Isoquinolino[4,3,2-de]phenanthridine: synthesis and its use in 1,3-dipolar cycloadditions to form nitrogen-containing polyaromatic hydrocarbons†
S.
Ito
*,
Y.
Tokimaru
and
K.
Nozaki
*
Department of Chemistry and Biotechnology, Graduate School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan. E-mail: ito_shingo@chembio.t.u-tokyo.ac.jp; nozaki@chembio.t.u-tokyo.ac.jp
First published on 4th November 2014
Abstract
The synthesis of the novel azomethine ylide, isoquinolino[4,3,2-de]phenanthridine, and its use in 1,3-dipolar cycloaddition with various alkenes and alkynes to form the corresponding fused pyrrolidines and pyrroles is reported.
Cycloadditions between 1,3-dipoles and unsaturated dipolarophiles are an indispensable tool for the construction of 5-membered heterocyclic structures.1 An especially impressive demonstration of their versatility and efficiency led to the concept of “click chemistry” using the Huisgen reaction.2 Depending on the combination between 1,3-dipoles and dipolarophiles, a wide variety of heterocyclic rings can be synthesized. Among the various 1,3-dipoles, azomethine ylides are particularly useful intermediates for preparing pyrrolidines and pyrroles, which are important building blocks for many natural products and pharmaceuticals.3 However, only a few examples of 1,3-dipolar cycloaddition of azomethine ylides focus on the preparation of polyaromatic hydrocarbons (PAHs),4 despite the increasing importance of nitrogen-containing PAHs.5–7 Our research group focuses on isoquinolino[4,3,2-de]phenanthridine (1) as a novel class of azomethine ylides to generate pyrrolidines and dihydropyrroles containing a fused isoquinolino[4,3,2-de]phenanthridine structure, which in turn can be converted to the corresponding pyrroles upon oxidative dehydrogenation (Fig. 1). Herein we report the synthesis of isoquinolino[4,3,2-de]phenanthridine (1) and its use in 1,3-dipolar cycloadditions with various alkenes and alkynes to form the corresponding pyrrolidines, 2,5-dihydropyrroles, and pyrroles.8,9 The method presented is highly effective for the synthesis of fused 1,2,3,4,5-pentaarylpyrroles via reactions with diarylacetylenes. To the best of our knowledge, only a few compounds with a fused 1,2,3,4,5-pentaarylpyrrole structure, such as 2,3-diaryl fused pyrroles (dibenzo[e,g]indoles10 and diacenaphtho[1,2-b:1′,2′-d]pyrroles11), 3,4-diaryl fused pyrroles (dibenzo[e,g]isoindoles12,13 and acenaphtho[1,2-c]pyrroles14), and pyrrole-fused azacorronenes,5b have been reported.
 | ||
| Fig. 1 Structure of isoquinolino[4,3,2-de]phenanthridine 1 and its cycloaddition with dipolarophiles. | ||
Scheme 1 illustrates the synthesis of isoquinolino[4,3,2-de]phenanthridine (1) starting from 2,6-dibromo-4-tert-butylaniline (2). Initially, a palladium-catalyzed Suzuki–Miyaura reaction of 2 with 1,3-dihydro-1-hydroxy-2,1-benzoxaborole generated 2,6-diarylaniline 3.15 A subsequent intramolecular cyclization of 3 by treatment with hydrogen chloride in 1,4-dioxane–1,2-dichloroethane at 100 °C afforded the cyclized product 4 as a yellow crystalline powder in good yield (74%). When 4 was dissolved in carbon tetrachloride under exposure to irradiation of ambient light, the color of the reaction mixture changed from yellow to red, suggesting the formation of a radical species such as 4′ (Scheme 2). Since this reaction was not observed in the dark, the reaction might be initiated by a homolytic cleavage of a Cl3C–Cl bond.16 Gradually 4 was converted to iminium chloride 5, presumably through the chlorinated intermediate 4′′. Conversion of 4 and 4′ into 5 was found to be accelerated by addition of hydrogen chloride, which led to a color change from red to yellow. Compound 5 exhibited characteristically unsymmetrical NMR resonances in chloroform-d with a distinctive iminium proton observed at 11.6 ppm. Subsequent deprotonation of compound 5 was accomplished by treatment with triethylamine under inert conditions to generate isoquinolino[4,3,2-de]phenanthridine 1in situ.17
