Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Superconductivity in Sm-doped [n]phenacenes (n = 3, 4, 5)†
Gianluca A.
Artioli
a,
Franziska
Hammerath‡
b,
Maria Cristina
Mozzati
b,
Pietro
Carretta
b,
Federica
Corana
c,
Barbara
Mannucci
c,
Serena
Margadonna
d and
Lorenzo
Malavasi
*a
aDepartment of Chemistry and INSTM, University of Pavia, Pavia 27100, Italy. E-mail: lorenzo.malavasi@unipv.it
bDepartment of Physics and CNISM, University of Pavia, Pavia 27100, Italy
cCentro Grandi Strumenti, University of Pavia, Pavia 27100, Italy
dDepartment of Chemistry, University of Oslo, Oslo 0371, Norway
First published on 25th November 2014
Abstract
We report here the discovery of a new aromatic hydrocarbon superconductor, Sm-doped chrysene, with Tc ∼ 5 K, and compare its behavior with those measured in the full series of Sm-doped [n]phenacene superconductors, with n = 3, 4, 5, thus determining the trend of Tc as a function of the number of fused benzene rings and for an odd or even number of units.
Research activity in the field of superconductivity has moved in the last few years towards materials containing light elements in order to have cheap, lightweight and versatile superconductors with respect to the existing materials. In particular, carbon-based superconductors have attracted significant interest, which has been strongly revitalized by the discovery of a superconducting transition temperature, Tc, of ∼18 K in K-doped picene, i.e. a hydrocarbon consisting of five fused benzene rings arranged in a zigzag manner.1 Picene is the n = 5 member of the [n]phenacenes class of polycyclic aromatic hydrocarbons (PAHs). To date, only a few other hydrocarbon systems have shown a superconducting behavior: alkali-metal-doped phenanthrene,2 with a Tc of about 5 K, Sr1.5phenanthrene and Ba1.5phenanthrene with Tc of 5.6 and 5.4 K, respectively,3 K-doped coronene with Tc around 7 K,4 and K-doped 1,2:8,9-dibenzopentacene (Tcca. 30 K).5 Finally, in 2012, superconductivity was induced by rare-earth doping in phenanthrene, with the Tc approaching 6 K for La and Sm intercalated compounds.6 Together with these few experimental reports, a rich literature on the theoretical study of different PAHs also appeared in recent times.7–10 Despite the absence of reliable experimental data to date, the theoretical models available suggest that electron–electron correlations play an important role in doped PAHs and that superconductivity in these systems cannot be explained by considering only the electron–phonon interactions.11
We have recently reviewed12 the actual status of the research on polycyclic aromatic hydrocarbon superconductors pointing out the very uncommon situation where two research groups (i.e., those that discovered the different superconducting phenacenes) are the only research groups capable of producing such samples, in spite of the rather simple synthetic procedures, namely solid state reactions between the metal and the organic materials sealed under vacuum.
In this communication we present a new PAH superconductor, Sm1chrysene, together with the first systematic investigation of Sm-doped [n]phenacenes (n = 3, 4, 5), corresponding to phenanthrene, chrysene and picene, thus confirming the presence of a superconducting fraction in Sm1phenanthrene and in a new doped picene system (Sm1picene).
The samples have been synthesized by means of solid state reactions between the Sm metal (NewMet, 99.9% – grinded to fine powder in a glove box) and the organic substrates. All manipulations were carried out in an argon-filled glove box. Phenanthrene and chrysene were purchased from Aldrich (>98%) and purified by sublimation prior to use, and dried and grinded to fine powder, while picene was prepared according to a new synthetic route we recently developed.16 The reactions were conducted in quartz tubes sealed under high vacuum (ca. 10−5 Pa). The reaction temperature was 240 °C for phenanthrene, 330 °C for chrysene and 420 °C for picene. Samples were left in the furnace for about 96 h. After the reactions, all the samples became black and all the successive manipulations and measurements were carried out with the samples sealed under vacuum.
The possible presence of a superconducting state in the Sm-doped phenacenes studied here has been investigated by means of SQUID magnetization measurements in magnetic fields of a few Oersteds, both in zero-field cool (ZFC) and field-cool (FC) protocols.
