Carbon nanotube/gold nanoparticle composite-coated membrane as a facile plasmon-enhanced interface for sensitive SERS sensing†
Kun
Zhang
,
Ji
Ji
,
Xiaoni
Fang
,
Ling
Yan
and
Baohong
Liu
*
Department of Chemistry, State Key Lab of Molecular Engineering of Polymers and Institutes of Biomedical Sciences, Fudan University, Shanghai 200433, China. E-mail: bhliu@fudan.edu.cn
First published on 10th October 2014
Abstract
The facile assembly of three-dimensional (3D) plasmonic substrates has been demonstrated. The assembly is based on the homogeneous decoration of multi-walled carbon nanotube/gold nanoparticle (CNT/AuNP) hybrid nanocomposites on a commercial polyvinylidene difluoride (PVDF) membrane, which is achieved via simple filtration. The CNT/AuNP hybrids with a unique 1D/0D structure remarkably improve the coverage and uniformity of plasmonic nanostructures on the membrane. The effective inter-particle and inter-tube coupling creates a multitude of hot spots within the probe area, and can produce a strong SERS effect. Moreover, the flexible membrane-based scaffold can efficiently collect and concentrate trace targets from large-volume sample solutions at milliliter-scale. The membrane-based SERS sensor shows high sensitivity and good reproducibility. The SERS sensor is employed to detect various molecular contaminants in aqueous samples, demonstrating its excellent field-testing capabilities for applications ranging from food safety to environmental monitoring.
Introduction
Surface-enhanced Raman spectroscopy (SERS) has attracted significant attention as a powerful technique for the detection and identification of trace chemicals in the fields of clinical diagnosis,1–3 environmental control,4,5 and homeland security.6,7 Considerable effort has therefore been dedicated to the engineering of SERS substrates to create numerous hot spots, and to maximize the amplification of Raman signals.8,9 Various approaches based on top-down and bottom-up techniques, such as lithography and self-assembly, have been used to fabricate SERS substrates with very large enhancement factors.10–13 To further facilitate the development of SERS assays for practical applications, another significant requirement is that the enhancing substrates should be facile, low-cost and portable. Several distinctive works have been reported for this purpose.14–16 Despite the improvement of these studies, the employment of rigid substrates (for example, glass and silicon wafers) limits the scope of their applications, especially for on-site analysis in the fields of public safety and environmental monitoring, because of the inflexibility and limited shelf life.Considering the many shortcomings associated with conventional rigid plasmonic substrates, flexible supporting materials such as filter paper and filter membranes have attracted considerable research interests because of the advantages that they hold in terms of low cost and high sample-collecting efficiency.17–19 Moreover, these flexible substrates are also typically eco-friendly and biodegradable, making them easy to handle after the completion of detection. Several approaches using physical vapor depositions,20 printing techniques21 and in situ syntheses22 have been reported for the impregnation of plasmonic nanoparticles on cellulose paper for SERS-based sensing applications. These methods, while offering opportunities for the fabrication of highly SERS-active substrates, are usually accompanied by some drawbacks of their own, including requirements for expensive equipment, the preparation of highly concentrated nanoparticle solutions, and tedious synthesis procedures. Flexible SERS substrates can also be obtained by dipping filter paper in a solution of gold (Au) nanorods.23 Despite the apparent simplicity of this technique, the preparation of these substrates also requires rather high-concentrations of nanorod suspensions, and a period of two days is required to ensure the sufficient adsorption of the nanorods. Recently, Yu et al. reported the preparation of flexible SERS substrates simply by filtering silver nanoparticles through a filter membrane.24 In this process, nanoparticles were first trapped in the pores of the membrane to form a SERS substrate in which the analytes were concentrated from a large volume of samples. The micro-uniformity of the substrate should be improved because of the inhomogeneous distribution of the filter pores. Au nanoworms with a large length-width ratio were also synthesized and trapped on commercial filter membranes, which significantly improved the reproducibility of the substrate because of the even distribution of the nanoworms on the surface of the filter membrane.25
In this work, we report that carbon nanotube-supported Au nanoparticle (CNT/AuNP) composites can be used as basic Raman-enhancing units to fabricate three-dimensional (3D) plasmonic substrates via simple filtration through PVDF membranes. The carbon nanotubes not only provide a large surface area for the anchoring of AuNPs and the enrichment of target molecules, but also effectively improve the uniformity of the substrate because of their large length-to-width ratio.26 The dense assembly of AuNPs on the nanotubes and the effective inter-tube coupling forms a large number of SERS hot spots, resulting in strong SERS responses. The established substrates are simple-to-fabricate, easy-to-use, cost-efficient and flexible, and show good potential for practical SERS applications.