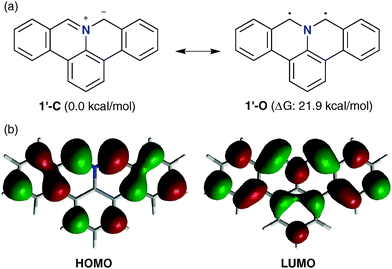
In order to elucidate the properties of 1, we performed density functional theory (DFT) calculations using the B3LYP hybrid functional at the 6-31G(d,p) basis set on the model compound 1′, in which the t-butyl group of 1 is replaced by a hydrogen (Fig. 2). Compound 1′ can adopt a closed-shell zwitterionic structure as (1′-C) or an open-shell biradical structure as (1′-O). Our calculations revealed that 1′-C is by 21.9 kcal mol−1 more stable than 1′-O, indicating that 1 should exist in the azomethine ylide form (Fig. 2a). Azomethine ylides are electron-rich species with high-lying HOMOs and LUMOs; accordingly, they preferentially react with electron-deficient dipolarophiles whereby the HOMO of the dipoles interacts with the LUMO of the dipolarophiles.3d The calculated HOMO of 1′-C is predominantly located in both α-positions of the nitrogen atom, suggesting that 1 reacts with an unsaturated dipolarophile in these positions (Fig. 2b).
Indeed, we observed that azomethine ylide 1 underwent 1,3-dipolar cycloadditions, when it was generated in situ and concurrently treated with internal alkynes.18 The reaction with dimethyl acetylenedicarboxylate (DMAD) at room temperature afforded 2,5-dihydropyrrole 7a in 60% yield (Scheme 1). In the 1H NMR spectrum, the benzylic proton of 7a was observed at 5.35 ppm in chloroform-d. The cycloadditions with diarylacetylenes, Ar–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–Ar (Ar = Ph or C6H4-4-CO2Me), proceeded at a high temperature of 100 °C produced pyrroles 8b and 8c in 30 and 20% yields, respectively, via subsequent dehydrogenation of 7.9 All the compounds were characterized by 1H and 13C NMR spectroscopic analyses.
C–Ar (Ar = Ph or C6H4-4-CO2Me), proceeded at a high temperature of 100 °C produced pyrroles 8b and 8c in 30 and 20% yields, respectively, via subsequent dehydrogenation of 7.9 All the compounds were characterized by 1H and 13C NMR spectroscopic analyses.
The solid-state structures of 7a, 8b, and 8c were confirmed by single crystal X-ray crystallographic analysis (Fig. 3). Suitable crystals were obtained by slowly evaporating dichloromethane solutions of these compounds. The top view of 7a indicates that the three benzene rings and the 2,5-dihydropyrrole ring are not aligned in a coplanar fashion. The dihedral angles between the central benzene and the adjacent benzene rings are 23.7° (C14–C15–C17–C18) and −18.8° (C9–C10–C11–C12). In the case of 8b and 8c, the three benzene rings and the pyrrole ring share a highly coplanar geometry with dihedral angles between the pyrrole moiety and the adjacent benzene rings of less than 3°. The C–N bond lengths and C1–N1–C4 angles in these pyrrole structures are consistent with those of previously reported fused 1,2,3,4,5-pentaarylpyrroles.11,14
 | ||
| Fig. 3 X-ray structures of 7a, 8b, and 8c. Hydrogen atoms for all structures and t-butyl groups for top views are omitted for clarity. | ||
Azomethine ylide 1 was found to react with electron-deficient alkenes. The reaction with methyl acrylate produced pyrrolidine 6d in 61% yield as an inseparable mixture of endo and exo adducts (ca. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). N-Phenylmaleimide also reacted with 1 to give pyrrolidine 6e in moderate yield. The product was obtained as a mixture of diastereomers which could be separated using silica gel column chromatography to afford the endo isomer in 26% yield and the exo isomer in 22% yield. As fullerene C60 is known to be a good dipolarophile, the 1,3-dipolar cycloaddition of 1 with C60 was also investigated in order to form a hybrid of pyrrolidine and C60.19 The reaction of 1 with C60 at 40 °C afforded adduct 9, which was successfully purified by preparative HPLC with a buckyprep® column, in 65% yield (Scheme 3). In the 1H NMR spectrum of 9, three singlet resonances were observed for the t-butyl group (1.46 ppm), for the benzylic protons (6.26 ppm), and the aromatic protons in ortho positions to the t-butyl group (7.74 ppm). Moreover, two doublet (7.95/7.73 ppm) and two triplet (7.52/7.40 ppm) resonances were observed for the aromatic protons of the peripherally fused benzo groups. The 13C NMR spectrum of 9 shows 45 signals (32 signals for the C60 unit and 13 signals for the isoquinolino[4,3,2-de]phenanthridine unit), indicating that compound 9 is of Cs symmetry. The UV/Vis spectrum of 9 showed a local-maximum signal at 433 nm, which is a characteristic band for 6
1). N-Phenylmaleimide also reacted with 1 to give pyrrolidine 6e in moderate yield. The product was obtained as a mixture of diastereomers which could be separated using silica gel column chromatography to afford the endo isomer in 26% yield and the exo isomer in 22% yield. As fullerene C60 is known to be a good dipolarophile, the 1,3-dipolar cycloaddition of 1 with C60 was also investigated in order to form a hybrid of pyrrolidine and C60.19 The reaction of 1 with C60 at 40 °C afforded adduct 9, which was successfully purified by preparative HPLC with a buckyprep® column, in 65% yield (Scheme 3). In the 1H NMR spectrum of 9, three singlet resonances were observed for the t-butyl group (1.46 ppm), for the benzylic protons (6.26 ppm), and the aromatic protons in ortho positions to the t-butyl group (7.74 ppm). Moreover, two doublet (7.95/7.73 ppm) and two triplet (7.52/7.40 ppm) resonances were observed for the aromatic protons of the peripherally fused benzo groups. The 13C NMR spectrum of 9 shows 45 signals (32 signals for the C60 unit and 13 signals for the isoquinolino[4,3,2-de]phenanthridine unit), indicating that compound 9 is of Cs symmetry. The UV/Vis spectrum of 9 showed a local-maximum signal at 433 nm, which is a characteristic band for 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 ring-bridged 58 π-electron fullerenes.20 All the observed data are consistent with an addition of azomethine ylide 1 onto the 6
6 ring-bridged 58 π-electron fullerenes.20 All the observed data are consistent with an addition of azomethine ylide 1 onto the 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 ring junction of C60.
6 ring junction of C60.
Furthermore, we found that the cycloadditions were affected by the polarity of the solvent. The reaction of 1 in acetonitrile at 80 °C produced the dimerized product 10 in 3.5% yield (Scheme 4).21 This homo-coupling reaction of azomethine ylide 1 could be potentially applied to the synthesis of nitrogen-containing nanographene or graphene nanoribbons via subsequent oxidative cyclization and dehydrogenation.
In summary, we have developed a new method to synthesize the azomethine ylide, isoquinolino[4,3,2-de]phenanthridine 1, which undergoes 1,3-dipolar cycloadditions with various alkenes and alkynes. This study presents a potentially useful method to form nitrogen-containing PAHs with fused pyrrole structures.
A part of this work was supported by “Nanotechnology Platform” (No.12024046) of MEXT, Japan. The authors would like to thank Prof. Eiichi Nakamura, Dr Koji Harano, and Ms Utako Takeda (The University of Tokyo) for the preparative HPLC separation of compound 9.
Notes and references
- (a) S. Kanemasa, Heterocycles, 2010, 82, 87 CrossRef CAS PubMed; (b) Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products, ed. A. Padwa and W. H. Pearson, John Wiley & Sons, New York, 2002 Search PubMed; (c) 1,3-Dipolar Cycloaddition Chemistry, ed. A. Padwa, John Wiley & Sons, New York, 1984 Search PubMed.