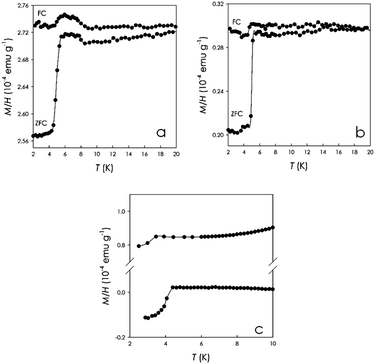
Fig. 1(a)–(c) present the SQUID measurements at 6 Oe on three distinct Sm1phenanthrene sample batches.
As can be seen from Fig. 1(a) and (b), the susceptibility of the samples displays a clear drop at around 5.0–5.2 K (taken at the onset) in the ZFC curves. For the sample shown in Fig. 1(c) (upper curve) the drop is less pronounced and falls at a slightly lower temperature. Finally, the curve presented in the lower part of Fig. 1(c) shows the result obtained for the same sample whose curve is shown in the upper part of the panel but after pressing the sample by means of a laboratory hydrostatic press. In this case, as can be seen from the plot, the M/H curve displays a diamagnetic signal with a Tc around 4.4 K. The drop in the M/H vs. T curves shown in Fig. 1(a) and (b) is a characteristic feature of a potentially superconducting material with Tc ∼ 5 K; however, a clear diamagnetic signal is not always observed in the samples of Sm1phenanthrene reported in Fig. 1, in spite of the same reliable preparation route, suggesting that the shielding fractions are relatively small. It should be stressed that, for all the PAH superconductors reported to date, it has been observed that the shielding fractions are very low with suggested bulk superconductivity coming from the increase of these shielding fractions upon pressing the powdered samples.1,2 It has been discussed and proposed that these small shielding fractions in powder samples could arise from the penetration of the magnetic field into the superconducting phase because of the smaller size of crystallites than the London penetration depth.2 The few cases where very high shielding fractions were observed have been the object of paper retraction (see ref. 13 and 14) or subject to some criticism due to the presence of the spurious metallic La phase, which is known to be a superconductor with a Tc of about 6 K. In this work, the choice of using Sm has been motivated by the fact that Sm is not superconducting and, accordingly, one can avoid a misleading interpretation of the results. However, since La is often present as an impurity in other rare-earths, we checked by SQUID magnetometry measurements that the starting Sm rod used to synthesize the Sm-doped phenacene did not show any trace of superconductivity (Fig. S1, ESI†).
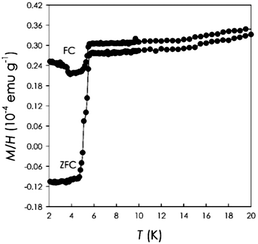
The next series of Sm-phenacenes investigated was Sm1chrysene. Chrysene is the n = 4 member of the phenacene family and in the current literature there are no reports regarding doped chrysene. Fig. 2 shows a representative susceptibility curve for Sm1chrysene at 6 Oe.
It is possible to observe a clear superconducting transition with a Tc around 5.4 K yielding a diamagnetic signal at low temperature, with a shielding fraction approaching 1%, in agreement with the data on doped picene and phenanthrene.1,2
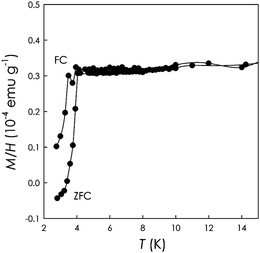
Finally, we prepared and measured Sm1picene. Fig. 3 shows the FC/ZFC measurement performed on Sm1picene at 6 Oe.
The Sm1picene has a sharp superconducting transition at around 4 K with a magnetic susceptibility at 2 K of about −0.05 × 10−4 emu g−1 which is not very far from the value of −0.2 × 10−4 emu g−1 found for the 7 K superconducting phase of K3picene.1
We carried out XRD measurements on the new organic superconductor synthesized, i.e. Sm1chrysene, by sealing the sample under vacuum within a capillary. The result of the XRD investigation (Cu-radiation) is presented in Fig. 4. The XRD pattern of the doped sample corresponds quite nicely with that of pristine chrysene. Vertical red lines refer to a monoclinic unit cell based on that of pure chrysene (i.e., space group I2/c, a = 8.341 Å, b = 6.18 Å, c = 25.05 Å, β = 116.2°). The lattice parameters determined are a = 8.3860(5) Å, b = 6.2610(4) Å, c = 25.401(1) Å, β = 116.4(3)°. With respect to pure chrysene, there is a slight expansion of the unit cell in the three directions with a cell volume of 299.616 Å3 of the doped sample with respect to 293.750 Å3 of the pristine chrysene (i.e., ca. +2%). Some minor impurities of SmH2 (estimated to be below 5%) have also been detected in the diffraction pattern (marked with asterisks).