Experimental
Materials and methods
Durapore PVDF filter membranes (0.22 μm, 13 mm) were purchased from Sigma-Aldrich and used with syringe filter holders from Xinya Purification Factory (Shanghai, China) for fabricating the SERS substrates. Multiwalled carbon nanotubes with a diameter 20–50 nm were purchased from Shenzhen Nanotech Port Co. (Shenzhen, China). Hydrogen tetrachloroaurate (HAuCl4·4H2O), trisodium citrate dihydrate (Na3C6O7·2H2O), ammonium hydroxide (NH4·OH), H2SO4, HNO3, tetraethoxysilane (TEOS), 3-aminopropyl-trimethoxysilane (APTMS), 4-nitrothiophenol (4-NTP), malachite green (MG) and Rhodamine 6G (R6G) were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). 2,2′-dipyridyl (DP) and paraquat were purchased from Sigma-Aldrich. All the chemicals were of analytical grade and used as received without further purification.Synthesis of AuNPs
AuNPs were prepared according to Frens' method,27i.e., the reduction of hydrogen tetrachloroaurate by sodium citrate. In brief, 300 mL of HAuCl4 (0.01%) was heated to boiling, and 3 mL of citrate solution was added. The mixture was allowed to boil for an additional 15 min accompanied by a color change from pale yellow, blue, and finally to a brilliant red, indicating the formation of monodisperse spherical particles. The particles were centrifuged and redispersed in 15 mL of deionized water for use. The as-prepared colloidal Au particles were ca. 40 nm in diameter and had an extinction maximum at about 532 nm, as characterized by scanning electron microscopy (SEM) (taken with a Nova NanoSEM NPE207 field emission electron microscope at an acceleration voltage of 3 kV).Preparation of CNT/AuNP composites
CNT/AuNP hybrids were prepared according to the literature method.28 Prior to the synthesis, multiwalled carbon nanotubes were treated with H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HNO3 (3
HNO3 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume ratio). In a typical procedure, 2 mL of ammonium (28 wt% in water) and 2 mL H2O were added to 40 mL of a CNT ethanol solution (5 mg of CNT). After this, 0.1 mL of TEOS was added and sonicated for 8 h and maintained overnight at room temperature. Then, 40 μL of APTMS was added to the previous solution and stirred for 12 h. After centrifugation and washing 3 times with ethanol and water, respectively, 0.1 mL CNTs with an abundance of amino groups on their side walls were mixed with 1.5 mL of the Au colloid and stirred for 3 h to obtain the CNT/AuNP composites. The prepared CNT/AuNP composites were dispersed in 15 mL of water and stored at 4 °C until use.
1 in volume ratio). In a typical procedure, 2 mL of ammonium (28 wt% in water) and 2 mL H2O were added to 40 mL of a CNT ethanol solution (5 mg of CNT). After this, 0.1 mL of TEOS was added and sonicated for 8 h and maintained overnight at room temperature. Then, 40 μL of APTMS was added to the previous solution and stirred for 12 h. After centrifugation and washing 3 times with ethanol and water, respectively, 0.1 mL CNTs with an abundance of amino groups on their side walls were mixed with 1.5 mL of the Au colloid and stirred for 3 h to obtain the CNT/AuNP composites. The prepared CNT/AuNP composites were dispersed in 15 mL of water and stored at 4 °C until use.
