy and lifetime studies reveal that there is only blue fluorescence at room temperature (with ns lifetimes), but the colour change is due to the emergence of a lower-energy phosphorescence band, which emits red light (with μs lifetimes) under fluidic conditions due to solvent stabilisation of the excited state and emits green light (with ms lifetimes) in rigid frozen solvents (Scheme 1).
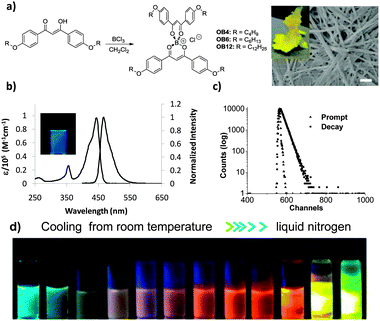
The synthesis of these alkyl-chain substituted OBDKs (OB4–OB12) is straight-forward: the corresponding β-diketones react readily with BCl3 in CH2Cl2 at room temperature and the products are obtained with high yields as bright-yellow, fibrous solids which also form paper-like textures after drying (Fig. 1a and Fig. S1, ESI†). These boronium compounds, which possess extremely polar cores and bear non-polar exteriors, exhibit solvent-dependent solubility. Complexes OB4 and OB6 are soluble in most moderately (e.g., CH2Cl2 and CHCl3) to very (e.g., CH3OH) polar organic solvents at room temperature. OB12 is soluble in CH2Cl2 but forms macroscopic particles in alcohols. OB18 (not shown) is only dissolvable at much elevated temperatures and is thus excluded from the current study. The boronium compounds OB4–OB12 were characterized by 1H-NMR and high-resolution mass spectrometry (Fig. SA–F, ESI†). In solution, tetra-coordinated boronium-diketone complexes exhibit extremely high extinction coefficients at absorption maxima (e.g., OB4 in CH2Cl2, λmax = 444 nm; ε ∼ 1.0 × 105 M−1 cm−1, Fig. 1b), indicating a very strong π–π* process.23 The π–π* transition also generates fairly intense fluorescence in the blue-light region which is even visible under ambient light conditions (e.g., OB4 in CH2Cl2, λF = 467 nm; τF = 1.85 ns; ΦF = 0.72, Fig. 1c). As expected, the length of alkyl chains exerts no influence on the electronic transitions of these complexes in dilute solution, since all three complexes (OB4, OB6, and OB12) share essentially the same absorption and fluorescence emission spectra (Fig. S2, ESI†).
The dramatic luminescence emission colour change of OBDKs with temperature (Fig. 1d) was discovered by serendipity when we tried to collect their emission spectra at −196 °C in methanol/ethanol glass when the cooling process was monitored. As can be readily observed by the naked eye, OB4 (OB6 is similar) in a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 methanol/ethanol mixture exhibits aqua-blue-coloured emission under UV irradiation (λex = 365 nm) at room temperature; however, as the temperature starts to decrease, the emission colour starts to turn white, indicating increased contribution in the red region. Although the intensity is severely quenched at the beginning of the cooling process, it increases quickly as temperature decreases even further. Gradually, an orange-red colour replaces white until the solution forms a clear glass, where the emission suddenly becomes yellow-green. The emission spectra of OB4 and OB6 in a 4
1 methanol/ethanol mixture exhibits aqua-blue-coloured emission under UV irradiation (λex = 365 nm) at room temperature; however, as the temperature starts to decrease, the emission colour starts to turn white, indicating increased contribution in the red region. Although the intensity is severely quenched at the beginning of the cooling process, it increases quickly as temperature decreases even further. Gradually, an orange-red colour replaces white until the solution forms a clear glass, where the emission suddenly becomes yellow-green. The emission spectra of OB4 and OB6 in a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