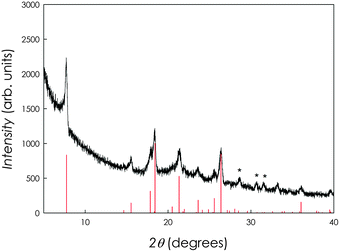 | ||
| Fig. 4 XRD pattern for the Sm1chrysene sample. Vertical red bars refer to the monoclinic unit cell relative to the pristine chrysene. | ||
Such impurities could not be completely removed by additional thermal treatments. Moreover, prolonged and further thermal treatments lead to sample decomposition. It should be stressed, however, that the diffraction analysis of these new organic superconductors is not simple because of the common formation of impurities and/or amorphous phases, as also reported, for example, for K3picene.1 Still, one can conclude that our structural results show an expansion of the Sm-doped chrysene which is compatible with metal doping within the organic substrate unit cell, in contrast with K3picene where the doped samples show a contraction of the pristine organic material unit cell,1 but in agreement with the structural data on rare-earth-doped phenanthrene.4 One can further notice that our structural results show a trend analogous to the one observed in the corresponding acene materials (i.e., pentancene is a 5-ring member of the acene family while picene is the 5-ring member of the phenacene family), as well as with the theoretical calculations.15,16 Finally, the amount of spurious phases was very low and in some cases (as in Sm1phenanthrene) no impurities were detected, suggesting that nominal and experimental stoichiometries could be close, even though a strict control on the experimental stoichiometries cannot be provided at this stage.
To summarize, in this work we prepared Sm-doped [n]phenacenes (n = 3, 4, 5) with Sm which was selected because it can provide the requested electron doping with a low metal doping, thus – in principle – making the sample preparation easier. In addition, Sm is not superconducting thus avoiding a misinterpretation of the susceptibility measurements. The investigation of the Sm-doped [n]phenacene family with n = 3, 4, 5 indicated that superconductivity is actually found in all the samples. In this work we reported, for the first time, the evidence of a superconducting transition in doped chrysene (i.e., the n = 4 member of the phenacene family) as well as the first preparation of Sm-doped picene. In addition, the data on Sm1phenatrene are the first confirmation, together with the data reported by the research group that discovered superconductivity in this phase, that a superconducting transition is actually present in doped phenanthrene. Furthermore, our systematic study suggests that the critical temperatures observed for the series investigated here are very close to each other and around 4.5–5.5 K, with a slight decrease of Tc from 5.5 to 4.0 K passing from phenanthrene to picene. A Tc of 5 K for Sm1phenanthrene is in agreement with the data previously reported in the literature.6 For the other two samples, i.e. Sm1chrysene and Sm1picene, there are no literature data to be used for comparison. However, if one should only take into account the pure effect of electron doping, a higher Tc should be expected for Sm1picene on the basis of the results reported for K3picene.1 Clearly, other effects play a role as a function of the intercalating metal and thus a simple comparison with other data is not straightforward. Due to the same synthesis protocol and the most possible reproducible approach in the preparation of the samples reported here, the substantial invariance of the Tc by changing the number of aromatic rings seems to be solid within the framework of the present work. Such new results reported in this work open important questions regarding the mechanism of superconductivity in PAHs. Electron–phonon models predict a reduction of Tc with the increasing number of carbon atoms, as a consequence of the decrease of the electron–phonon interaction by increasing the number of carbon atoms in phenanthrene edge-type hydrocarbons17 while the models considering the electron correlation as the basis of the effective attraction between added electrons, predict a progressive increase of Tc.11 It is clear that making available, in this work, the first set of experimental data as a function of carbon atoms, will represent a very important playground for further modelling of the superconductivity mechanism in PAHs.
To conclude, in the present work we reported the evidence of superconductivity in Sm-doped [n]phenacenes (n = 3, 4, 5) with Tc's around 4.5–5.5 K with small differences by changing the number of carbon atoms. Shielding fractions are small but consistent with those reported for other doped PAH systems.1,2 The observation of very close Tc's as a function of the number of benzene rings, and for [n]phenacenes with an even and odd n-value for the same level of electron doping, raises new questions that open the way for further and new theoretical investigations on the electron pairing mechanism in PAHs.
We gratefully acknowledge financial support from the Cariplo Foundation through project 2013-0632.
Notes and references
- R. Mitsuhashi, Y. Suzuki, Y. Yamanari, H. Mitamura, T. Kambe, N. Ikeda, H. Okamoto, A. Fujiwara, M. Yamaji, N. Kawasaki, Y. Maniwa and Y. Kubozono, Nature, 2010, 464, 76 CrossRef CAS PubMed.
- X. Wang, R. H. Liu, Z. Gui, Y. L. Xie, Y. J. Yan, J. J. Ying, X. G. Luo and X. H. Chen, Nat. Commun., 2011, 2, 507 CrossRef CAS PubMed.
- X. F. Wang, Y. J. Yan, Z. Gui, R. H. Liu, J. J. Ying, X. G. Luo and X. H. Chen, Phys. Rev. B, 2011, 84, 214523 CrossRef.
- Y. Kubozono, H. Mitamura, X. Lee, X. He, Y. Yamanari, Y. Takahashi, Y. Suzuki, Y. Kaji, R. Eguchi, K. Akaike, T. Kambe, H. Okamoto, A. Fujiwara, T. Kato, T. Kosugi and H. Aoki, Phys. Chem. Chem. Phys., 2011, 13, 16476 RSC.
- M. Xue, T. Cao, D. Wang, Y. Wu, H. Yang, X. Dong, J. He, F. Li and G. F. Chen, Sci. Rep., 2012, 2, 389 Search PubMed.
- X. F. Wang, X. G. Luo, J. J. Ying, Z. J. Xiang, S. L. Zhang, R. R. Zhang, Y. H. Zhang, Y. J. Yan, A. F. Wang, P. Cheng, G. J. Ye and X. H. Chen, J. Phys.: Condens. Matter, 2012, 24, 345701 CrossRef CAS PubMed.
- S. Shahab and E. Tosatti, Phys. Rev. B, 2014, 90, 075143 CrossRef.
- F. Capitani, M. Hoppner, B. Joseph, L. Malavasi, G. A. Artioli, L. Baldassarre, A. Perucchi, M. Piccinini, S. Lupi, P. Dore, L. Boeri and P. Postorino, Phys. Rev. B, 2013, 88, 144303 CrossRef.
- J. A. Verges, P. L. de Andres, E. San-Fabian, G. Chiappe, E. Louis and A. Guijiarro, Phys. Rev. B, 2012, 85, 165102 CrossRef.
- G. Giovannetti and M. Capone, Phys. Rev. B, 2011, 83, 134508 CrossRef.
- Z. Huang, C. Zhang and H. Q. Kun, Sci. Rep., 2012, 2, 922 Search PubMed.
- G. A. Artioli and L. Malavasi, J. Mater. Chem. C, 2014, 2, 1577 RSC.
- J. Ying, X. Wang, Y. Yan, Z. Xiang, X. Luo, Z. Sun and X. Chen, Phys. Rev. B, 2013, 87, 179901 CrossRef.
- Y. Kasahara, Y. Takeuchi and Y. Iwasa, Phys. Rev. B, 2013, 87, 179902 CrossRef.
- P. L. de Andres, A. Guijarro and J. A. Verges, Phys. Rev. B, 2011, 84, 144501 CrossRef.
- Q.-W. Huang, G.-H. Zhong, J. Zhang, X.-M. Zhao, C. Zhang, H.-Q. Lin and Z.-J. Chen, J. Chem. Phys., 2014, 140, 114301 CrossRef PubMed.
- T. Kato, K. Yoshizawa and K. Hirao, J. Chem. Phys., 2002, 116, 3420 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: A susceptibility curve for the pure Sm metal used for the synthesis of samples and GC/MS spectra of organic starting materials. See DOI: 10.1039/c4cc07879a |
| ‡ Present address: Institut für Festkörperphysik, Technische Universität Dresden, 01069 Dresden, Germany. |
| This journal is © The Royal Society of Chemistry 2015 |