SERS assay
Our SERS substrate was fabricated as described in the previously reported process, in which a volume of CNT/AuNP suspension was passed through the PDVF membrane using a syringe type filter holder.24 The membrane traps and concentrates CNT/AuNP, leading to a uniform plasmonic substrate. After this, the membrane was immersed into appropriate volume of sample solutions, allowing analyte molecules to be efficiently adsorbed on CNT/AuNP hybrids or to the membrane. Then, the SERS spectra were recorded from the surface of the membrane when it was not completely dried using a Labram-1B Raman spectrometer from Yobin Yvon with a laser (2 mW) excitation wavelength of 632.8 nm. A 10-second integration time was used, with averaging of 3 signal acquisitions.Results and discussion
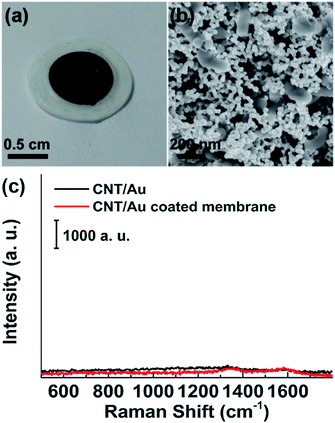
Gold nanoparticles with an average size of 40 nm (ESI Fig. S1a†) were used for the preparation of CNT/AuNP hybrids because of the size-dependent SERS activity and the intrinsic chemical stability of AuNPs. The diameter of the carbon nanotubes increased from ∼60 nm to ∼200 nm after silylation with TEOS and APTMS, suggesting that the CNTs have been coated with a layer of SiO2 (ESI Fig. S1b and S1c†). This structure provided a large surface area and numerous binding sites for the assembly of AuNPs onto CNTs (ESI Fig. S1d†).In a SERS assay, background signals from the enhancing substrate itself may severely interfere with the exaction of the band information of the analyte molecules. In the present work, CNT/AuNP hybrids were used as the Raman-enhancing units, and they produced negligible SERS response when illuminated by laser radiation (Fig. 1c). As shown in Fig. 1a, a deposition of the CNT/AuNP hybrids on the membrane surface resulted in a homogeneously distributed nanostructured coating with a dark brown color. After the filtration of SERS-active CNT/AuNP hybrids, the membrane also showed lower background features (Fig. 1c). The SEM image clearly indicated that the porous membrane was fully coated with layers of CNT/AuNP hybrids, producing a unique nanoporous structure with a large surface area that was beneficial for the adsorption of the analyte molecules. The SEM images also demonstrated that an ultrahigh coverage of AuNPs was achieved on the membrane. Although some CNT terminals were not fully covered by the Au particles, the coverage was at least three times higher than that reported for a previous study in which pure silver nanoclusters were used.24 In addition, an abundant Au clusters were formed because of the cross-linking of the CNT/AuNP hybrids. This inter-tube coupling created a large number of hot-spots, making our CNT/AuNP membrane an excellent SERS substrate.
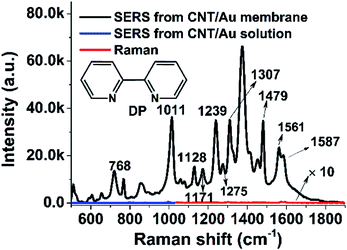
Fig. 2 shows the SERS spectra for the adsorption of 2,2′-dipyridyl (DP) on the CNT/AuNP-decorated membrane and the corresponding nanomaterial suspensions containing DP. No obvious Raman signal was obtained from the CNT/AuNP aqueous solution. After the assembly of the nanohybrids on the membrane surface, a lot of molecular information was observed in the SERS spectrum for the adsorbed DP corresponding well with the literature values.29 To allow a quantitative description of the enhancing ability of the CNT/AuNP-composite-treated membrane, Raman signals were measured for 10 mM of DP deposited on the bare membrane and 10 μM of DP deposited on the membrane onto which the CNT/AuNP hybrids were assembled. The two spectra are shown in Fig. 2b. The enhancement factor (EF) was determined according to the following equation:
