Evaluation of Analytical Instrumentation. Part XXV: Differential Scanning Calorimetry
S.
Gaisford
*
UCL School of Pharmacy, University College London, London WC1N 1AX, UK. E-mail: s.gaisford@ucl.ac.uk
First published on 29th January 2015
Abstract
The Analytical Methods Committee has received and approved the following report from the Instrumental Criteria Sub-Committee.
Introduction
This report was prepared for the Analytical Methods Committee (AMC) by the author with contributions and critical review from other members of the Instrumental Criteria Sub-Committee: S. Greenfield (Chair), C. B. Braungardt, S. J. Hill, K. E. Jarvis, G. Lord, M. Sargent (Vice Chair), P. J. Potts and M. West. The Thermal Methods Group, to whom the AMC expresses its thanks, also reviewed the report prior to publication.All users of analytical equipment should be familiar with the basic principles of its operation, the main areas of application, key features and aspects of day-to-day use. The aim of this report is to provide a general overview of the technique followed by an objective evaluation of the instrumentation and its capabilities in tabular form. The table of selection criteria is a unique aspect of this series of reports, providing a checklist of features to be considered in purchasing and using complex analytical instrumentation. Most features relate to the instrumentation itself but more general factors such as installation in the laboratory, service requirements and manufacturer support are also considered. A brief description is provided for each feature together with an explanation of its importance and, if appropriate, guidance on how it may be assessed.
Other reports
The Analytical Methods Committee has published the following reports in the series:Part I. Atomic absorption Spectrophotometers, Primarily for use with Flames, Anal. Proc., 1984, 21, 45. Revised in Analyst, 1998, 123, 1407.
Part II. Atomic absorption Spectrophotometers, Primarily for use with Electrothermal Atomizers, Anal. Proc., 1985, 22, 128. Revised in Analyst, 1998. 123, 1415.
Part III. Polychromators for use in Emission Spectrometry with ICP Sources, Anal. Proc., 1986, 23, 109.
Part IV. Monochromators for use in Emission Spectrometry with ICP Sources, Anal. Proc., 1987, 24, 3.
Part V. Inductively Coupled Plasma Sources for use in Emission Spectrometry, Anal. Proc., 1987, 24, 266.
Part VI. Wavelength Dispersive X-ray Spectrometers, Anal. Proc., 1990, 27, 324.
Part VII. Simultaneous Wavelength Dispersive X-ray Spectrometers, Anal. Proc., 1991, 28, 312.
Part VIII. Instrumentation for Gas–Liquid Chromatography, Anal. Proc., 1993, 30, 296.
Part IX. Instrumentation for High-performance Liquid Chromatography. Analyst, 1997, 122, 387.
Part X. Instrumentation for Inductively Coupled Plasma Mass Spectrometry, Analyst, 1997, 122, 393.
Part XI. Instrumentation for Molecular Fluorescence Spectrometry, Analyst, 1998, 123, 1649.
Part XII. Instrumentation for Capillary Electrophoresis, Analyst, 2000, 125, 361.
Part XIII. Instrumentation for UV-VIS-NIR Spectrometry, Analyst, 2000, 125, 367.
Part XIV. Instrumentation for Fourier Transform Infrared Spectrometry, Analyst, 2000, 125, 375.
Part XV Instrumentation for Gas Chromatography-Ion-Trap Mass Spectrometry, Analyst, 2001, 126, 953.
Part XVI. Evaluation of General User NMR Spectrometers, Accred. Qual. Assur., 2006, 11, 130–137.
Part XVII. Instrumentation for Inductively Coupled Plasma Atomic Emission Spectrometers, Accred. Qual. Assur, 2005, 10, 155–159.
Part XVIII. Differential Scanning Calorimetry, Accred. Qual. Assur, 2005, 10, 160–163.
Part XIX. CHNS Elemental Analysers, Accred. Qual. Assur, 2006, 11, 569–576.