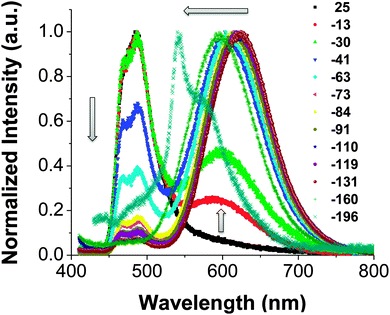
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 methanol/ethanol mixture at different temperatures were recorded. Considering the inconsistency of temperature readings obtained using digital thermometers of different manufacturers, we therefore prepared cooling baths from various organic solvent–liquid nitrogen mixtures of known temperatures (Table S1, ESI†). As shown in Fig. 2, the emission spectra of OB4 were directly collected through an optical fiber attached to a diode-array fluorospectrometer (signal amplification was not necessary due to high emission intensity). At room temperature, the sample exhibits a single fluorescence band at ∼480 nm. Consistent with visual observations, the emission spectra can clearly capture the appearance and successive weighing-in of a second, much more red-shifted emission band at ∼620 nm (broad), when the temperature gradually decreases, in addition to the band at 480 nm. When the cooling bath temperature is below −70 °C, the emission spectrum is overwhelmed by the second red band and the relative intensity of the first aqua blue band appears to be insignificant. When the solution forms a clear and rigid glass, a major emission band is peaked at 542 nm with a vibronic shoulder at 589 nm. The separation of the two peaks is by 1400–1500 cm−1, which could be caused by C
1 methanol/ethanol mixture at different temperatures were recorded. Considering the inconsistency of temperature readings obtained using digital thermometers of different manufacturers, we therefore prepared cooling baths from various organic solvent–liquid nitrogen mixtures of known temperatures (Table S1, ESI†). As shown in Fig. 2, the emission spectra of OB4 were directly collected through an optical fiber attached to a diode-array fluorospectrometer (signal amplification was not necessary due to high emission intensity). At room temperature, the sample exhibits a single fluorescence band at ∼480 nm. Consistent with visual observations, the emission spectra can clearly capture the appearance and successive weighing-in of a second, much more red-shifted emission band at ∼620 nm (broad), when the temperature gradually decreases, in addition to the band at 480 nm. When the cooling bath temperature is below −70 °C, the emission spectrum is overwhelmed by the second red band and the relative intensity of the first aqua blue band appears to be insignificant. When the solution forms a clear and rigid glass, a major emission band is peaked at 542 nm with a vibronic shoulder at 589 nm. The separation of the two peaks is by 1400–1500 cm−1, which could be caused by C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibrations of the benzene ring, and is indicative of the π–π* nature of the emission.24 The green emission in rigid alcohol glass is very likely due to the blue shift of the 620 nm emission under fluid conditions, since a clear trend of gradual blue shifts is evident along with the cooling process. An almost same luminescence thermochromism behavior can be observed for OB6 (Fig. S3, ESI†).
C vibrations of the benzene ring, and is indicative of the π–π* nature of the emission.24 The green emission in rigid alcohol glass is very likely due to the blue shift of the 620 nm emission under fluid conditions, since a clear trend of gradual blue shifts is evident along with the cooling process. An almost same luminescence thermochromism behavior can be observed for OB6 (Fig. S3, ESI†).