| EF = (ISERS × NRaman)/(IRaman × NSERS) | (1) |
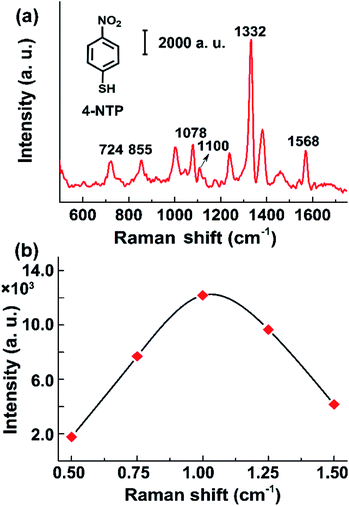
Apparently, the amount of CNT/AuNP hybrids at the membrane surface has a significant influence on the SERS activity. To determine the optimal volume of CNT/AuNP, suspensions of the enhancing material with volumes of 0.5, 1, 1.25, and 1.5 mL were, respectively, passed through four pieces of PVDF membrane, which were then immersed in 200 μL solution of 10−6 mol L−1 of 4-NTP for 60 min, to allow sufficient adsorption of Raman reporters. Fig. 3a shows the SERS spectrum of 4-NTP on the CNT/AuNP-coated filter membrane. The characteristic 4-NTP bands can be clearly identified in the spectrum because of the large signal enhancement.30,31 The SERS intensity of the most prominent band at 1332 cm−1 is compared in Fig. 3b. The peak intensity significantly increased with increasing CNT/AuNP volume, and it reached the largest value when 1 mL of CNT/AuNP was loaded. However, further increases in the CNT/AuNP volume resulted in a rapid decrease in the SERS response. These observations are not difficult to explain. With increases in the volume of the CNT/AuNP suspension, the membrane became darker in color, indicating a steady increase in the membrane surface coverage with the CNT/AuNP hybrids, which was advantageous for creating a multitude of hot spots. However, loading with large amount of CNT/AuNP led to the formation of a thick carbonaceous cake, instead of a three-dimensional plasmonic network, on the membrane. This could have caused the surface properties of the membrane to exhibit characteristics similar to that of a bulk metal. This in turn led to the loss of the SERS activity (Fig. 3b).
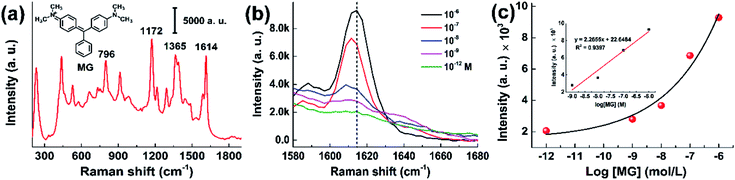
In order to investigate the detection performance of the CNT/AuNP-treated membrane, SERS spectra were collected for different concentrations of malachite green (MG). This dye was initially chosen in our study because its maximum absorption peak is very close to the 632.8 nm wavelength of the laser source used in the measurements. Thus, resonance enhancement could be achieved, and the signal strength could be further improved. A typical SERS spectrum of MG is presented in Fig. 4a, where the prominent peaks at 1172, 1365 and 1615 cm−1 were due to the in-plane modes of C–H ring bending, N-phenyl stretching and C–C ring stretching, respectively. The medium 796 cm−1 band was attributed to the out-of-plane modes of C–H rings.32Fig. 4b shows the intensity changes observed for the band at 1615−1 cm for MG concentrations ranging from 10−8 to 10−12 M. Although the concentration was reduced to pM levels, the characteristic band could still be recognized. Fig. 4c shows a plot of the SERS intensity at 1615 cm−1 as a function of the logarithmic MG concentration. It was found that the relationship between the signal response and the MG concentration was well described by an exponential equation. At higher concentrations (from 10−9 to 10−6 M), the band intensity linearly increased with the MG concentration, providing a correlation coefficient of R2 = 0.9397. Different from the monolayer adsorption isotherm, this nonlinear relationship between the signal response and the concentration was probably due to the multi-layer and porous feature of SERS substrates (Fig. 1b). We speculate that the data in Fig. 4c possibly described the first inflection point of the isotherm. As the detection limit is the main consideration in the present work, the signal responses at very high concentrates were not investigated further.
