Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dendrimeric calcium-responsive MRI contrast agents with slow in vivo diffusion†
Serhat
Gündüz
ab,
Nobuhiro
Nitta
c,
Sandip
Vibhute
b,
Sayaka
Shibata
c,
Martin E.
Mayer
d,
Nikos K.
Logothetis
be,
Ichio
Aoki
c and
Goran
Angelovski
*a
aMR Neuroimaging Agents Group, Max Planck Institute for Biological Cybernetics, 72076 Tübingen, Germany. E-mail: goran.angelovski@tuebingen.mpg.de
bDepartment for Physiology of Cognitive Processes, Max Planck Institute for Biological Cybernetics, 72076 Tübingen, Germany
cMolecular Imaging Center, National Institute of Radiological Sciences (NIRS), 4-9-1 Anagawa, Inage-ku, Chiba 263-8555, Japan
dInstitute for Organic Chemistry, Faculty of Science, University of Tübingen, 72076 Tübingen, Germany
eDepartment of Imaging Science and Biomedical Engineering, University of Manchester, Manchester M13 9PT, UK
First published on 31st October 2014
Abstract
We report a methodology which enables the preparation of dendrimeric contrast agents sensitive to Ca2+ when starting from the monomeric analogue. The Ca-triggered longitudinal relaxivity response of these agents is not compromised by undertaking synthetic transformations, despite structural changes. The in vivo MRI studies in the rat cerebral cortex indicate that diffusion properties of dendrimeric contrast agents have great advantages as compared to their monomeric equivalents.
Smart or responsive contrast agents (SCAs) can have a significant role in magnetic resonance imaging (MRI). These are molecular sensors capable of altering their magnetic properties upon changing their local environment, thus indicating a modification in the physiology of the studied biological system. In turn, SCAs enable the assessment of biological processes in vivo at the cellular and molecular levels, with an outstanding spatial and temporal resolution.
A number of SCAs for the use in MRI have been developed so far.1–3 Nevertheless, their broader application is still pending, with only a few demonstrating their potential in vivo.4–6 In neuroimaging, SCAs enable a new functional MRI (fMRI) method that measures brain function to be developed.7,8 For this purpose, Ca2+ and various neurotransmitter molecules are the most preferred SCA targets due to their critical involvement in neuronal signaling.5,9
However, when using SCAs for fMRI, temporally resolved information within the range of seconds is necessary to successfully follow the dynamics of Ca2+ transients or neurotransmitter release. In such cases, the ability to compare the signal intensity (SI) from two types of scan series (with and without brain tissue stimulation) is crucial for the success of the fMRI experiment.5 Principally, one aims to have no or minimal SCA concentration change during the two consecutive experiments, so that the SI change may be associated only with the target concentration change. Otherwise, the inability to control the local SCA concentration may lead to ambiguous results. The recorded MRI signal in T1- and T2-weighted imaging depends on the agent concentration and relaxivity (r1 or r2 for T1- or T2-based SCA, respectively),2 as well as its background signal (the MR signal of tissue in the absence of an SCA). Therefore, the signal changes are usually a consequence of the SCA concentration change in a particular voxel rather than its relaxivity change, which prevents any conclusions on actual SCA activity. Few attempts to combine quantitative MRI techniques have been made to allow better control of the local SCA concentration.10–12
An option to control the SCA concentration and minimize its changes may be by affecting SCA diffusion properties. Our previous studies show that monomacrocyclic conventional contrast agents (Magnevist®, Dotarem®) or Ca-sensitive SCAs diffuse very fast into the rat cerebral cortex, unless Ca-induced aggregation and the resulting increase in the size of the agent causes slower diffusion.13–15 A fast SI decrease or aggregate formation complicates or completely prevents Ca-dependent fMRI, demanding the optimization of the SCA only beyond the r1 changes.