It is not unusual for highly polar fluorophores to exhibit temperature-dependent charge-transfer fluorescence due to changes associated with solvent dielectric properties with temperature.25 In such circumstances, the luminescence emission maxima normally show a steady red-shift as a function of temperature, which can be predicted by the Lippert–Mataga equation.26,27 For OBDKs however, it is noted that an additional peak at a much lower energy region (λ ∼ 620 nm) starts to weigh in as temperature drops, switching the emission colour from aqua blue to orange-red. This bimodal distribution of luminescence could be due to excimer-like emission, as has been noted in difluoroboron diketone systems28 or for platinum diketone materials.29,30 However, an excimer is not likely since it is diffusion-controlled and does not form efficiently at high viscosity (low temperature).31 As a result, the emergence of phosphorescence is highly plausible for the observed luminescence thermochromism since low temperatures are favored conditions for phosphorescence. To confirm that phosphorescence is indeed the lower-energy band, we videotaped the emission spectral changes using a diode-array spectrometer at a 100 ms interval (Fig. S4, ESI†). Firstly, at −196 °C, the emission band at 542 nm is apparently phosphorescence since it has a lifetime of 960 ms and a strong “after-glow” could be observed by the naked eye. Next, when warming the OB4 sample from −196 °C to room temperature, it is evident that the structured 542 nm band in rigid glass continuously evolves into the structureless 620 nm red emission band under fluidic conditions. As a result, the red emission is very likely to be phosphorescence as well. We performed a single point lifetime measurement at −80 °C and the measured lifetime at 620 nm is 0.21 μs. Finally, an increase in the relative intensity of the red band can also be detected at −20 °C after the solution is de-aerated in three freeze–pump–thaw cycles (Fig. S5, ESI†) since molecular oxygen is known to be a good triplet quencher.32 However, room temperature phosphorescence for this sample is not evident even when the solution has been thoroughly degassed, suggesting strong thermal quenching at elevated temperatures (Fig. S5, ESI†).
In summary, we have synthesized a series of organoboronium bisdiketonates (OBDKs) substituted with different alkyl chains. These boron materials are found to exhibit quite interesting thermochromic luminescence, where in 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 methanol/ethanol the emission colour shifts from aqua blue at room temperature to orange-red at lower temperatures and eventually to yellowish green when the solution forms a clear glass. The mechanism of temperature-sensitive emission is explained as the emergence of a phosphorescence band at lower energy. Under fluidic conditions, the phosphorescence is solvated and thus red-shifted; in the frozen glass matrix, however, the non-solvated green phosphorescence dominates the emission spectrum. These organoboronium complexes have potential in wide-range temperature sensing applications.
1 methanol/ethanol the emission colour shifts from aqua blue at room temperature to orange-red at lower temperatures and eventually to yellowish green when the solution forms a clear glass. The mechanism of temperature-sensitive emission is explained as the emergence of a phosphorescence band at lower energy. Under fluidic conditions, the phosphorescence is solvated and thus red-shifted; in the frozen glass matrix, however, the non-solvated green phosphorescence dominates the emission spectrum. These organoboronium complexes have potential in wide-range temperature sensing applications.
The work was supported by the Natural Science Foundation of China (21222405). We thank Mr Joshua D. Vaughn for taking the photos used in figures and Prof. J. N. Demas (University of Virginia) for helpful discussions.
Notes and references
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Springer Science, New York, USA, 4th edn, 2006, ch. 6 Search PubMed.
- C. Reichardt, Chem. Rev., 1994, 94, 2319–2358 CrossRef CAS.
- C. Pietsch, U. S. Schubert and R. Hoogenboom, Chem. Commun., 2011, 47, 8750–8765 RSC.
- W. Struve, P. Rentzepis and J. Jortner, J. Chem. Phys., 1973, 59, 5014–5019 CrossRef CAS PubMed.
- N. Mataga and Y. Murata, J. Am. Chem. Soc., 1969, 91, 3144–3152 CrossRef CAS.
- A. D. Kummer, C. Kompa, H. Niwa, T. Hirano, S. Kojima and M. E. Michel-Beyerle, J. Phys. Chem. B, 2002, 106, 7554–7559 CrossRef CAS.
- B. Dunn and J. I. Zink, J. Mater. Chem., 1991, 1, 903–913 RSC.
- P. Bosch, F. Catalina, T. Corrales and C. Peinado, Chem. – Eur. J., 2005, 11, 4314–4325 CrossRef CAS PubMed.
- Y. Luo, P. Wu, J. Hu, S. He, X. Hou and K. Xu, Anal. Methods, 2012, 4, 1310–1314 RSC.
- L. Yin, C. He, C. Huang, W. Zhu, X. Wang, Y. Xu and X. Qian, Chem. Commun., 2012, 48, 4486–4488 RSC.