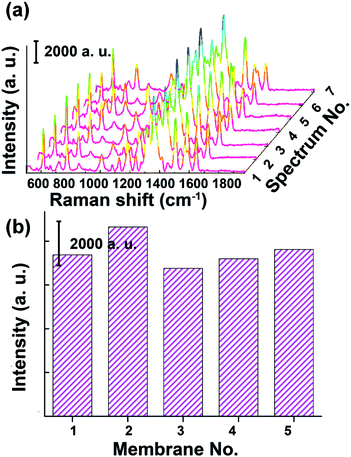
Apart from the enhancing capability, the reproducibility of a SERS substrate is another important parameter for evaluating the practicability of SERS sensors. To investigate the signal homogeneity produced by our CNT/AuNP membrane, the SERS spectra for 10−6 M of R6G were measured from seven randomly selected positions on the membrane. As presented in Fig. 5a, the main Raman bands of R6G, which were associated with the in-plane modes of ring C–C bending (the band at 612 cm−1) and aromatic C–C stretching vibrations (the bands at 1360, 1509, and 1648 cm−1), were significantly enhanced in all of the spectra.33 The intensity distribution of the SERS peak at 1509 cm−1 was calculated. A relative standard deviation (RSD) of 15.8% for Raman intensities at different positions was obtained, indicating a good reproducibility due to the large coverage of AuNPs distributed on the membrane surface.34,35 To further evaluate the influence of the filtration process on the reproducibility of the membrane-based SERS sensor, we also investigated the signal variations from different membranes (Fig. 5b). The RSD of 9.4% revealed that the filtration process could be used as a simple method for the batch fabrication of SERS substrates with good reproducibility. The variation of the signal intensity was considered to be mainly due to the surface unevenness of the membrane after it was treated with the CNT/AuNP hybrids (Fig. 1b), which made it difficult to focus the probe laser in the same plane. On the other hand, it was difficult to ensure that the dye molecules were completely and uniformly adsorbed on the membrane, and the spot diameter of the laser used in the experiment was only approximately 1 μm, giving rise to further contributions to the signal fluctuations. Despite these limitations, the experimental results were comparable with previously reported literature values for paper-based SERS sensors, and clearly demonstrated the good reproducibility of such CNT/AuNP membrane.
In addition to the sensitivity and reproducibility, the stability of the SERS sensor was also evaluated. It is known that the plasmonic substrate is usually exposed to aqueous solution for loading the target molecules for detection. The membrane-based sensor was immersed into water for 48 h, and a little change in the capability for Raman amplification was observed (ESI Fig. S2a†). Furthermore, the membrane still provided stable SERS signals after treating with acid (0.1 M HCl), alkali (1 M NaOH), oxidant (oxygen flow), and UV radiation for 20 min (ESI Fig. S2b†). The high stability might be explained by the chemical inertness of gold and carbon.
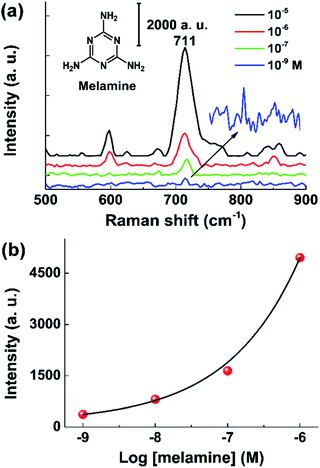
To demonstrate the utility of the CNT/AuNP membrane as a facile and efficient SERS sensor in real applications, the membrane was employed to analyse some hazardous organic compounds. We first performed the on-site detection of melamine in water samples because of ever-increasing food safety considerations. Fig. 6a presents the SERS spectra of melamine with concentrations ranging from 1 nM to 1 μM. As the data shows, the characteristic peak at 711 cm−1 could still be clearly identified even at a concentration as low as 1 nM (0.126 ppb).36Fig. 6b shows a plot of the intensity of the SERS band at 711 cm−1 as a function of the logarithmic concentration of melamine. Similar to the case of MG, the experimental results could be fitted well with an exponential function. The detection performance for 1 nM (0.126 ppb) completely met the current requirements of FDA for the accepted levels of melamine (2.5 ppm). This sensor was further applied to detect melamine in complex samples. As shown in Fig. S3,† as low as 1 nM of melamine spiked in liquid milk could be clearly detected, which was comparable to other SERS-based methods (ESI Table S1†). Fig. 7 shows the test results for paraquat, a bipyridylium herbicide that is extremely dangerous for human health because of its acute and chronic toxicity. The SERS signal intensities varied strongly depending on the concentration of paraquat, and could be described well by a linear equation y = 14.1968x + 133.1575, giving a correlation coefficient of R2 = 0.9968 (Fig. 7a and b).