We have therefore sought to attach one of our very active Ca-sensitive SCAs to a dendrimer molecule in order to slow down its diffusion in vivo (Fig. 1). The necessary synthetic transformations should yield a molecule bigger in size (e.g. an increase in the molecular weight), although the responsive part of the SCA must be retained, as well as its Ca-sensitivity and MRI activity. Furthermore, such a molecule would enable the delivery of a high local concentration of active species (SCA monomeric units), which is crucial for observing maximal MRI signal changes.16
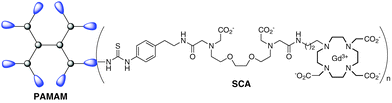 | ||
| Fig. 1 Dendrimeric smart contrast agent DSCA consisting of a PAMAM G1 dendrimer coupled to Gd-complexes of a DO3A-unit bearing an EGTA-derived Ca-chelator. | ||
For this purpose, we have prepared a reactive monomacrocyclic molecule that bears an ethylene glycol tetraacetic acid (EGTA)-derived Ca-chelator coupled to a 1,4,7,10-tetraazacyclododecane-1,4,7-tricarboxylic acid (DO3A) unit for Ca-sensing and reporting purposes, respectively. This class of SCAs recently showed extremely advantageous properties, with both the mono- and bismacrocyclic SCA analogues exhibiting high r1 changes in the presence of Ca2+.16,17 Moreover, studies in a complex cell culture model suggest that the T1 change in vivo may exceed several percent, more than the current fMRI methodology allows. Furthermore, the SCA does not affect the normal cellular signaling, and Ca2+ transients persist even in its presence.
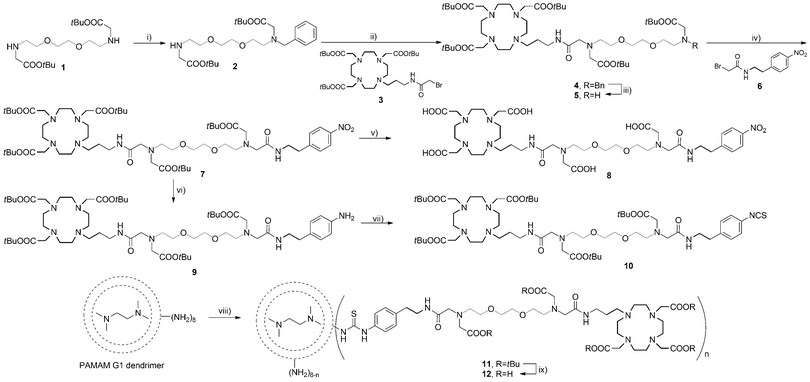
To allow the SCA coupling to the poly(amidoamine) (PAMAM) dendrimer, we have synthetically modified the EGTA-derived core and coupled an aryl-isothiocyanate (NCS) group to the terminus. The NCS group selectively reacts with the amine surface of the PAMAM dendrimer building a stable thiourea group, and this coupling reaction is a common choice for the preparation of dendrimeric MRI agents.18,19 The synthetic route for the preparation of the desired dendrimer is shown in Scheme 1. The preparation commenced from amine 1 which was monoalkylated with benzyl chloride in the presence of cesium carbonate in dimethylformamide to obtain amine 2. The macrocyclic bromide 3 that bears a DO3A chelator and the (propyl)acetamide linker was attached to amine 2 in the presence of potassium carbonate in acetonitrile to provide benzyl-protected amine 4. The reductive removal of the benzyl group was achieved by Pd(OH)2-catalyzed hydrogenation to yield amine 5. The precursor 6 for the introduction of the NCS group was prepared by alkylation of 4-nitrophenylethylamine with bromoacetyl bromide. Amine 5 was subsequently alkylated with bromide 6 to obtain macrocycle 7, which was used in two different reactions. The tert-butyl esters in the first portion of 7 were hydrolyzed with formic acid to give 8, a chelator for Gd3+. Upon preparation of Gd8, this complex was used to compare its r1 response with the r1 response of the desired dendrimeric SCA. Namely, Gd8 already contains a Ca-chelator and can alone act as an SCA.