Part XX. Instrumentation for Energy Dispersive X-ray Fluorescence Spectrometry, Accred. Qual. Assur, 2006, 11, 610–624.
Part XXI. NIR instrumentation for process control, Accred. Qual. Assur, 2006, 11, 236–237.
Part XXII. Instrumentation for liquid chromatography/mass spectrometry, Accred. Qual. Assur, 2007, 12, 3–11.
Part XXIII. Portable XRF instrumentation, Accred. Qual. Assur, 2008, 13, 453–464.
Part XXIV. Instrumentation for quadrupole ICP-MS, Anal. Methods, 2010, 2, 1206–1221.
An overview of differential scanning calorimetry
Introduction
In differential scanning calorimetry (DSC) the difference in power (ΔP, in mW) required to heat a sample (S) and an inert reference (R) is determined as a function of temperature (T). There are many commercially available DSC instruments, the designs of which usually vary only in terms of furnace arrangement and/or the number and positioning of thermocouples. Early instrument designs plotted the difference in temperature between S and R (differential thermal analysis, DTA) but this term is no longer in common use. DSC instruments are compact, with a footprint typically no larger than 0.5 × 0.5 m2.DSC is one of a group of techniques that make measurements at controlled temperature. Collectively these are known by the term thermoanalytical and the field is termed thermal analysis (TA).1 The International Confederation of Thermal Analysis and Calorimetry (ICTAC) defines the nomenclature and calibration methods used throughout the TA field.
Key areas of application
DSC has widespread application in many diverse fields because it does not require the sample material to possess any specific functional or chemical property, save that it changes its heat content when undergoing a phase change. The small size of the sample pans limits the sample mass to ca. 5–10 mg and so solids are usually studied. Large volume instruments are available for solutions. The instrument can detect thermally-driven phase transitions (such as melting, crystallisation and glass transitions) as well as the loss of volatile components. It is possible to purchase DSC instruments capable of studying solutions, but these have larger cells and operate at slower heating rates, and so are not considered in this report.Key features
All DSC instruments control temperature (T) with respect to time (t). The heating rate (β) can be:– Linear (dT/dt = β)
– Modulated (e.g. dT/dt = sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β)
β)
– Stepped (e.g. a number of isothermal periods at discrete temperatures)
– Sample-controlled (e.g. the sample response governs β).
All DSC instruments use a heater (termed a furnace) to supply power to the sample and reference materials. Where a common (or single) furnace is used to heat S and R, the instrument is of a heat-flux design and where separate furnaces are used to heat S and R, the instrument is of a power-compensation design. Knowledge of the arrangement is important. Heat-flux instruments tend to have a greater thermal mass and so usually have lower maximum heating rates (typically up to ca. 200 °C min−1). Power-compensation instruments, having smaller thermal mass furnaces, can achieve faster heating rates (typically up to ca. 750 °C min−1). Solid-state (i.e. etched silicon chip) calorimeters, possessing very little thermal mass, can achieve heating rates of the order of 106 °C s−1. Similarly, measurements may be performed at defined cooling rates. The cooling rates achievable will be dependent upon the cooling system (typically either a refrigerated cooling system (RCS) or a liquid-nitrogen circulator). Again, the maximum rates attainable will be fastest for solid-state systems and slowest for heat-flux instruments. A power-compensation instrument equipped with an RCS would typically cool to −60 °C at −100 °C min−1.