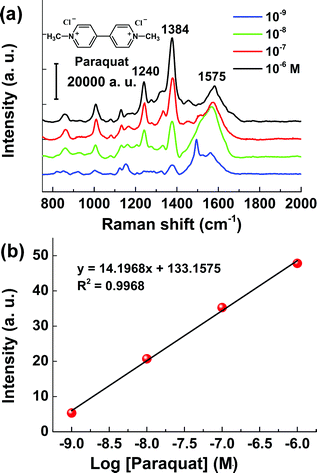 | ||
| Fig. 7 (a) SERS spectra of paraquat with concentrations ranging from 1 nM to 1 μM. (b) Linear plot of the intensity 1384 cm−1 peak versus logarithmic paraquat concentration. | ||
Conclusions
In conclusion, we have demonstrated a very simple, cost-effective and highly efficient SERS sensor by decorating the filter membrane with carbon nanotube/gold nanoparticle hybrid composites. The CNT/AuNP hybrids with a large surface area were highly advantageous for the trapping and concentration of target molecules. The densely assembled gold nanoparticles on the carbon nanotubes and the effective inter-tube coupling provided a large number of SERS hot spots, ensuring strong SERS enhancement. Using an inexpensive filter membrane as the supporting material avoided the low sample collection efficiencies associated with conventional rigid substrates. The flexible SERS substrate exhibited high sensitivity and reproducibility, and it was successfully applied to achieve the trace detection of various compounds with relevance to fields ranging from food safety to environmental protection. Thus, the CNT/AuNP coated membrane reported here shows great potential as a novel, flexible and efficient SERS platform for real-life applications.Acknowledgements
This work is supported by NSFC (21375022, 21175028 and 21105014) and State key lab of Molecular Engineering of Polymers.Notes and references
- W. Lu, A. K. Singh, S. A. Khan, D. Senapati, H. Yu and P. C. Ray, J. Am. Chem. Soc., 2010, 132, 18103–18114 CrossRef CAS PubMed.
- M. Vendrell, K. K. Maiti, K. Dhaliwal and Y.-T. Chang, Trends Biotechnol., 2013, 31, 249–257 CrossRef CAS PubMed.
- C. Jiang, R. Liu, G. Han and Z. Zhang, Chem. Commun., 2013, 49, 6647–6649 RSC.
- Y. Du, R. Liu, B. Liu, S. Wang, M.-Y. Han and Z. Zhang, Anal. Chem., 2013, 85, 3160–3165 CrossRef CAS PubMed.
- A. KumaráSingh and P. ChandraáRay, Chem. Commun., 2011, 47, 10326–10328 RSC.
- S. S. Dasary, A. K. Singh, D. Senapati, H. Yu and P. C. Ray, J. Am. Chem. Soc., 2009, 131, 13806–13812 CrossRef CAS PubMed.
- B. D. Piorek, S. J. Lee, M. Moskovits and C. D. Meinhart, Anal. Chem., 2012, 84, 9700–9705 CrossRef CAS PubMed.
- A. Campion and P. Kambhampati, Chem. Soc. Rev., 1998, 27, 241–250 RSC.
- H. H. Wang, C. Y. Liu, S. B. Wu, N. W. Liu, C. Y. Peng, T. H. Chan, C. F. Hsu, J. K. Wang and Y. L. Wang, Adv. Mater., 2006, 18, 491–495 CrossRef CAS.
- N. A. Abu Hatab, J. M. Oran and M. J. Sepaniak, ACS Nano, 2008, 2, 377–385 CrossRef CAS PubMed.
- D. Choi, Y. Choi, S. Hong, T. Kang and L. P. Lee, Small, 2010, 6, 1741–1744 CrossRef CAS PubMed.
- R. G. Freeman, K. C. Grabar, K. J. Allison, R. M. Bright, J. A. Davis, A. P. Guthrie, M. B. Hommer, M. A. Jackson, P. C. Smith and D. G. Walter, Science, 1995, 267, 1629–1632 CrossRef CAS PubMed.