The nitro group in the second portion of 7 was reduced to the amine by Pd/C-catalyzed hydrogenation in ethanol to give aniline 9. This was finally converted into isothiocyanate 10 using thiophosgene to obtain an amine-reactive SCA precursor that can be coupled to the PAMAM generation 1 (G1) dendrimer. The coupling of 10 to the dendrimer surface was achieved with triethylamine in dimethylformamide to obtain 11. The product was purified by removing excess of unreacted 10 using a lipophilic Sephadex column with methanol as the eluent. The final dendrimeric ligand 12 was prepared by hydrolyzing tert-butyl esters with formic acid. The purification of 12 was performed by size-exclusion chromatography using a hydrophilic Sephadex column with water as the eluent. Dendrimer 12 was analyzed by 1H NMR spectroscopy and MALDI-TOF/MS. The appearance of aromatic protons in the 1H NMR spectrum is the evidence for NCS ligand-dendrimer conjugate formation (these peaks do not exist in the 1H NMR spectra of the commercial PAMAM dendrimers). Gd3+ was introduced into DO3A chelators of the dendrimeric ligands using GdCl3·6H2O to obtain DSCA. Excess of Gd3+ from undesired complexation with the Ca-chelating part or the dendrimer core was removed using ethylenediaminetetraacetic acid (EDTA). The final product DSCA was purified by centrifugation using 3 KDa molecular weight cut-off filters to remove GdEDTA and excess of free EDTA, and was confirmed by MALDI-TOF/MS.
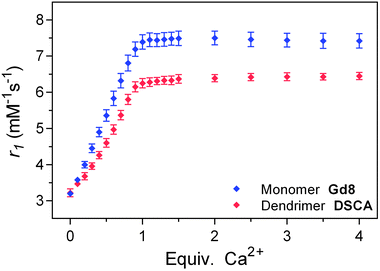
The activity of prepared monomeric Gd8 and dendrimeric DSCA was investigated by relaxometric titrations at 7 T (300 MHz) and 25 °C in a buffered medium. Solutions of Gd8 and DSCA with a known starting concentration of Gd3+ were prepared and the longitudinal T1 relaxation times were determined upon addition of defined amounts of Ca2+. The initial r1 relaxivities of Gd8 and DSCA were similar, showing that no gain in r1 at high magnetic field (7 T) can be achieved for a Gd3+ complex with a large molecular weight.20 Nevertheless, differences in the maximal r1 enhancement were obtained upon saturation of the SCA solutions with Ca2+ (Fig. 2). Gd8 and DSCA exhibited around 130% and 100% increase in r1, respectively. These results are in line with our previous observations reported on SCAs with the same EGTA-derived Ca-chelator, but with different sizes and flexibilities.17,21 The r1 response in the flexible monomacrocyclic SCA is higher than in the sterically hindered bismacrocyclic SCA, which could be explained by reduced flexibility for intramolecular conformational changes in DSCA. However, it is very important to note that the high activity of monomeric SCAs incorporated in the dendrimeric system is retained. This opens a new realm of possibilities for attachment of NCS-containing monomeric SCAs to various nano-size carriers (e.g. functionalized dendrimers and nanoparticles), which should allow broader utilization of such responsive MRI probes.
The diffusion properties of Gd8 and DSCA in the central nervous system (CNS) were investigated using MRI in vivo. Gd8 or DSCA was administered into the rat cerebral cortex (the primary somatosensory forelimb region, S1FL). Twenty minutes after administration, thirty T1-weighted MR images were acquired over a period of 4 hours (see ESI†). Although the Gd8 injection site showed a robust signal increase in the cortex in 0.3 h, the signal enhancement rapidly attenuated after 1 h. In contrast, a larger signal increase was observed in the DSCA-injected region in 0.3 h, as compared to Gd8, and the signal enhancement was maintained over 3 h (Fig. 3a). The longitudinal changes in the SI for Gd8 (n = 3) and DSCA (n = 3) were compared as a function of time. The SI in selected regions of interest (ROIs) from the SCA-injected sites in the cortex was normalized showing desired diffusion trends for Gd8 and DSCA (Fig. 3b and ESI†). The SI of DSCA was significantly higher than that of Gd8 at all time-points after injection. The slope of fitted logarithmic curves of Gd8 (−0.18) was steeper than that of DSCA (−0.14). These results clearly indicate that the dendrimeric DSCA has a longer retention time in the CNS than the monomer Gd8.