Basic principles
Sample and reference materials are sealed in pans (or crucibles) and placed in the instrument before being heated (or cooled) in accordance with a user-defined programme. Pans are typically made of pressed aluminium (for experiments up to 600 °C) or aluminium oxide or gold (if higher experimental temperatures are required). Stainless steel, gold-plated stainless steel high-pressure crucibles and/or sealed glass crucibles are used for decomposition studies at high temperatures.DSC measurements
DSC measurements comprise contributions from two sources; heat capacity (Cp) effects and any other processes (phase transformations or chemical reactions) that the sample might undergo (represented by the generic term f(T,t)). | (1) |
If the sample undergoes a phase change or chemical process, or there is a change in heat capacity, there will be a concomitant event in the DSC data. Typical DSC data, in this case an endotherm resulting from melting of a pure compound, are shown in Fig. 1. It should be noted that endotherms may be plotted positive or negative depending upon whether the instrument calculates ΔP as S–R or R–S. The convention varies between manufacturers and so the direction should be indicated on the y-axis. Several parameters may be determined, including the heat of fusion (ΔHfus), obtained by integrating the area under the curve (note: with respect to time not temperature), the extrapolated onset temperature (To), the peak maximum temperature (Tm), and the change in heat capacity (ΔCp).
DSC data show a dependency upon heating rate, faster rates resulting in larger but broader peaks, Fig. 2. Thus, as the heating rate increases, sensitivity increases but at the expense of resolution (separation of events). Selection of the most appropriate heating rate will depend on the nature of the sample and will often involve a compromise between sensitivity and resolution. Typically, heating rates of 10–20 °C min−1 are used.
Modulated temperature DSC (MTDSC)
In this case the linear (underlying or average) heating rate is modulated by a periodic function. The modulation can have any form but is typically sinusoidal, square or sawtooth. In the case of sinusoidal modulation (Fig. 3), the dependence of temperature with time is described by:2T = T0 + βt + AT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ωt ωt | (2) |
dq/dt = Cp(β + AT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ω ω![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos(ωt)) + f′ (t,T) + C cos(ωt)) + f′ (t,T) + C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin(ωt) sin(ωt) | (3) |
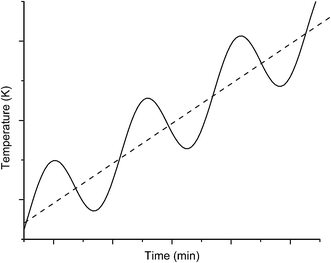 | ||
| Fig. 3 Sample temperature as a function of time in MTDSC (solid line, calculated using eqn (2)) and the corresponding underlying linear heating rate (dotted line). | ||
One component, Cp(β + AT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ω
ω![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos(ωt)), is dependent upon heat capacity effects which, as discussed earlier, should be reversible, and so is termed the reversing heat flow. Heat capacity effects can be considered to occur instantaneously (except for glass transitions); since this term follows a cosine function it should therefore be 0° out of phase with the modulation in heating rate (assuming endothermic events are plotted in the positive direction – if plotted in the negative direction there will be a 180° phase lag).3
cos(ωt)), is dependent upon heat capacity effects which, as discussed earlier, should be reversible, and so is termed the reversing heat flow. Heat capacity effects can be considered to occur instantaneously (except for glass transitions); since this term follows a cosine function it should therefore be 0° out of phase with the modulation in heating rate (assuming endothermic events are plotted in the positive direction – if plotted in the negative direction there will be a 180° phase lag).3
The second term, f′(t,T) + C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin(ωt), is dependent upon a kinetic response (i.e. any process that the sample undergoes, which may be a physical transformation or a chemical reaction, which takes a small, but finite, time). Many processes might contribute to the kinetic response (for instance, melting, crystallisation, polymorph transformation etc.). Some of these processes are reversible (melting) and some are essentially irreversible (transformation to a more stable polymorph for instance), but over the time and temperature scales of a typical MTDSC experiment all of these events can be considered to proceed in the forward direction only and so are termed non-reversing. Since the kinetic response follows a sine function it should be 90° out of phase with the heat capacity term.
sin(ωt), is dependent upon a kinetic response (i.e. any process that the sample undergoes, which may be a physical transformation or a chemical reaction, which takes a small, but finite, time). Many processes might contribute to the kinetic response (for instance, melting, crystallisation, polymorph transformation etc.). Some of these processes are reversible (melting) and some are essentially irreversible (transformation to a more stable polymorph for instance), but over the time and temperature scales of a typical MTDSC experiment all of these events can be considered to proceed in the forward direction only and so are termed non-reversing. Since the kinetic response follows a sine function it should be 90° out of phase with the heat capacity term.
