Palladium/norbornene chemistry: an unexpected route to methanocarbazole derivatives via three Csp3–Csp2/Csp3–N/Csp2–N bond formations in a single synthetic sequence†
Farnaz
Jafarpour
*,
Nafiseh
Jalalimanesh
,
Mahdieh
Teimouri
and
Mitra
Shamsianpour
School of Chemistry, College of Science, University of Tehran, P.O. Box 14155-6455, Tehran, Iran. E-mail: jafarpour@ut.ac.ir
First published on 4th November 2014
Abstract
A Catellani reaction terminated by palladacycle amination leading to methanocarbazoles is reported. The outcome is the formation of three different Csp3–Csp2/Csp3–N/Csp2–N bonds through a Heck reaction/C–H activation/double amination cascade in one process.
Selective C–C and C–N bond formation through palladium catalyzed C–H bond functionalization because of its viable and environmentally benign features is one of the most fundamental topics in modern chemistry.1 Among the considerable efforts made, the Catellani reaction represents a promising protocol for selective multifunctionalization of aromatics through sequential aromatic ortho C–H activation/C–C bond formation followed by C–N or C–O bond construction.2 This type of cascade reaction which has led to many synthetic applications is based on the cooperative action of norbornene and palladium.3 The unusual reactivity of norbornene in these reactions contributes to the construction of a palladacycle intermediate through stereoselective cis, exo insertion into the arylpalladium bond.4 The intermediacy of the Pd(IV) species plays a crucial role and directs the subsequent reaction steps selectively to the assembly of the product scaffold. Generally, in ortho-substituted iodoarenes regioselective Csp2–Csp2 rather than Csp2–Csp3 coupling of Pd(IV) intermediates at the carbon ortho to the original C–halide bond occurs which ensures the generation of a biaryl linkage (ortho-effect).5 It has been also recently discovered that certain chelating groups may offset this ortho-effect and lead to a Csp2–Csp3 migratory coupling of arene and norbornene.6 In this context, recently Catellani and Derat developed a palladium/norbornene-catalyzed reaction of iodoarenes and ortho-bromoanilines which led to the formation of dibenzoazepine derivatives with a contrast deviation from Catellani's previous report on the formation of N-substituted carbazoles via the reaction of the same reagents having acetylated or sulfonylated amino groups (Scheme 1, path b versus path a).6d,7 Chelation of the unprotected amino group of bromoaniline to palladium played the key role for Csp2–Csp3 bond formation followed by the subsequent cyclization to the nitrogen-containing seven-membered ring.
Based on these results and in a continuation of our efforts aimed at the development of palladium-catalyzed cascade reactions, herein we report our findings on a new outcome from the palladium-catalyzed reaction of iodoarenes, anilines and either norbornene or norbornadiene (Scheme 1, path c). The presented study uncovers a different chemo- and regioselective annulation reaction to provide an interesting formation of methanocarbazole derivatives via a cascade intermolecular Heck-type reaction/intramolecular C–H activation/direct intermolecular amination. This process involves three Csp3–Csp2/Csp3–N/Csp2–N bond formations in one procedure and involves the unexpected direct amination of palladacycle which was not achieved previously. This multistep reaction proceeds in an ordered sequence and is chemo-, regio-, and stereoselective where an ortho-amination overtakes the conventional ipso-amination. The other remarkable feature of this reaction compared to commonly utilized palladium catalyzed C–H aminations,8 is the direct conversion of aromatic ortho C–H bond to C–N bond under atom economical, synthetically convenient and mild reaction conditions without the requisite for an external oxidant.
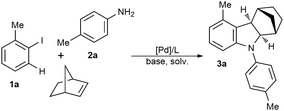
In a typical experiment 2-iodotoluene 1a, p-toluidine 2a and norbornene, were reacted in the presence of PdCl2, PPh3, Cs2CO3 in DME at 120 °C for 24 h. Surprisingly, 1,4-methanocarbazole 3a was obtained albeit in 22% yield (Table 1, entry 1). The reaction was optimized with respect to palladium sources and ligands where Pd(OAc)2/PPh3 proved to be the most effective catalytic system (entries 2–6). Employment of harder bases such as K2CO3, further increased the efficiency of the annulation reaction (entry 7). Next some other reaction variables including solvent and temperature were examined and the ideal results were obtained in DMF heating at 140 °C (entries 8–11). Finally, decreasing the amount of norbornene to 2 equivalents, improved the yield (entry 12). The control experiment in the absence of the catalyst resulted in the recovery of the starting materials.