- F. L. Yap, P. Thoniyot, S. Krishnan and S. Krishnamoorthy, ACS Nano, 2012, 6, 2056–2070 CrossRef CAS PubMed.
- L. L. Qu, D. W. Li, J. Q. Xue, W. L. Zhai, J. S. Fossey and Y. T. Long, Lab Chip, 2012, 12, 876–881 RSC.
- X. Jiang, Y. Lai, M. Yang, H. Yang, W. Jiang and J. Zhan, Analyst, 2012, 137, 3995–4000 RSC.
- L. X. Quang, C. Lim, G. H. Seong, J. Choo, K. J. Do and S. K. Yoo, Lab Chip, 2008, 8, 2214–2219 RSC.
- L. Polavarapu and L. M. Liz-Marzán, Phys. Chem. Chem. Phys., 2013, 15, 5288–5300 RSC.
- C. H. Lee, L. Tian and S. Singamaneni, ACS Appl. Mater. Interfaces, 2010, 2, 3429–3435 CAS.
- K. R. Wigginton and P. J. Vikesland, Analyst, 2010, 135, 1320–1326 RSC.
- R. Zhang, B.-B. Xu, X.-Q. Liu, Y.-L. Zhang, Y. Xu, Q.-D. Chen and H.-B. Sun, Chem. Commun., 2012, 48, 5913–5915 RSC.
- W. W. Yu and I. M. White, Analyst, 2013, 138, 1020–1025 RSC.
- Y. Meng, Y. Lai, X. Jiang, Q. Zhao and J. Zhan, Analyst, 2013, 138, 2090–2095 RSC.
- C. H. Lee, M. E. Hankus, L. Tian, P. M. Pellegrino and S. Singamaneni, Anal. Chem., 2011, 83, 8953–8958 CrossRef CAS PubMed.
- W. W. Yu and I. M. White, Analyst, 2012, 137, 1168–1173 RSC.
- W. EwenáSmith, Analyst, 2012, 137, 2297–2299 RSC.
- Y. Sun, K. Liu, J. Miao, Z. Wang, B. Tian, L. Zhang, Q. Li, S. Fan and K. Jiang, Nano Lett., 2010, 10, 1747–1753 CrossRef CAS PubMed.
- G. Frens, Nature, 1973, 241, 20–22 CAS.
- S. Guo, J. Li, W. Ren, D. Wen, S. Dong and E. Wang, Chem. Mater., 2009, 21, 2247–2257 CrossRef CAS.
- M. Lopez-Ramirez, L. Guerrini, J. V. Garcia-Ramos and S. Sanchez-Cortes, Vib. Spectrosc., 2008, 48, 58–64 CrossRef CAS PubMed.
- J. Huang, Y. Zhu, M. Lin, Q. Wang, L. Zhao, Y. Yang, K. X. Yao and Y. Han, J. Am. Chem. Soc., 2013, 135, 8552–8561 CrossRef CAS PubMed.
- X. Yang, D. Tryk, K. Hasimoto and A. Fujishima, Appl. Phys. Lett., 1996, 69, 4020–4022 CrossRef CAS PubMed.
- Y. Zhang, Y. Huang, F. Zhai, R. Du, Y. Liu and K. Lai, Food Chem., 2012, 135, 845–850 CrossRef CAS PubMed.
- T. T. B. Quyen, C.-C. Chang, W.-N. Su, Y.-H. Uen, C.-J. Pan, J.-Y. Liu, J. Rick, K.-Y. Lin and B.-J. Hwang, J. Mater. Chem. B, 2014, 2, 629–636 RSC.
- W. L. Zhai, D. W. Li, L. L. Qu, J. S. Fossey and Y. T. Long, Nanoscale, 2012, 4, 137–142 RSC.
- L. L. Qu, Q. X. Song, Y. T. Li, M. P. Peng, D. W. Li, L. X. Chen, J. S. Fossey and Y. T. Long, Anal. Chim. Acta, 2013, 792, 86–92 CrossRef CAS PubMed.
- Z.-Q. Wen, G. Li and D. Ren, Appl. Spectrosc., 2011, 65, 514–521 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4an01473a |
| This journal is © The Royal Society of Chemistry 2015 |