The utility of MTDSC comes in being able to deconvolute the overall heat-flow signal into these two components. Deconvolution requires calculation of the underlying heat flow signal (which is the average of the modulated response – equivalent to the heat flow signal that would be recorded for a normal DSC experiment performed at the same underlying linear heating rate). This can be considered equal to:
| Underlying heat flow = Cpβ + f′(t,T) | (4) |
Heat capacity reflects the rise in temperature for a given input of heat and so can be determined by comparing the amplitude of the modulated heat-flow (Amhf) with the amplitude of the modulated heating rate (Amhr):
 | (5) |
The amplitudes are determined using a Fourier Transform. From eqn (4) and (5) it can be seen that the heat capacity (reversing) component of the underlying heat flow is given by:
 | (6) |
Hence the kinetic response (non-reversing) component of the heat flow can be determined by difference:
 | (7) |
Heat flow data recorded with MTDSC thus allows separation of processes into reversing or non-reversing events. This aids both identification and isolation (if multiple events occur at the same temperature) of processes, although being a mathematical routine it is possible to introduce artifacts into the reversing and non-reversing data if the modulation parameters are not selected carefully.
Experimental considerations
Most DSC instruments purge the air space around the sample and reference pans with a gas (typically nitrogen or helium). The purge gas serves many functions. Firstly, if the instrument is operated at sub-ambient temperatures, the gas, being dry, prevents condensation or freezing of water. It serves as a heat-transfer medium to ensure the pan and contents are at a temperature as close as possible to the heating block (if greater heat transfer is needed, helium is used as the purge gas). Finally, if any gaseous degradation products are emitted from the pan, the purge gas ensures they are carried out to waste and do not condense on the instrument.
Instruments should be calibrated for temperature and enthalpy upon installation and then performance verified on a daily or weekly basis (recalibration being required if the verification test indicates it). Calibration is performed with a certified reference material (CRM). Typically, CRMs for DSC are highly pure materials with well-established melting points and heats of fusion. IUPAC recommends a number of CRMs for DSC calibration (Table 1). Of these, indium is the most widely used material, although calibration with at least two CRMs, possessing melting points over the range at which measurements are to be performed, is advised. CRMs should be used under an inert gas and disposed of after use (the exception is indium, which can be reused if not heated above 180 °C). Gallium will react with aluminium and indium will alloy with gold and so care is needed when selecting pan material. As a general point, uncertainty of heat of fusion measurements will be determined by the uncertainty of the balance used to weigh the sample material.
| CRM | Melting temperature (°C) | Enthalpy of fusion (J g−1) |
|---|---|---|
| Cyclopentane | −93.4 | 8.63 |
| Gallium | 29.8 | 79.9 |
| Benzoic acid | 123.0 | 148.0 |
| Indium | 156.6 | 28.6 |
| Tin | 231.9 | 60.4 |
| Zinc | 419.53 | 107.50 |
| Aluminium | 660.3 | 398.0 |
MTDSC requires selection of additional experimental parameters (underlying heating rate and frequency and amplitude of oscillation). Proper selection is vital to ensure artifacts are not introduced to the data post-deconvolution. In particular, MTDSC assumes that the response of the sample varies linearly with the modulation in temperature. It also assumes that any changes in the underlying heat flow are slow relative to the time scale of the modulation (this allows averaging of the data, required to recover the underlying heat flow). This means there must be many modulations over the course of a transition (a minimum of six is usually recommended). If these conditions cannot be met then deconvolution cannot be achieved. Melting of a pure material is an example where deconvolution usually fails, because as a material melts its temperature will not rise until melting has finished; thus, during melting the temperature of the sample cannot be modulated. Selection of the modulation parameters therefore requires some prior knowledge of the transitions through which the sample will progress and it may be that several experiments will need to be performed with varying parameters to optimise the data. Typical starting values are an underlying heating rate of 2 °C min−1, frequency 30–60 s and amplitude 0.5–1 °C.