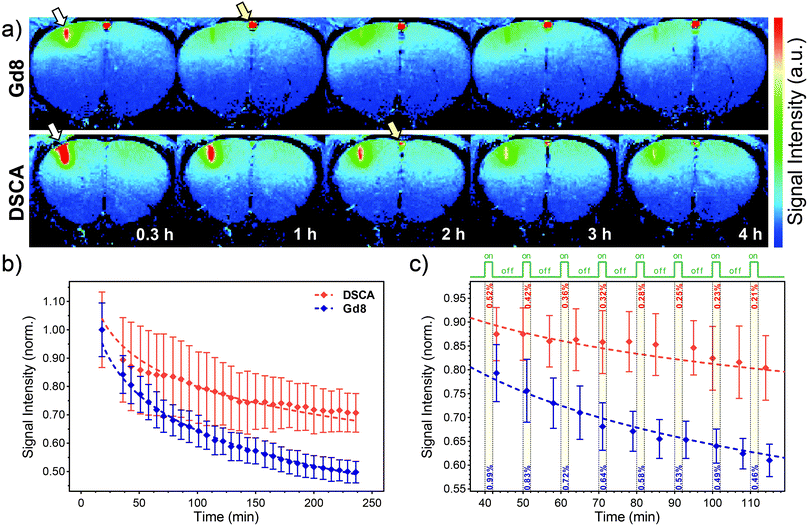 | ||
| Fig. 3 In vivo MRI after Gd8 or DSCA intracerebral administration. (a) Color-scaled typical T1-weighted MR images 0.3, 1, 2, 3, and 4 h after SCA injection (upper: Gd8, lower: DSCA). White arrows indicate the injection site in the cortex. Yellow arrows show vessels on the surface of the brain. (b) Typical longitudinal signal alteration at the injection site after administration of Gd8 (blue diamonds) and DSCA (red diamonds); dashed lines show logarithmic fits explained in the text and ESI.† ROIs (30 voxels) were defined around the injection site in the cortex (0.375 × 1.25 mm2). (c) Selected regions from single experiments for small ROIs (9 voxels, 0.1–0.2 mm from the injection site), showing a decrease in the SI only due to diffusion of Gd8 or DSCA during the hypothesized 2 min stimulation of the cortex (green line and yellow bars on the graph). Dashed lines show logarithmic fits performed for shown experimental points. Values for estimated SI changes were calculated from fits and shown within yellow bars. | ||
The usefulness of this property was further estimated by a more specific analysis of MRI experiments. The maximal SI changes in dynamic, T1-weighted fMRI experiments based on this concept are expected in regions with higher or similar SCA concentration relative to the local extracellular Ca-concentration ([SCA] ≥ [Ca2+]e).16 We therefore selected smaller ROIs closer to the injection site where higher SCA concentrations would be expected shortly after the intracerebral injection (40–90 min). The SI changes caused only by SCA diffusion were estimated every 10 min for a 2 min duration of a hypothesized ‘stimulus-on’ period (e.g. by performing trains of periodic electrical stimuli5). The results show an almost halved SI change of DSCAvs.Gd8 due to diffusion, with the SI change always <0.5% for DSCA (Fig. 3c and ESI†). This is very encouraging because the loss of signal is still below the detection threshold valid for this type of experiment.5 We note that at later time points the SI changes indeed become smaller, although the local SCA concentration also decreases below the level that may report the Ca2+ fluctuation changes.
In conclusion, we have developed a very useful technique to slow down the diffusion rate of SCAs and stabilize the measured SI during in vivo MRI in the CNS. The strategy we established allows synthetic modifications of the existing SCAs and their covalent attachment to functional nano-size carriers, while retaining their activity and bioresponsiveness. The resulting molecular probes allow more convenient functional imaging studies. The dynamic MRI with the use of responsive, slowly diffusing contrast agents holds great promise to visualize Ca-signaling during neuronal activity and may lead to valuable insights for understanding brain function.
The financial support from the Max-Planck Society, the Turkish Ministry of National Education (PhD fellowship to S.G.) and European COST CM1006 and TD1004 Actions is gratefully acknowledged. This research was partly supported by Kakenhi and Center of Innovation (COI) Program, Japan Science and Technology Agency (JST), Japan.
All animal experiments were approved by the Animal Welfare Committee of NIRS (07-1067-9) and were in full compliance with the Law for the Humane Treatment and Management of Animals (Law No. 105, Japan).
Notes and references
- E. L. Que and C. J. Chang, Chem. Soc. Rev., 2010, 39, 51–60 RSC.
- C. Q. Tu, E. A. Osborne and A. Y. Louie, Ann. Biomed. Eng., 2011, 39, 1335–1348 CrossRef PubMed.
- M. C. Heffern, L. M. Matosziuk and T. J. Meade, Chem. Rev., 2014, 114, 4496–4539 CrossRef CAS PubMed.
- A. Y. Louie, M. M. Huber, E. T. Ahrens, U. Rothbacher, R. Moats, R. E. Jacobs, S. E. Fraser and T. J. Meade, Nat. Biotechnol., 2000, 18, 321–325 CrossRef CAS PubMed.
- T. Lee, L. X. Cai, V. S. Lelyveld, A. Hai and A. Jasanoff, Science, 2014, 344, 533–535 CrossRef CAS PubMed.
- M. G. Shapiro, G. G. Westmeyer, P. A. Romero, J. O. Szablowski, B. Kuster, A. Shah, C. R. Otey, R. Langer, F. H. Arnold and A. Jasanoff, Nat. Biotechnol., 2010, 28, 264–270 CrossRef CAS PubMed.
- A. P. Koretsky, NeuroImage, 2012, 62, 1208–1215 CrossRef PubMed.
- A. Jasanoff, Trends Neurosci., 2007, 30, 603–610 CrossRef CAS PubMed.
- A. Jasanoff, Curr. Opin. Neurobiol., 2007, 17, 593–600 CrossRef CAS PubMed.
- E. Gianolio, R. Napolitano, F. Fedeli, F. Arena and S. Aime, Chem. Commun., 2009, 6044–6046 RSC.
- L. Frullano, C. Catana, T. Benner, A. D. Sherry and P. Caravan, Angew. Chem., Int. Ed., 2010, 49, 2382–2384 CrossRef CAS PubMed.
- E. Gianolio, L. Maciocco, D. Imperio, G. B. Giovenzana, F. Simonelli, K. Abbas, G. Bisi and S. Aime, Chem. Commun., 2011, 47, 1539–1541 RSC.
- G. E. Hagberg, I. Mamedov, A. Power, M. Beyerlein, H. Merkle, V. G. Kiselev, K. Dhingra, V. Kubìček, G. Angelovski and N. K. Logothetis, Contrast Media Mol. Imaging, 2014, 9, 71–82 CrossRef CAS PubMed.
- I. Mamedov, S. Canals, J. Henig, M. Beyerlein, Y. Murayama, H. A. Mayer, N. K. Logothetis and G. Angelovski, ACS Chem. Neurosci., 2010, 1, 819–828 CrossRef CAS PubMed.
- J. Henig, I. Mamedov, P. Fouskova, E. Tóth, N. K. Logothetis, G. Angelovski and H. A. Mayer, Inorg. Chem., 2011, 50, 6472–6481 CrossRef CAS PubMed.
- G. Angelovski, S. Gottschalk, M. Milošević, J. Engelmann, G. E. Hagberg, P. Kadjane, P. Andjus and N. K. Logothetis, ACS Chem. Neurosci., 2014, 5, 360–369 CrossRef CAS PubMed.
- P. Kadjane, C. Platas-Iglesias, P. Boehm-Sturm, V. Truffault, G. E. Hagberg, M. Hoehn, N. K. Logothetis and G. Angelovski, Chem. – Eur. J., 2014, 20, 7351–7362 CrossRef CAS PubMed.
- C. A. Boswell, P. K. Eck, C. A. S. Regino, M. Bernardo, K. J. Wong, D. E. Milenic, P. L. Choyke and M. W. Brechbiel, Mol. Pharmaceutics, 2008, 5, 527–539 CrossRef CAS PubMed.
- M. M. Ali, M. Woods, P. Caravan, A. C. L. Opina, M. Spiller, J. C. Fettinger and A. D. Sherry, Chem. – Eur. J., 2008, 14, 7250–7258 CrossRef CAS PubMed.
- J. B. Livramento, C. Weidensteiner, M. I. M. Prata, P. R. Allegrini, C. Geraldes, L. Helm, R. Kneuer, A. E. Merbach, A. C. Santos, P. Schmidt and E. Toth, Contrast Media Mol. Imaging, 2006, 1, 30–39 CrossRef CAS PubMed.
- G. Angelovski, P. Fouskova, I. Mamedov, S. Canals, E. Toth and N. K. Logothetis, ChemBioChem, 2008, 9, 1729–1734 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Methods, synthetic procedures, selected spectroscopic and MRI data. See DOI: 10.1039/c4cc07540d |
| This journal is © The Royal Society of Chemistry 2015 |