- P. Chen and F. Jäkle, J. Am. Chem. Soc., 2011, 133, 20142–20145 CrossRef CAS PubMed.
- K. Parab, K. Venkatasubbaiah and F. Jäkle, J. Am. Chem. Soc., 2006, 128, 12879–12885 CrossRef CAS PubMed.
- Y. Qin, I. Kiburu, S. Shah and F. Jäkle, Macromolecules, 2006, 39, 9041–9048 CrossRef CAS.
- Y. Qin, C. Pagba, P. Piotrowiak and F. Jäkle, J. Am. Chem. Soc., 2004, 126, 7015–7018 CrossRef CAS PubMed.
- Y. Qin, I. Kiburu, S. Shah and F. Jäkle, Org. Lett., 2006, 8, 5227–5230 CrossRef CAS PubMed.
- Y. Nagata, H. Otaka and Y. Chujo, Macromolecules, 2008, 41, 737–740 CrossRef CAS.
- A. Nagai, J. Miyake, K. Kokado, Y. Nagata and Y. Chujo, J. Am. Chem. Soc., 2008, 130, 15276–15278 CrossRef CAS PubMed.
- Y. Nagata and Y. Chujo, Macromolecules, 2008, 41, 2809–2813 CrossRef CAS.
- A. Nagai, K. Kokado, Y. Nagata and Y. Chujo, Macromolecules, 2008, 41, 8295–8298 CrossRef CAS.
- G. Zhang, J. Chen, S. J. Payne, S. E. Kooi, J. Demas and C. L. Fraser, J. Am. Chem. Soc., 2007, 129, 8942–8943 CrossRef CAS PubMed.
- G. Zhang, J. Lu, M. Sabat and C. L. Fraser, J. Am. Chem. Soc., 2010, 132, 2160–2162 CrossRef CAS PubMed.
- S. Xu, R. E. Evans, T. Liu, G. Zhang, J. Demas, C. O. Trindle and C. L. Fraser, Inorg. Chem., 2013, 52, 3597–3610 CrossRef CAS PubMed.
- X. Zhang and G. Zhang, Anal. Methods, 2012, 4, 2641–2643 RSC.
- E. A. Chandross, J. Ferguson and E. McRae, J. Chem. Phys., 1966, 45, 3546–3553 CrossRef CAS PubMed.
- M. Goes, M. de Groot, M. Koeberg, J. W. Verhoeven, N. R. Lokan, M. J. Shephard and M. N. Paddon-Row, J. Phys. Chem. A, 2002, 106, 2129–2134 CrossRef CAS.
- S. Kong, L. Xiao, Z. Chen, X. Yan, B. Qu, S. Wang and Q. Gong, New J. Chem., 2010, 34, 325–330 RSC.
- H.-H. Lin, Y.-C. Chan, J.-W. Chen and C.-C. Chang, J. Mater. Chem., 2011, 21, 3170–3177 RSC.
- X. Sun, X. Zhang, X. Li, S. Liu and G. Zhang, J. Mater. Chem., 2012, 22, 17332–17339 RSC.
- L. Deng, P. T. Furuta, S. Garon, J. Li, D. Kavulak, M. E. Thompson and J. M. Fréchet, Chem. Mater., 2006, 18, 386–395 CrossRef CAS.
- J.-Y. Cho, B. Domercq, S. Barlow, K. Y. Suponitsky, J. Li, T. V. Timofeeva, S. C. Jones, L. E. Hayden, A. Kimyonok and C. R. South, Organometallics, 2007, 26, 4816–4829 CrossRef CAS.
- S. Pispas, Soft Matter, 2011, 7, 474–482 RSC.
- G. Zhang, G. M. Palmer, M. W. Dewhirst and C. L. Fraser, Nat. Mater., 2009, 8, 747–751 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and all relevant characterization data. See DOI: 10.1039/c4cc08289c |
| This journal is © The Royal Society of Chemistry 2015 |