Selection criteria for DSC instruments
Table 2 summarises key features of DSC instrumentation and includes criteria to be considered when purchasing DSC equipment. It also provides some guidance on instrumental requirements for different applications, for example measurements above or below ambient temperature.| Feature | Definition of feature and guidance for assessment | Reasoning |
|---|---|---|
| 1 Instrumental criteria | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| (a) Instrument design | DSC instruments are available in two designs and the configuration affects the performance envelope | The furnace arrangement defines the maximum heating and cooling rates that can be achieved |
| Determine whether the instrument is of heat-flux or power-compensation design. Consider the types of sample to be studied and the maximum heating rates that might be used | ||
| (i) Heat-flux instrument | A single furnace is used to heat both the sample and reference materials | The furnace has a large thermal mass and so maximum heating and cooling rates likely to be reduced, although the instrument will probably be more robust |
| Determine the maximum heating and cooling rates the instrument can achieve | ||
| (ii) Power-compensation instrument | Individual furnaces are used to heat the sample and reference materials | The furnaces have smaller thermal masses, so maximum heating and cooling rates likely to be increased |
| Determine the maximum heating and cooling rates the instrument can achieve | ||
| (b) General design features | ||
| (i) Ease of dismantling | The user may be able to perform a cleaning routine if the instrument is easily disassembled | Volatile samples or materials that sublime can leave deposits on the DSC assembly and cover. If these areas are easy to reach, the user can remove these deposits manually, improving instrument performance and lifetime |
| Determine the ease with which the furnace lid can be removed and the DSC assembly reached | ||
| (ii) Thermocouple arrangement | Thermocouples measure temperature. Multiple thermocouples wired in series form a thermopile | A greater number of thermocouples will reduce the need for exact positioning of pans. |
| Determine the number and location of thermocouples under the sample and reference pans | ||
| (iii) Purge gas | The purge gas flows over the sample and reference pans, improving thermal contact with the instrument and removing volatile components | Nitrogen is typically used but helium will increase thermal contact, improving resolution of events that occur at similar temperatures. May require cylinders of both gasses. |
| Determine if one (or more) purge gases can be used and whether the software can switch between them automatically | ||
| (iv) Purge gas flow rate | The rate at which the purge gas flows over the sample and reference materials | High flow rates will require cylinders to be changed more frequently and may reduce the number of experiments that can be performed overnight with an autosampler |
| Determine the minimum rate at which the purge gas flows through the DSC | ||
| (iii) Control of flow rate | The method by which purge gas flow rate is modulated | Mass-flow controllers will give a more consistent flow-rate. The flow rate will affect thermal contact of the sample and reference pans with the instrument |
| Is the flow rate controlled by a regulator on a cylinder or by mass-flow controllers? | ||
| (c) Cooling system | The cold-stage against which the furnace(s) operates. | The cold-stage determines the lowest operating temperature of the instrument and the maximum cooling rates attainable |
| Determine the type of cold stage(s) available. Consider the types of sample to be investigated and the lowest temperatures/cooling rates needed | ||
| (i) Circulating water/oil bath | An external bath that circulates cooling water or oil around the DSC | Minimum temperature limited to freezing point of cooling fluid. Heat removal proportional to flow rate |
| Determine if an external circulating water/oil bath is required and whether it can be controlled with the software | ||
| (ii) Refrigerated cooling system (RCS) | An electronic cooling system utilising a refrigerant gas | Minimum temperature is likely to be around −130 °C. Useful for sub-ambient experiments down to ca. −90 °C and will achieve faster cooling rates than a circulating bath |
| Determine if an RCS unit is available and whether it can be controlled with the software | ||
| (iii) Liquid nitrogen | Liquid nitrogen is circulated around the cold stage with an external pump | Will achieve lowest cold-stage temperature (ca. −196 °C) and so fastest cooling rates. Needs training in handling and use of liquid nitrogen. May require oxygen monitor and special signage in laboratory |
| Determine if a liquid nitrogen circulator is available and whether it can be controlled with the software | ||