- (a) V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596 CrossRef CAS; (b) C. W. Tornøe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057 CrossRef PubMed . For reviews, ; (c) A. Lauria, R. Delisi, F. Mingoia, A. Terenzi, A. Martorana, G. Barone and A. M. Almerico, Eur. J. Org. Chem., 2014, 3289 CrossRef CAS; (d) T. Jin, M. Yan and Y. Yamamoto, ChemCatChem, 2012, 4, 1217 CrossRef CAS; (e) F. Amblard, J. H. Cho and R. F. Schinazi, Chem. Rev., 2009, 109, 4207 CrossRef CAS PubMed.
- (a) J. Adrio and J. C. Carretero, Chem. Commun., 2014, 50, 12434 RSC; (b) J. Adrio and J. C. Carretero, Chem. Commun., 2011, 47, 6784 RSC; (c) G. Pandey, P. Banerjee and S. R. Gadre, Chem. Rev., 2006, 106, 4484 CrossRef CAS PubMed; (d) I. Coldham and R. Hufton, Chem. Rev., 2005, 105, 2765 CrossRef CAS PubMed; (e) C. Nájera and J. M. Sansano, Curr. Org. Chem., 2003, 7, 1105 CrossRef.
- (a) H. Mehrabi and J. Pishahang, Synth. Commun., 2014, 44, 76 CrossRef CAS; (b) T. Topinka, M. Potáček, J. Dostál and J. Marek, Collect. Czech. Chem. Commun., 1994, 59, 2641 CrossRef; (c) J. Dostál, M. Potáček, O. Humpa and J. Marek, Bull. Soc. Chim. Belg., 1994, 103, 343 CrossRef.
- For recent examples of azacorronenes, see (a) B. He, A. B. Pun, L. M. Klivansky, A. M. McGough, Y. Ye, J. Zhu, J. Guo, S. J. Teat and Y. Liu, Chem. Mater., 2014, 26, 3920 CrossRef CAS; (b) M. Takase, T. Narita, W. Fujita, M. S. Asano, T. Nishinaga, H. Benten, K. Yoza and K. Müllen, J. Am. Chem. Soc., 2013, 135, 8031 CrossRef CAS PubMed; (c) J. Wei, B. Han, Q. Guo, X. Shi, W. Wang and N. Wei, Angew. Chem., Int. Ed., 2010, 49, 8209 CrossRef CAS PubMed.
- For reviews on azacene chemistry, see (a) U. H. F. Bunz, J. U. Engelhart, B. D. Lindner and M. Schaffroth, Angew. Chem., Int. Ed., 2013, 52, 3810 CrossRef CAS PubMed; (b) U. H. F. Bunz, Chem. – Eur. J., 2009, 15, 6780 CrossRef CAS PubMed.
- For recent examples of other nitrogen-containing PAHs, see (a) K. Goto, R. Yamaguchi, S. Hiroto, H. Ueno, T. Kawai and H. Shinokubo, Angew. Chem., Int. Ed., 2012, 51, 10333 CrossRef CAS PubMed; (b) Q. Tan, S. Higashibayashi, S. Karanjit and H. Sakurai, Nat. Commun., 2012, 3, 891 CrossRef PubMed; (c) C. Tong, W. Zhao, J. Luo, H. Mao, W. Chen, H. S. O. Chan and C. Chi, Org. Lett., 2012, 14, 494 CrossRef CAS PubMed; (d) T. P. Vaid, J. Am. Chem. Soc., 2011, 133, 15838 CrossRef CAS PubMed; (e) Y. Kou, Y. Xu, Z. Guo and D. Jiang, Angew. Chem., Int. Ed., 2011, 50, 8753 CrossRef CAS PubMed; (f) D. Wu, W. Pisula, V. Enkelmann, X. Feng and K. Müllen, J. Am. Chem. Soc., 2009, 131, 9620 CrossRef CAS PubMed; (g) D. Wu, W. Pisula, M. C. Haberecht, X. Feng and K. Müllen, Org. Lett., 2009, 11, 5686 CrossRef CAS PubMed.
- During the preparation of the present manuscript, Feng and Müllen et al. reported the synthesis of isoquinolino[4,3,2-de]phenanthridine (1) as a model segment for nitrogen-doped zigzag-edge peripheries and its use in 1,3-dipolar cycloadditions to generate 2,5-dihydropyrroles. R. Berger, A. Giannakopoulos, P. Ravat, M. Wagner, D. Beljonne, X. Feng and K. Müllen, Angew. Chem., Int. Ed., 2014, 53, 10520 CrossRef CAS PubMed.
- For the construction of benzo[7,8]indolizino[6,5,4,3-def]phenanthridine structure, see: J. Zhou, W. Yang, B. Wang and H. Ren, Angew. Chem., Int. Ed., 2012, 51, 12293 CrossRef CAS PubMed.
- (a) J.-O. Lim, S.-H. Hwang, Y.-K. Kim, Y.-H. Kwak, H.-J. Jung and J.-H. Lee, US Pat., 2011/0240978, 2011 Search PubMed; (b) H.-J. Jung, S.-H. Hwang, Y.-K. Kim, Y.-H. Kwak, J.-O. Lim and J.-H. Lee, US Pat., 2011/0240977, 2011 Search PubMed.
- Y. Nagasaka, C. Kitamura, H. Kurata and T. Kawase, Chem. Lett., 2011, 40, 1437 CrossRef CAS.
- J. Rigaudy and J. Baranne-Lafont, Tetrahedron Lett., 1966, 7, 2431 CrossRef.
- (a) T. Schaefer, P. Murer, F. Wendeborn, B. Schmidhalter, K. Bardon, A. Ricci and J. Pommerehne, PCT Int. Pat., WO2008119666, 2008 Search PubMed; (b) F. Wendeborn, B. Schmidhalter, T. Schaefer, P. Murer and K. Bardon, PCT Int. Pat., WO2008031743, 2008 Search PubMed.
- X. Chen, J. Jin, Y. Wang and P. Lu, Chem. – Eur. J., 2011, 17, 9920 CrossRef CAS PubMed.
- For related procedure, see: M. Albrecht, Synthesis, 2008, 2451 CrossRef CAS PubMed.
- (a) S. A. Markaryan, Arm. Khim. Zh., 1987, 40, 334 CAS; (b) C. Radlowski and W. V. Sherman, J. Phys. Chem., 1970, 74, 3043 CrossRef CAS.
- Since azomethine ylides are generally unstable, only few examples of isolated azomethine ylides—typically having electron-withdrawing groups for stabilization—have so far been reported. (a) D. J. Lee, H. S. Han, J. Shin and E. J. Yoo, J. Am. Chem. Soc., 2014, 136, 11606 CrossRef CAS PubMed; (b) E. Lopez-Calle, M. Keller and W. Eberbach, Eur. J. Org. Chem., 2003, 1438 CrossRef CAS and references cited therein; (c) J. P. Freeman, Chem. Rev., 1983, 83, 241 CrossRef CAS; (d) H. Seidl, R. Huisgen and R. Knorr, Chem. Ber., 1969, 102, 904 CrossRef CAS.
- A reaction with a terminal alkyne, phenylacetylene, under the same conditions afforded a complex mixture of products.
- (a) N. Tagmatarchis and M. Prato, Synlett, 2003, 768 CAS; (b) M. Prato and M. Maggini, Acc. Chem. Res., 1998, 31, 519 CrossRef CAS; (c) M. Maggini, G. Scorrano and M. Prato, J. Am. Chem. Soc., 1993, 115, 9798 CrossRef CAS.
- (a) B. Kräutler and J. Maynollo, Tetrahedron, 1996, 52, 5033 CrossRef; (b) Y. Rubin, S. Khan, D. I. Freedberg and C. Yeretzian, J. Am. Chem. Soc., 1993, 115, 344 CrossRef CAS; (c) F. Diederich, U. Jonas, V. Gramlich, A. Herrmann, H. Ringsdorf and C. Thilgen, Helv. Chim. Acta, 1993, 76, 2443 CrossRef.
- Feng and Müllen, et al. obtained dimer 10 in 51% yield by a reaction using tributylamine as a base and dimethylsulfoxide as a solvent at 190 °C. See ref. 8.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and analytical data, including X-ray crystallographic data for 7a, 8b, and 8c. CCDC 1019909–1019911. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4cc06643j |
| This journal is © The Royal Society of Chemistry 2015 |