| Entry | Base | [Pd] | L | Solv. | T [°C] | Yield [%] |
|---|---|---|---|---|---|---|
| a Reaction conditions: p-toluidine 2a (0.2 mmol), 2-iodotoluene (2 equiv.), catalyst (10 mol%), ligand (22 mol%), base (2 equiv.), norbornene (3 equiv.) in a solvent (0.1 M) at 120 °C for 24 h. b Norbornene (2 equiv.). | ||||||
| 1 | Cs2CO3 | PdCl2 | PPh3 | DME | 120 | 22 |
| 2 | Cs2CO3 | Pd(OAc)2 | PPh3 | DME | 120 | 30 |
| 3 | Cs2CO3 | Pd(dba)2 | PPh3 | DME | 120 | 15 |
| 4 | Cs2CO3 | Pd(OAc)2 | PBu3 | DME | 120 | 29 |
| 5 | Cs2CO3 | Pd(OAc)2 | P(OEt)3 | DME | 120 | 28 |
| 6 | Cs2CO3 | Pd(OAc)2 | P(o-Tol)3 | DME | 120 | 29 |
| 7 | K2CO3 | Pd(OAc)2 | PPh3 | DME | 120 | 35 |
| 8 | K2CO3 | Pd(OAc)2 | PPh3 | DMF | 120 | 47 |
| 9 | K2CO3 | Pd(OAc)2 | PPh3 | DMSO | 120 | 33 |
| 10 | K2CO3 | Pd(OAc)2 | PPh3 | DMA | 120 | 21 |
| 11 | K2CO3 | Pd(OAc)2 | PPh3 | DMF | 140 | 57 |
| 12 | K 2 CO 3 | Pd(OAc) 2 | PPh 3 | DMF | 140 | 63 |
Gratifyingly, using Pd(OAc)2 (10 mol%), PPh3 (22 mol%), K2CO3 (2 equiv.), norbornene (2 equiv.) at 140 °C in DMF for 24 h led to the formation of 3a in 63% isolated yield. 1,4-Methanocarbazoles (fused indolines) are ubiquitous structural motifs in natural and synthetic compounds which exhibit various biological and pharmaceutical activities.9 For example, N-phenyl indoline derivatives exhibit antidepressant activities10 and hexahydrocarbazoles have a typical antipsychotic character.11 Also indoline-based organic dyes have been proved to exhibit high efficiencies in dye-sensitized solar cells.12 Accordingly, new and efficient synthetic routes to these structural motifs would find significant utility in organic synthesis.
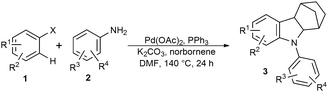
With the optimized conditions in hand, the scope of the annulation reaction to construct various methanocarbazoles was examined. As is summarized in Table 2, a wide range of substituted iodoarenes and anilines were found to be compatible with this domino Heck reaction/double amination protocol. First the reactivity of ortho-substituted iodoarenes, 2-iodotoluene 1a and 4-iodo-m-xylene 1b with various anilines were explored where the latter behaved better resulting in the desired product 3b, in 81% yield (entry 2).
| Entry | Product | Yield [%] | |
|---|---|---|---|
| a Optimized conditions. b Yields for X = Br in parentheses. | |||
| 1 |

|
3a: R2 = H, R3 = H, R4 = 4-Me | 63 |
| 2 | 3b: R2 = 7-Me, R3 = H, R4 = 4-NO2 | 81 | |
| 3 | 3c: R2 = H, R3 = H, R4 = 4-OMe | 58 | |
| 4 | 3d: R2 = H, R3 = 3-NO2, R4 = H | 48 | |
| 5 | 3e: R2 = H, R3 = H, R4 = H | 44 | |
| 6 | 3f: R2 = H, R3 = 3-NO2, R4 = 4-F | 57 | |
| 7 |

|
3g: R3 = H, R4 = 4-NO2 | 73 (62)b |
| 8 | 3h: R3 = 3-Cl, R4 = 4-Cl | 50 (37)b | |
| 9 | 3i: R3 = H, R4 = 4-Cl | 45 (36)b | |
| 10 | 3j: R3 = H, R4 = 4-CF3 | 48 (35)b | |
| 11 |

|
3k: R3 = 4-NO2 | 74 |
| 12 |

|
3l: R1 = H, R2 = 7-Me, R3 = 4-NO2 | 62 |
| 13 | 3m: R1 = CF3, R2 = H, R3 = 4-NO2 | 47 | |
| 14 | 3n: R1 = H, R2 = H, R3 = 4-NO2 | 56 (55)b | |
| 15 | 3o: R1 = H, R2 = H, R3 = 2-Me | Trace | |
Various electron-rich and -deficient anilines with methoxy, nitro and fluoro substituents were also tolerated under the optimized reaction conditions, affording the annulated products in moderate to good yields (entries 3–6). It is noteworthy that methanocarbazole derivatives of 4-nitroanilines may have potential applications in construction of pigments and synthetic organic dyes containing a new type of indoline structure.13
Intriguingly, even iodoarenes lacking ortho-substituents reacted well with anilines under the optimized reaction conditions to give the same annulated products. 4-Methoxy-substituted iodoarenes reacted successfully with anilines to afford the related methanocarbazoles in moderate to good yields (entries 7–10). Notably, bromoarenes were also tolerated under the reaction conditions where comparable yields of 3g–3j were attained employing 1-bromo-4-methoxybenzene. Furthermore, chlorinated anilines also survived the reaction conditions and afforded 3h and 3i in moderate yields (entries 8 and 9). Albeit in moderate yields, this is a synthetically interesting result as such substituents are versatile handles for further functionalization and facilitating the practical synthesis of highly functionalized molecules. Notably, the more sterically encumbered 2-iodonaphthalene was also successfully converted to the corresponding methanocarbazole 3k in 74% yield (entry 11). Even the ortho-trifluoromethyl substituted iodoarene, which, due to its inferior reactivity, is rarely used as a coupling partner in direct arylation reactions, was compatible with the reaction conditions (entry 13). Besides substituted haloarenes, iodo- and bromobenzene were also found to be amenable to the sequential Heck/amination reaction (entry 14). Unfortunately, more electron deficient haloarenes bearing nitro groups were not tolerated under the reaction conditions, affording only the N-arylated products. A competing reaction was also observed using 4-nitroaniline. An amino palladation of norbornene was followed by a sequential Heck reaction/cyclization to furnish dimethanocarbazole 4 (Scheme 2).14 Other anilines gave no or negligible amounts of such byproducts.
 | ||
| Scheme 2 Amino-palladation/Heck reaction/cyclization reaction leading to the side product dimethanocarbazole. | ||
It is significant that 1,3-bis(4-nitrophenyl)urea also survived the reaction conditions where annulation and deprotection of anilines proceeded smoothly leading to the desired indolines in moderate to good yields (Scheme 3).
Lastly, the reaction of iodoarene 1b and norbornadiene with a higher reactivity compared to norbornene was explored. Pleasingly, the norbornadiene adduct 5 was constructed in a satisfactory yield (Scheme 4).
Our plausible mechanistic hypothesis shown in Scheme 5, seems to involve an interconversion between Pd0 and PdII species. The first step involves oxidative addition of aryl halide to Pd(0) followed by stereoselective insertion of norbornene into the arylpalladium bond, leading to the formation of the cis, exo-arylnorbornylpalladium species which readily undergoes cyclization via C–H bond activation to the five membered palladacycle 6. The activation of the unsaturated carbon–hydrogen bond of arene can be induced by its coordination to the metal, which makes the bond susceptible to the amination reaction. Next, association of the aniline to the palladium species15 (instead of oxidative addition of haloarene to form the Pd(IV) species) and subsequent reductive elimination, converts intermediate 7 to product 3 and Pd(0). Likely, nitrogen chelation to the palladium plays the key role for the selective addition of aniline to the palladacycle and formation of the indolines instead of unsymmetrical biaryls. Two alternative routes to the desired methanocarbazoles were also considered which were discarded according to experimental evidence (see the ESI† for details).
In conclusion, we have uncovered a simple route to construct three Csp3–Csp2/Csp3–N/Csp2–N bonds in a single synthetic process based on the ordered sequences of Heck reaction/C–H activation/amination cascade which leads to the formation of interesting methanocarbazole derivatives, using readily accessible starting materials. Consecutive construction of double C–N bonds which is not often feasible in the absence of oxidants and complicated ligands is readily achieved using this course and may be applicable in construction of interesting fused heterocycles. Furthermore, a deviation from the conventional palladium/norbornene catalyzed reactions is launched.
Notes and references
- For selected reviews on metal-catalyzed C–H bond functionalizations see: (a) J. Wencel-Delord, T. Droge, F. Liu and F. Glorius, Chem. Soc. Rev., 2011, 40, 4740 RSC; (b) C. S. Yeung and M. V. Dong, Chem. Rev., 2011, 111, 1215 CrossRef CAS PubMed; (c) J. F. Hartwig, Chem. Soc. Rev., 2011, 40, 1992 RSC; (d) L. McMurray, F. OHara and M. Gaunt, Chem. Soc. Rev., 2011, 40, 1885 RSC; (e) T. W. Lyons and M. Sanford, Chem. Rev., 2010, 110, 1147 CrossRef CAS PubMed; (f) D. A. Colby, R. G. Bergman and J. A. Ellman, Chem. Rev., 2010, 110, 624 CrossRef CAS PubMed; (g) L.-M. Xu, B.-J. Li, Z. Yang and Z.-J. Shi, Chem. Soc. Rev., 2010, 39, 712 RSC; (h) L. Ackermann, R. Vicente and A. R. Kapdi, Angew. Chem., Int. Ed., 2009, 48, 9792 CrossRef CAS PubMed; (i) C.-J. Li, Acc. Chem. Res., 2009, 42, 335 CrossRef CAS PubMed; (j) X. Chen, K. M. Engle, D.-H. Wang and J.-Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094 CrossRef CAS PubMed; (k) R. Giri, B.-F. Shi, K. M. Engle, N. Maugel and J.-Q. Yu, Chem. Soc. Rev., 2009, 38, 3242 RSC; (l) I. V. Seregin and V. Gevorgyan, Chem. Soc. Rev., 2007, 36, 1173 RSC; (m) L.-C. Campeau and K. Fagnou, Chem. Soc. Rev., 2007, 36, 1058 RSC; (n) L.-C. Campeau and K. Fagnou, Chem. Commun., 2006, 1253 RSC; (o) O. Daugulis, V. G. Zaitzev, D. Shabashov, Q.-N. Pham and A. Lazareva, Synlett, 2006, 3382 CrossRef CAS PubMed.
- (a) M. Catellani, F. Frignani and A. Rangoni, Angew. Chem., Int. Ed., 1997, 36, 119 CrossRef CAS; (b) M. Catellani and M. C. Fagnola, Angew. Chem., Int. Ed., 1994, 33, 2421 CrossRef ; for selected reviews, see: ; (c) P. Sehnal, R. J. K. Taylor and I. J. S. Fairlamb, Chem. Rev., 2010, 110, 824 CrossRef CAS PubMed; (d) G. P. Chiusoli, M. Catellani, M. Costa, E. Motti, N. Della Ca' and G. Maestri, Coord. Chem. Rev., 2010, 254, 456 CrossRef CAS PubMed; (e) A. Martins, B. Mariampillai and M. Lautens, Top. Curr. Chem., 2010, 292, 1 CrossRef CAS; (f) K. Muniz, Angew. Chem., Int. Ed., 2009, 48, 9412 CrossRef CAS PubMed; (g) M. Catellani, E. Motti and N. Della Ca, Acc. Chem. Res., 2008, 41, 1512 CrossRef CAS PubMed; (h) D. Alberico, M. E. Scott and M. Lautens, Chem. Rev., 2007, 107, 174 CrossRef CAS PubMed; (i) M. Lautens, D. Alberico, C. Bressy, Y.-Q. Fang, B. Mariampillai and T. Wilhelm, Pure Appl. Chem., 2006, 78, 351 CrossRef CAS; (j) M. Catellani, E. Motti, F. Faccini and R. Ferraccioli, Pure Appl. Chem., 2005, 77, 1243 CrossRef CAS; (k) M. Catellani, Top. Organomet. Chem., 2005, 14, 21 CAS.
- For selected examples of Catellani reaction see: (a) P.-X. Zhou, L. Zheng, J.-W. Ma, Y.-Y. Ye, X.-Y. Liu, P.-F. Xu and Y.-M. Liang, Chem. – Eur. J., 2014, 20, 6745 CrossRef CAS PubMed; (b) Z.-Y. Chen, C.-Q. Ye, H. Zhu, X.-P. Zeng and J.-J. Yuan, Chem. – Eur. J., 2014, 20, 4237 CrossRef CAS PubMed; (c) H. Weinstabl, M. Suhartono, Z. Qureshi and M. Lautens, Angew. Chem., Int. Ed., 2013, 52, 5305 CrossRef CAS PubMed; (d) L. Jiao and T. Bach, Angew. Chem., Int. Ed., 2013, 52, 6080 CrossRef CAS PubMed; (e) Z. Dong and G. Dong, J. Am. Chem. Soc., 2013, 135, 18350 CrossRef CAS PubMed; (f) H. Liu, M. El-Salfiti and M. Lautens, Angew. Chem., Int. Ed., 2012, 51, 9846 CrossRef CAS PubMed; (g) F. Jafarpour and N. Jalalimanesh, Tetrahedron, 2012, 68, 10286 CrossRef CAS PubMed; (h) D. I. Chai, P. Thansandote and M. Lautens, Chem. – Eur. J., 2011, 17, 8175 CrossRef CAS PubMed; (i) L. Jiao and T. Bach, J. Am. Chem. Soc., 2011, 133, 12990 CrossRef CAS PubMed; (j) M. Blanchot, D. A. Candito, F. Larnaud and M. Lautens, Org. Lett., 2011, 13, 1486 CrossRef CAS PubMed; (k) D. A. Candito and M. Lautens, Org. Lett., 2010, 12, 3312 CrossRef CAS PubMed; (l) G. Maestri, M.-H. Larraufie, E. Derat, C. Ollivier, L. Fensterbank, E. Lacote and M. Malacria, Org. Lett., 2010, 12, 5692 CrossRef CAS PubMed; (m) E. Motti, N. Della Ca, S. Deledda, E. Fava, F. Panciroli and M. Catellani, Chem. Commun., 2010, 46, 4291 RSC; (n) P. Thansandote, E. Chong, K.-O. Feldmann and M. Lautens, J. Org. Chem., 2010, 75, 3495 CrossRef CAS PubMed; (o) F. Jafarpour and H. Hazrati, Adv. Synth. Catal., 2010, 352, 363 CrossRef CAS; (p) N. Della Ca, E. Motti, A. Mega and M. Catellani, Adv. Synth. Catal., 2010, 352, 1451 CrossRef CAS; (q) D. A. Candito and M. Lautens, Angew. Chem., Int. Ed., 2009, 48, 6713 CrossRef CAS PubMed; (r) A. Rudolph and M. Lautens, Angew. Chem., Int. Ed., 2009, 48, 2656 CrossRef CAS PubMed; (s) F. Jafarpour and P. T. Ashtiani, J. Org. Chem., 2009, 74, 1364 CrossRef CAS PubMed; (t) Y. B. Zhao, B. Mariampillai, D. A. Candito, B. Laleu, M. Z. Li and M. Lautens, Angew. Chem., Int. Ed., 2009, 48, 1849 CrossRef CAS PubMed; (u) K. M. Gericke, D. I. Chai, N. Bieler and M. Lautens, Angew. Chem., Int. Ed., 2009, 48, 1447 CrossRef CAS PubMed; (v) N. Della Ca, G. Maestri and M. Catellani, Chem. – Eur. J., 2009, 15, 7850 CrossRef CAS PubMed; (w) G. Maestri, N. Della Ca and M. Catellani, Chem. Commun., 2009, 4892 RSC; (x) N. Della Ca', E. Motti and M. Catellani, Adv. Synth. Catal., 2008, 350, 2513 CrossRef; (y) D. G. Hulcoop and M. Lautens, Org. Lett., 2007, 9, 1761 CrossRef CAS PubMed; (z) F. Jafarpour and M. Lautens, Org. Lett., 2006, 8, 3601 CrossRef CAS PubMed.
- (a) M. Catellani, C. Mealli, E. Motti, P. Paoli, E. Perez-Carreno and P. S. Pregosin, J. Am. Chem. Soc., 2002, 124, 4336 CrossRef CAS PubMed; (b) C.-S. Li, C.-H. Cheng, F.-L. Liao and S.-L. Wang, Chem. Commun., 1991, 710 RSC.
- G. Maestri, E. Motti, N. Della Ca, M. Malacria, E. Derat and M. Ctellani, J. Am. Chem. Soc., 2011, 133, 8574 CrossRef CAS PubMed.
- For exceptions to ortho-effect see: (a) M. Cheng, J. Yan, F. Hu, H. Chen and Y. Hu, Chem. Sci., 2013, 4, 526 RSC; (b) S. A. Lopez and K. N. Houk, J. Org. Chem., 2013, 78, 1778 CrossRef CAS PubMed; (c) M. H. Larraufie, G. Maestri, A. Beaume, E. Derat, C. Ollivier, L. Fensterbank, C. Courillon, E. Lacote, M. Catellani and M. Malacria, Angew. Chem., Int. Ed., 2011, 50, 12253 CrossRef CAS PubMed; (d) N. Della Ca, G. Maestri, M. Malacria, E. Derat and M. Catellani, Angew. Chem., Int. Ed., 2011, 50, 12257 CrossRef CAS PubMed.
- N. Della Ca', G. Sassi and M. Catellani, Adv. Synth. Catal., 2008, 350, 2179 CrossRef.
- For reviews of C–H amination, see: (a) S. H. Cho, J. Y. Kim, J. Kwak and S. Chang, Chem. Soc. Rev., 2011, 40, 5068 RSC; (b) H. M. L. Davies and J. R. Manning, Nature, 2008, 451, 417 CrossRef CAS PubMed; (c) A. R. Dick and M. S. Sanford, Tetrahedron, 2006, 62, 2439 CrossRef CAS PubMed; (d) H. M. L. Davies and M. S. Long, Angew. Chem., Int. Ed., 2005, 44, 3518 CrossRef CAS PubMed; (e) P. Mueller and C. Fruit, Chem. Rev., 2003, 103, 2905 CrossRef CAS PubMed.
- (a) E. Fattorusso and O. Taglialatela-Scafati, Modern Alkaloids: Structure, Isolation, Synthesis and Biology, Wiley-VCH, Weinheim, 2008 Search PubMed; (b) H. Kazuhiro and T. Kawassaki, Nat. Prod. Rep., 2007, 24, 843 RSC; (c) P. M. Dewick, Medicinal Natural Products: A Biosynthetic Approach, John Wiley & Sons, New York, 2nd edn, 2002 Search PubMed.
- (a) S. Archer, D. W. Wylie, L. S. Harris, T. R. Lewis, J. W. Schulenberg, M. R. Bell, R. K. Kullnig and A. Arnold, J. Am. Chem. Soc., 1962, 84, 1306 CrossRef CAS; (b) D. W. Wylie and S. Archer, J. Med. Pharm. Chem., 1962, 5, 932 CrossRef CAS.
- (a) J. M. Walker, W. D. Bowen, F. O. Walker, R. R. Matsumoto, B. de Costa and K. C. Rice, Pharmacol. Rev., 1990, 42, 355 CAS; (b) M. R. Munetz, S. C. Schulz, M. Bellin and I. Harty, Drug Dev. Res., 1989, 16, 79 CrossRef; (c) W. M. Welch, C. A. Herbert, A. Weissman and B. K. Koe, J. Med. Chem., 1986, 29, 2093 CrossRef CAS; (d) J. Lehmann, F. Knoch and N. Jiang, Arch. Pharm., 1993, 326, 947 CrossRef CAS.
- (a) H.-M. Cheng and W.-F. Hsieh, Energy Environ. Sci., 2010, 3, 442 RSC; (b) D. Kuang, S. Uchida, R. Humphry-Baker, S. M. Zakeeruddin and M. Grätzel, Angew. Chem., Int. Ed., 2008, 47, 1923 CrossRef CAS PubMed; (c) T. Horiuchi, H. Miura, K. Sumioka and S. Uchida, J. Am. Chem. Soc., 2004, 126, 12218 CrossRef CAS PubMed.
- (a) W. Li, B. Liu, Y. Wu, S. Zhu, Q. Zhang and W. Zhu, Dyes Pigm., 2013, 99, 176 CrossRef CAS PubMed; (b) M. Ashraf, A. Teshome, A. J. Kay, G. J. Gainsford, M. D. H. Bhuiyan, I. Asselberghs and K. Clays, Dyes Pigm., 2012, 95, 455 CrossRef CAS PubMed; (c) Q. Li, L. Lu, C. Zhong, J. Shi, Q. Huang, X. Jin, T. Peng, J. Qin and Z. Li, J. Phys. Chem. B, 2009, 113, 14588 CrossRef CAS PubMed.
- J. L. Brice, J. E. Harang, V. I. Timokhin, N. R. Anastasi and S. S. Stahl, J. Am. Chem. Soc., 2005, 127, 2868 CrossRef CAS PubMed.
- (a) H. Zheng, Y. Zhu and Y. Shi, Angew. Chem., Int. Ed., 2014, 53, 11280 CrossRef CAS PubMed and references cited therein; (b) T. A. Ramirez, Q. Wang, Y. Zhu, H. Zheng, X. Peng, R. G. Cornwall and Y. Shi, Org. Lett., 2013, 15, 4210 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterization data. See DOI: 10.1039/c4cc06789d |
| This journal is © The Royal Society of Chemistry 2015 |