| (e) Heating/cooling rates | Maximum rate at which temperature can be changed | |
| (i) Maximum heating rate | The fastest heating rate that can be used to heat the sample | Faster heating rates reduce run-times, increase sensitivity (but reduce resolution) and allow identification of kinetic transitions. Power-compensation designs typically have faster maximum heating rates. Solid-state chip calorimeters have fastest heating rates (>106 °C min−1) |
| Determine the fastest heating rate. Make sure that the rate of data capture gives sufficient data resolution | ||
| (ii) Maximum cooling rate | The fastest rate at which the sample can be cooled | Faster cooling rates allow quicker sample turnover as well as ability to match processing conditions |
| Determine the fastest cooling rate. Remember that the rate will change depending upon the type of cooling system used. If liquid nitrogen to be used, consider gas handling and training | Liquid nitrogen cooled instruments will have the fastest cooling rates, but users must be trained in gas handling | |
| (iii) Temperature modulation | The ability to use a mathematical function to modulate the heating rate with | Temperature modulation allows deconvolution of the data into ‘reversing’ and ‘non-reversing’ signals. Particularly useful for identifying glass transitions |
| Determine if a modulation function is available in the software | ||
| (iv) Maximum operating temperature | The highest temperature S and R can reach. | Using a LN2 cold stage might limit the upper temperature. Inorganic materials might require higher temperatures than organic materials. Limit will affect selection of pan material |
| Determine the maximum operating temperature | ||
| (f) Pans | The container in which the sample and reference materials are enclosed | |
| (i) Pan material | The material from which the pans are constructed | Aluminium pans have good thermal conductivity and low reactivity so are a good general choice. Gold forms an alloy with indium. Aluminium reacts with gallium and can potentially alloy with many CRMs if overheated. Single-use pans are machine-pressed, so may have traces of lubricating oil, so should be washed prior to use |
| Determine the range of pans available. Typically pans are aluminium, but gold, aluminium oxide, stainless steel, glass or platinum may also be used. Some instruments use fixed crucibles (generally made of Hastelloy). | ||
| (ii) Pan seal | The type of seal between the pan lid and base | Samples that lose volatile components will behave differently in hermetic and non-hermetic pans. A pinhole is often created in the lid of a hermetic pan to allow controlled release of volatile components. Hermetically sealed pans can withstand pressure up to 300 kPa (∼3 atm). Pressure-seals formed with O-rings or washers. Can withstand pressures greater than 300 kPa. |
| Determine the range of pan seals. Usually pans are open (non-hermetic), air-tight (hermetic) or pressure-sealed. Hermetic seal usually formed by cold-welding lid to base in a press, so determine whether press is supplied with instrument | ||
| (iii) Pan designs | The range of pan designs available from the manufacturer | Different pans may be needed depending upon the application. These may need different presses. Some pans have locating pins so they are centred in the cell. Pans are generally not transferable between instruments from different manufacturers. Open pans allow simultaneous spectroscopic investigation |
| Determine the range of pans available from the manufacturer. Consider also that pans may be available from other suppliers | ||
| (iv) Pan cost | The cost of the pans | Pans are usually single-use, so are the largest consumable cost. Pans usually sold in packs. Some instruments, usually design for studying liquids, have either fixed crucibles or reusable stainless steel pans |
| Determine the cost of the pans from the manufacturer | ||
| (v) Press | The type of press used to seal the pan and lid | Easier to have a single press that can be used with all pan types and seals. Make sure the sealing force is consistent each time the press is operated |
| Determine the number of different presses required to seal the range of pans. Determine whether the press can be used with different pan types and whether it can form both hermetic and non-hermetic seals | ||
| (g) Sample loading | Factors affecting loading of the S and R pans in the instrument | |
| (i) Loading temperature | The temperature range over which pans can be loaded into the instrument | Some samples are temperature-sensitive and may need to be loaded at sub-ambient temperature. Consider also the temperature of the autosampler (part iii) |
| Determine the loading temperature range. Pay particular attention to whether samples can be loaded at sub-ambient temperature | ||
| (ii) Sensitivity to loading position | The effect on the data from placing the pans on different positions of the DSC sensors | Off-centre pans can affect heat flow measurement. Use of an autosampler ensures consistent pan positioning, as does use of pans with a locating pin. This effect is minimised with a greater number of thermocouples |
| Determine if there is a mechanism to ensure pans are loaded repeatedly | ||
| (iii) Autosampler | An accessory that will automatically load and remove pans from the instrument | An autosampler ensures consistent positioning of pan on sensor as well as provides capacity to run experiments overnight. Need to ensure samples are stable to temperature and/or relative humidity while waiting to be loaded if autosampler does not control these |
| Determine whether an autosampler is standard or available as an option | ||
| (h) Calibration | Certified reference materials (CRM) used to calibrate temperature and enthalpy | |
| (i) Temperature | CRMs for calibration of temperature | Usually indium plus either tin or zinc. Available from various agencies if not supplied by the manufacturer |
| Determine whether CRMs are supplied with the instrument | ||
| (ii) Enthalpy | CRMs for calibration of enthalpy | Usually indium. Available from various agencies if not supplied by the manufacturer |
| Determine whether CRMs are supplied with the instrument | ||
| (iii) Calibration routine in software | A feature within the software to automate calibration | Ensures calibration is performed consistently and keeps a record of calibration files |
| Determine whether there is an automated calibration routine in the software | ||
| (i) Data acquisition and control software | How are data captured, analysed and/or exported? | |
| (i) Computer hardware | The computer system used to run the instrument | If a laboratory has multiple instruments, then the ability to run all the instruments from one computer reduces space requirement. On-board computer might become obsolete – difficult to update/upgrade |
| Determine whether the instrument is run by a separate computer or by an on-board computer. If the former, determine whether the hardware specification is available | ||
| (ii) Data capture rate | The rate at which data are recorded by the software | If the data capture rate is too slow, then there may be too few data points at fast heating rates and resolution of data will be affected |
| Determine the maximum data capture rate and whether it can be altered in the operating software | ||
| (iii) Instrument parameters | The variable settings that affect the experiment and instrument operation | Are all functions accessible through software or are there manual alternatives/overrides? Where data are being recorded for regulatory submission, limited control over settings may be preferable |
| Determine whether the software gives full control over all instrument functions, settings and parameters. Pay particular attention to whether calibration adjustments can be made as instrument performance changes with time/use | ||
| (iv) Software | The software used to operate the instrument and process the data | A single license would mean that data files can only be opened on the instrument computer – might preclude data analysis on a separate computer unless data can be exported. Some data analysis routines can be automated (e.g. Peak finder, area integration, glass transition step height). Experimental data file might only be readable by manufacturer's software |
| Determine the software package(s) supplied with the instrument. Is there a single licensed copy or can all users have their own copy of the software? | ||
| Determine whether the software package includes the tools necessary to enable data analysis. Is there a separate analysis program? What is the default file extension? | ||
| (v) Data presentation | The method by which data are presented | Data are often needed for inclusion in a report or publication and may need to be formatted in a particular manner |
| Determine whether the software can be used to export the data as a graphic file | ||
| (vi) Data format | The file formats in which data can be exported | Data exported in an ASCII format can generally be imported into any generic analysis program |
| Determine the types of file formats in which the data can be exported | ||
| (j) Connectivity | Ability of the instrument to connect with other analyzers | |
| (i) Evolved gas analysis | The purge gas is directed into a connected mass spectrometer | Can identify molecular mass of volatile components, which aids interpretation of thermal events |
| Determine whether an evolved gas analyser is supplied with the instrument or available as an accessory | ||
| (ii) Simultaneous spectro-photometric analysis | Some instrument designs allow a spectrophotometric probe to be mounted above the sample pan | Spectrophotometric data can identify chemical species (and physical form) as a function of temperature. Requires open pans, which may affect quality of thermal data |
| Determine whether there is an accessory that allows the probe to be mounted | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| 2 Non-instrumental criteria | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| (a) Previous instruments | ||
| (i) Innovation | The company's record for developing instruments with innovative features | Demonstrates knowledge and understanding of the technique and user requirements |
| Determine the track record of the company in terms of instrument development and innovation | ||
| (ii) Reliability record | The company's record for instrument reliability | Reflects good design and manufacturing quality and indicates likely servicing and maintenance schedule |
| Determine the reliability record of the instrument. Consider asking to speak with existing customers | ||
| (iii) Similarity of operation, layout and design (including software) to existing instruments in the laboratory | The degree of similarity of the instrument to those already in use | Similarity of design and operation means that operators can draw on in-house expertise, resulting in reduced costs and time for training. It may also minimise the cost of spares and fittings |
| Determine any differences between the current instrument and any previous types. Check to see if any instruments are already in use in the workplace | ||
| (iv) Confidence in the supplier | The confidence gained from past experience or experience of other users | A good working relationship ensures the instrument will be used to its maximum potential and should minimize service and maintenance time |
| Talk with existing customers and users | ||
| (b) Servicing | ||
| (i) Service support | The availability of service support from supplier or third-party | Essential to ensure continued operation of instrument over planned lifetime with minimum down-time for service and maintenance |
| Determine whether a service plan is available or enquire about service call out response times and cost. Consider whether independent service engineers are available | ||
| (ii) Calibration and validation service | Availability of calibration and validation service from supplier or third-party | Often a requirement as part of a Quality Management System or if data to be used in regulatory submissions |
| Determine whether there is an installation, validation and calibration service available. | ||
| (iii) Availability, cost and delivery of spares and consumables | The range of stock carried by, or quickly available to, the supplier or third-party | Will reduce instrument down-time and define day-to-day operating costs |
| Determine spares availability and ask to speak to existing customers or users | ||
| (iv) Effectiveness of service support | The ability of a service agent to identify and fix faults based on previous user experience | Will reduce instrument down-time |
| Ask to speak to existing customers or users | ||
| (c) Technical support | ||
| (i) Applications department | Access to advice from the application specialists | Application specialists will be able to advise on experiment design and data interpretation with respect to specific sample types |
| Determine whether the supplier has a applications laboratory and whether it has application specialists | ||
| (ii) Technical information | The range and quality of technical information, including the instruction manual, application notes and lists of papers | Helps operators to design experiments and optimise measurements for new applications |
| Ask to see technical information | ||
| (iii) Telephone and internet assistance | Support available via telephone or internet, including software updates | Rapid availability of assistance reduces need for service call-out. Can be judged by reference to other users experience |
| Determine the level of technical support | ||
| (iv) Training | The training available during or following installation of instrument | Helps to ensure operators use instrument effectively |
| Determine whether training is provided upon installation and whether training can be offered to new users | ||
| (v) User meetings | Meetings, conferences and technical briefings organised for users of the instrument by the manufacturer or third-party | Other users are often the best source of advice for solving problems and developing new applications |
| Determine if user meetings are offered | ||
References
- Principles of thermal analysis and calorimetry, ed. P. J. Haines, Royal Society of Chemistry, Cambridge, 2002, ISBN 0854046100 Search PubMed.
- M. Reading, A. Luget and R. Wilson, Modulated differential scanning calorimetry, Thermochim. Acta, 1994, 238, 295–307 CrossRef CAS.
- S. R. Aubuchon and P. S. Gill, The utility of phase correction in modulated DSC, J. Therm. Anal. Calorim., 1997, 49, 1039–1044 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |


