Efficient dye-sensitized solar cell with a pure thin film of a hybrid polyoxometalate covalently attached organic dye as a working electrode in a cobalt redox mediator system†
Davud Karimian,
Bahram Yadollahi*,
Mahmoud Zendehdel * and
Valiollah Mirkhani
* and
Valiollah Mirkhani
Department of Chemistry, University of Isfahan, Isfahan 81746-73441, Iran. E-mail: m.zendehdel@sci.ui.ac.ir; yadollahi@chem.ui.ac.ir; Fax: +98-31-37932700; Tel: +98-31-37932748, +98-31-37932742
First published on 1st September 2015
Abstract
Polyoxometalates (POMs) are a class of inorganic metal–oxygen clusters comprising many elements, with unique structures and molecular weights, which possess similar energy band structures to metal oxides. In this research, for the first time, a Keggin-type hybrid polyoxometalate [SiW11O39(Si(CH2)3NH2)2O] (Hybrid-POM) is used as a pure semiconductor thin film in the working electrode of an efficient dye-sensitized solar cell (DSC) which was fabricated with a D35 organic dye as the sensitizer and a one-electron fast redox mediator cobalt complex. The D35 dye is attached to the surface of the POM by a strong amide bond (D35@Hybrid-POM). For comparison, two DSCs were also fabricated with mesoporous anatase TiO2, with and without a titania blocking layer, under the same conditions of electrolyte, sensitizer and counter electrode. J–V measurements of the DSCs were obtained under AM1.5G irradiation and photoelectrochemical parameters like open-circuit voltage (Voc), short-circuit current density (Jsc), fill factor (FF), voltage decay and the overall efficiency were measured and compared to the mesoporous titania cells. The J–V curves show a promising enhancement overall in the DSC with the D35@Hybrid-POM structure in comparison with other prevalent mesoporous titania cells. Incident photon to current conversion efficiency spectra of the D35@Hybrid-POM DSCs and mesoporous titania with TBL (titania blocking layer) working electrodes were also obtained. A maximum IPCE of about 91% was found for the DSC employing D35@Hybrid-POM which is about 30% higher than the DSC with the mesoporous titania layer. The voltage decay measurements, calculated electron lifetime and effective recombination order (β) showed that the electron lifetime becomes promisingly longer when we use the Hybrid-POM structure in the photoanode. Thus, these results show that the rates of recombination and electron transfer of the cobalt mediator system at the surface of Hybrid-POM are very slow.
1. Introduction
Polyoxometalates (POMs) are a nano-sized class of metal–oxygen clusters, which have attracted lots of attention in the last decades. These interesting materials have been widely used in various fields such as catalysis, medicine, materials science and analytical chemistry.1–4 This class of inorganic compounds has found these applications, not only because of the versatile structures, but also because of the redox properties, charge distribution, structure tunability and variety of shapes and distributions.5,6 Among these fascinating properties, the photophysical and photochemical ones have received considerable attention.7–9POMs can reversibly accept and transfer electrons without changing in structure, which makes them an interesting material in photonic and photovoltaic applications.10,11
One of the outstanding properties of POMs is their changeable photoelectric traits, which would be adjustable by changing the structure and composition.3 POMs can accept electrons and play an electron donor role after reduction and because of this, they have been widely used in photovoltaic cells.12–14
Dye-sensitized solar cells (DSCs) are a promising alternative to conventional inorganic semiconductor-based solar cells because of the environmentally friendly and inexpensive materials, and fabrication method.15–17 In typical DSCs, a mesoporous film of titania nanocrystals is sensitized with organic or metal–organic dyes which in most applications are surrounded by a redox mediator in an organic solvent, typically acetonitrile.18–20 In a DSC, hole (h+) transport is accomplished by a solution phase redox mediator, which permeates the pores of the semiconductor and serves to electrically connect the anode and cathode. A redox mediator which displays selective heterogeneous electron transfer kinetics where rapid reduction of the oxidized mediator can occur only at the cathode could minimize electron/hole recombination. In a particularly interesting report, DSCs using the easy-to-prepare cobalt(III/II) tris(4,4′-di-tert-butyl-2,2′-bipyridyl), [Co(t-Bu2bpy)3]3+/2+, as a redox shuttle exhibited excellent incident photon to current efficiencies (IPCEs) equal to ∼80% of those of a comparable cell employing I3−/I−.21 Several promising results have been obtained using Co(II)/Co(III) redox mediators with organic dyes and different semiconductor systems as the photoanode.22–24
In most of the fabricated dye-sensitised solar cells (DSCs) based on POMs, the POM is added in order to modify the efficiency of injection. Using a POM in combination with TiO2, ZnO or other metal oxides can reduce the recombination in the cells. In all of these composites, the presence of metal oxides is necessary for utilization as an under-layer for POMs in the synthesized solar cells.10,25 Additionally, in these cells, the dye is attached to the metal oxides through covalent coordination bonds. In two recent works, Wang and coworkers synthesized a POM–TiO2 composite and used this composite in a working electrode,13 while in the other work, Li made a Rh-substituted POM–TiO2 composite and utilized it in a DSC.26 It goes without saying that most of these systems suffered from many problems and low efficiency, which should be solved in newly designed DSC systems.
Herein, to overcome these problems and achieve a novel method for the synthesis of DSCs based on POMs, a new hybrid system in which there is no under-layer was designed and an organic sensitizer was attached covalently to the POM in the working electrode. In this system we used [SiW11O39]8− as the semiconductor in the working electrode instead of TiO2. An ordered thin film structure of Hybrid-POM with an intrinsic high surface area could be easily fabricated by conventional printing methods. In order to attach the dye to the POM through a net covalent bond, we installed a pendant amine group at the end of the POM by attaching (3-aminopropyl)triethoxysilane (APTES) at the vacant position of the lacunary POM. The formation of an amide bond between the functionalized POM and the carboxylic group of the D35 dye was our devised path for attaching the dye to the metal oxide with just a covalent bond.
2. Experimental details
2.1 Synthesis and fabrication of the D35@Hybrid-POM electrode
2.2 Fabrication of the mesoporous TiO2 electrodes
A mesoporous titania working electrode with and without a thin titania blocking layer was fabricated. The titania sol was prepared (for the titania blocking layer (TBL)) using a previously reported procedure.29,30 For preparing the working electrode, FTO glass substrates (Pilkington, TEC15) were also cleaned in an ultrasonic bath. The conducting glass substrates were coated in a dipping–withdrawing manner (withdrawing speed: 0.1 mm s−1) (one layer) with the titania sol and preheated at 105 °C for 10 min. Mesoporous TiO2 films were prepared with an area of 0.25 cm2 by the doctor-blade method from a colloidal TiO2 paste (Dyesol DSL 30 NRD-T) (two times) and sintered. The temperature gradient program had four levels of 180 (10 min), 320 (10 min), 390 (10 min), and 500 (60 min) °C. The thickness of the layers was checked with a Dektak profilometer (about 7 μm). After sintering, when the temperature came down to 90 °C, the electrode was immersed in a dye bath containing 0.2 mM D35 in ethanol for 24 h. Then the film was rinsed in ethanol to remove excess dye.2.3 Dye-sensitized solar cell fabrication
The solar cells were assembled using a 30 μm thick thermoplastic Surlyn frame with a platinized counter electrode (TEC8), which was prepared by spin-coating (10 μL cm−2) 4.8 mM H2PtCl6 solution in ethanol on the glass substrate followed by heating in air at 400 °C for 30 min. An electrolyte solution consisting of 0.22 M [Co(bpy)3](PF6)2, 0.033 M [Co(bpy)3](PF6)3, 0.1 M LiClO4, and 0.2 M 4-tert-butylpyridine (TBP) in acetonitrile was then introduced through two holes predrilled in the counter electrode, and the cell was sealed with thermoplastic Surlyn covers and a glass coverslip.2.4 Characterization methods
Powder X-ray diffraction (XRD) patterns of the samples were recorded on a Bruker D8 ADVANCE instrument (Germany, wavelength: 1.5406 Å (Cu Kα), voltage: 40 kV, current: 40 mA in the 2θ range from 4 to 80). Infrared (IR) spectra were collected on a FT infrared spectrometer (JASCO, FT/IR-6300 (400–4000 cm−1), Japan). UV-vis diffuse reflectance spectra (UV-vis/DRS) were recorded on a UV-vis spectrophotometer (JASCO, V-670 (190–2700 nm), Japan) using BaSO4 as a reference.Current–voltage (I–V) and voltage decay characteristics were measured using a homemade multifunctional potentiostat instrument and a solar simulator giving light with AM1.5G spectral distribution, which was calibrated using a certified reference solar cell (Fraunhofer ISE) to an intensity of 1000 W m−2. A black mask with an aperture slightly larger (0.6 × 0.6 cm2) than the active area of the solar cell (0.5 × 0.5 cm2) was applied on top of the cell to avoid significant additional contribution from light falling on the device outside the active area. Incident photon to current conversion efficiency (IPCE) spectra were recorded using a computer-controlled setup consisting of a xenon light source (Nikon Xenon XE High Intensity Light Lamp), a monochromator (Spectral Products CM110 Compact 1/8 Meter), and a potentiostat (model BHP 2061-C, Behpajooh, Iran), calibrated using a certified reference solar cell (Fraunhofer ISE).
3. Results and discussion
3.1 Synthesis of D35@Hybrid-POM
In order to make an efficient connection between the dye and the working electrode (POM), a covalent bond between the POM and D35 as the dye molecule was designed. In this regard, first a layer of [SiW11O39]8− was made on the FTO glass. In the next step, for making an appropriate linker to attach D35 to the POM, the POM was treated with (3-aminopropyl)triethoxysilane to prepare an amine pendant group on the POM. Then, the D35 dye was attached to this functionalized POM through an amide bond. Comparison of the FT-IR of Hybrid-POM and D35@Hybrid-POM clearly showed that the peak at 1626 cm−1 is proof for the formation of an amide bond (Fig. 1). Additionally, the existence of the characteristic peaks of the POM in the hybrid product spectra, which correspond to the Si–O, W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and W–O–W bonds, clearly demonstrates that the POM structure has remained intact during this synthesis.
O and W–O–W bonds, clearly demonstrates that the POM structure has remained intact during this synthesis.
3.2 Characterization of the Hybrid-POM thin film
The XRD pattern of the Hybrid-POM thin film is shown in Fig. 2a. The dominant peak at 7.5° presents the (111) plane of the Keggin crystalline phase of the POM. The powder XRD pattern of Hybrid-POM is compared to the simulated pattern using the data set obtained by single-crystal XRD analysis of K10A-α-[SiW9O34]·24H2O (Fig. 2b).31 It is suggested that the grains in the Hybrid-POM thin film have a strongly preferential orientation along the (111) direction in the working electrode in comparison with other SiWxOyPOM XRD patterns in the powder form.32,33 | ||
| Fig. 2 XRD patterns of the Hybrid-POM thin film (a) and simulated pattern using the data set obtained by single-crystal XRD analysis of K10A-α-[SiW9O34]·24H2O31 (b). | ||
The UV-vis reflection spectrum of the Hybrid-POM thin film was measured before and after loading of D35 and the spectra are shown in Fig. 3a. A strong reflection is present in the range of 290–485 nm with a maximum reflection value of 87% that is related to POM absorption at 275 nm. After loading of D35, an extra reflection took place in the region of 510–700 nm, which is related to the broad absorption of D35 at 530 nm.
 | ||
| Fig. 3 UV-vis reflection spectra of the Hybrid-POM thin film before and after loading of D35 (a). Transformed UV-vis DRS spectra in the Kubelka–Munk equation (b). | ||
The optical absorption coefficient (α) is calculated using reflectance data according to the Kubelka–Munk equation, F(R) = α = (1 − R)2/2R, where R is the percentage of reflected light.34 The incident photon energy (hν) and the optical band gap energy (Eg) are related to the transformed Kubelka–Munk function, [F(R)hν]p = A(hν − Eg), where Eg is the band gap energy, A is the constant that depends on transition probability and p is the power index that is related to the optical absorption process. Theoretically p equals 1/2 or 2 for an indirect or direct allowed transition, respectively. The band gap energies of both the Hybrid-POM and D35@Hybrid-POM films were determined to be 3.3 eV based on the indirect transition (Fig. 3b).
From the interference pattern, the refractive index nf can be calculated using eqn (1):35
 | (1) |
| nf = Vsns + (1 − Vs)nair | (2) |
Using this formula, Vs is calculated to be 0.35 and the porosity to be 0.65. These values are in good agreement with high porosity film structures.
The surface structure and film thickness of the Hybrid-POM film are evaluated by FE-SEM and TEM analysis. A high porosity structure for the film surface with an average hole diameter of 10 nm is presented in the FE-SEM image of the surface of the Hybrid-POM film (Fig. S1-a†). The cross-section FE-SEM image of the film layer shows a thickness of 6.38 μm. Furthermore, the TEM image of the Hybrid-POM nanoparticles shows a highly ordered arrangement for the polyoxometalate clusters toward the (111) crystalline direction (Fig. S1-c†).
The dye coverage based on mol per square centimeter was determined according to the optical absorption data of the dye films (Fig. S2†). Using the standard formulas, we obtained a dye coverage (Γ) of 2.5 × 10−7 (mol cm−2) for the coverage of D35 on the polyoxometalate surface. Additionally, having the dye coverage value for D35, we can calculate the Langmuir adsorption isotherm (Γmax) as 2.6 × 10−7 for a 6.4 μm thick polyoxometalate film. The amide covalent bonding of the D35 molecules on Hybrid-POM led to an increase of dye coverage compared to mp-TiO2.37
Through the BET analysis we obtained a total pore volume of 0.52 cm3 g−1 and a porosity of 71%. The BET results show an enhancement of the Hybrid-POM film’s porosity compared to that of the mp-TiO2 film.37 Using a surface area of 1033.5 cm2, the area occupied by a D35 molecule on the polyoxometalates is: 1033.5 × 1014/(2.6 × 10−7 × 6.023 × 1023) = 0.66 nm2.
3.3 Photovoltaic results
The D35@Hybrid-POM thin film was selected to be used as the working electrode in the dye-sensitized solar cells not only due to its appropriate band gap energy but also because the Keggin crystalline structure could improve the electron mobility inside the layer. Furthermore, the (3-aminopropyl)triethoxysilane linker can provide an efficient terminal for covalent bonding of D35 on the POM layer.The photovoltaic parameters of the devices were evaluated by the I–V measurement technique. Statistical variation box plots of the photovoltaic parameters for the Hybrid-POM-based DSCs are presented in Fig. 4. Furthermore, these parameters are compared to the titania-based DSCs with blocking layer (mp-TiO2 with TBL) and without any blocking layer (mp-TiO2 without BL). The power conversion efficiency (η) was derived from the equation η = JscVocFF/I0, where Jsc is the short-circuit current density, Voc is the open-circuit voltage, FF is the fill factor, and I0 is the photon flux illuminating the solar cells. The DSC parameters of selected cells were calculated and are listed in Table 1.
| Efficiency (%) | Voc (V) | Jsc (mA cm−2) | FF | Sample |
|---|---|---|---|---|
| 8.02 | 0.899 | 12.78 | 0.697 | D35@Hybrid-POM |
| 4.63 | 0.787 | 10.98 | 0.536 | Mp-TiO2 with TBL |
| 3.56 | 0.844 | 9.02 | 0.468 | Mp-TiO2 without BL |
Using the polyoxometalate structure, the photovoltage values of the cells are improved to 900 mV which is about 100 mV higher than the mp-TiO2-based cells. The statistical correlation between the Voc values shows a high variation for the mp-TiO2 samples without blocking layer. Comparison of the Jsc values shows a promising enhancement of the photocurrent in the Hybrid-POM device of about 13 mA cm−2, which is higher than other reported values for D35-based DSCs. On the other hand, fill factor values of the Hybrid-POM devices are improved to 70%. In total, the overall efficiencies of the Hybrid-POM devices are improved to 8% which is two-fold higher than the mp-TiO2-based devices.
Incident photon to current conversion efficiency (IPCE) spectra were recorded for the DSCs fabricated with D35@Hybrid-POM and mesoporous titania with blocking layer (see Fig. 5). The IPCE for the Hybrid-POM-based DSC shows higher values between 350 nm to 650 nm (absorption range of D35) in comparison with the mp-TiO2-based DSC. A maximum IPCE of about 91% was found for the DSCs employing D35@Hybrid-POM which is about 30% higher than the DSC with the mesoporous titania layer (61%). The high values of IPCE in the D35@Hybrid-POM-based DSC could be attributed to not only faster electron injection between D35 and the POM structure via the pure covalent bond, but also higher electron mobility (electron diffusion) inside the Keggin structure of the POM.
 | ||
| Fig. 5 Spectra of incident photon to current efficiency (IPCE) for the DSCs based on D35@Hybrid-POM and mesoporous titania with TBL working electrodes. | ||
The voltage, V, is the difference between the Nernstian potential of the solution, E(R+/R), and the quasi-Fermi level, EF/q, (i.e. the potential of the semiconductor) at the contact electrode. With an open circuit, the photocurrent density is exactly offset by recombination, Urec. Recombination is generally used to refer to electron transfer from the semiconductor to the oxidized dye and the oxidized form of the redox shuttle.38,39 The largest increase in Voc could be realized by making the solution potential more positive and/or increasing the quasi-Fermi energy of the semiconductor material. The open circuit photovoltage is thus highly sensitive to the properties of the redox couple and the electronic states of the semiconductor material. In the present work, all the DSCs were fabricated with a similar electrolyte medium, and thus the higher values of Voc could be attributed to the higher energy of the quasi-Fermi level of D35@Hybrid-POM, in comparison with mesoporous TiO2. Furthermore, the crystalline structure of the photoanode layer can directly affect the Voc values. Jadhav et al. have shown that photoelectric performance is strongly dependent on the combined effect of Voc and Jsc which shows the characteristic changes with structure texture and morphology as well as the pore size and specific surface area of the fabricated electrode.40
The maximum fill factor is strictly a function of the diode quality factor, γ, and Voc.41 There are different processes which can cause the apparent diode quality factor to exceed the ideal value of 1, including surface state mediated recombination. Direct electron transfer from the semiconductor substrate to the electrolyte (shunting) can impact the fill factor, especially in cases where outer-sphere redox shuttles are used;39 however, this can be overcome through the use of a blocking layer.42,43 The actual fill factor is also attenuated in the cell by any uncompensated series resistance (Rs). Including γ and Rs, the J–V characteristics can be described in terms of the diode equation written as:
 | (3) |
The high fill factor values of the cell with D35@Hybrid-POM could be attributed to stepping down of the recombination reaction between the POM surface and the electrolyte. The Keggin-type structure of Hybrid-POM provides a strong electron accepter cluster which can easily accept photoelectrons in the vacant d orbitals of the tungsten metal core. Thus, injected electrons could quickly diffuse into the space charge double layer of Hybrid-POM. Thereupon, the amount of injected electrons on the interfacial (electrode/electrolyte) double layer will be reduced, improving the shunt resistance of the electron recombination process at the uncoated surface of the semiconductor. Furthermore, the very high photocurrent values that were obtained for the cell with D35@Hybrid-POM, is another promising result for the cluster-type structure of the photoanode layer. [SiW11O39]8− clusters with diameters of about 1–1.5 nm are strongly attached together44 and this assembling of the clusters led to an increase of the trap states and a promising improvement of electron diffusion into the semiconductor layer. On the other hand, the very strong attachment of the D35 dye with the (3-aminopropyl)triethoxysilane linker by a covalent bond, in line with the hole site of the lacunary POM, can improve the rate of electron injection of the photoexcited electrons to the conduction bond of Hybrid-POM and lead to an increase of the photocurrent.
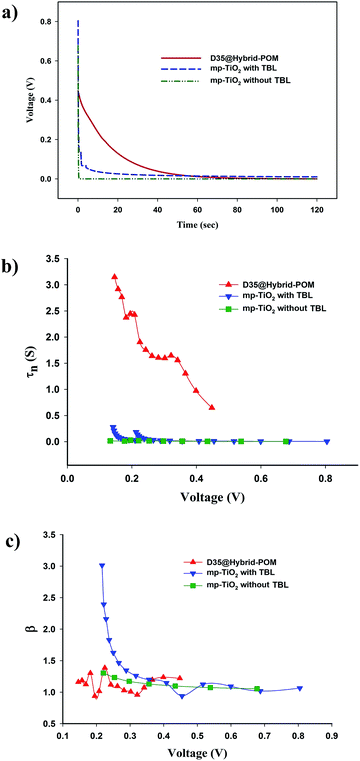
By the open-circuit voltage decay technique we could gain deeper knowledge of the dye regeneration and recombination process in DSCs.45,46 Voltage decay curves of the DSCs with D35@Hybrid-POM, and mesoporous titania with TBL and without TBL are shown in Fig. 6a. The DSC with the D35@Hybrid-POM layer in the photoanode, shows a very slow decay of open-circuit voltage in comparison with the two other cells. The transient (V(t)) electron lifetimes in the DSCs were calculated and are shown in Fig. 6b. Fig. 6b shows that the DSC with D35@Hybrid-POM has higher τe values from 0.5 to 3.2 seconds in the voltage range of 0.50 to 0.10 V but the electron lifetimes of the DSCs with titania are below 0.3 seconds.
Wang et al. presented the relation between the electron lifetime and electron transport process in a polyoxometalate–TiO2 composite by open-circuit voltage decay and electrochemical impedance spectroscopy.13 The high electron lifetimes in the DSC with the Hybrid-POM layer could be directly related to higher electron transport inside the photoanode layer. The effective recombination orders (β) were calculated using a model, including trapping effects, and are presented in Fig. 6c.47 The β values of D35@Hybrid-POM and titania without blocking layer cells are approximately constant at about 1.2 in all the voltage ranges which indicates a constant mechanism for the electron transfer. The voltage decay and electron lifetime results of the DSC with D35@Hybrid-POM are in good agreement with the J–V measurement results, which show a very slow recombination reaction at the surface of POM clusters.
The results from electrochemical impedance spectroscopy analysis of the DSCs (shown in Fig. S3†) are consistent with the above analysis. Nyquist plots of the DSCs containing mp-TiO2 and Hybrid-POM were evaluated at different voltages. The distributed interfacial charge transfer resistance (Rct) is strongly affected by changing the photoanode layer. Rct is significantly increased by changing mp-TiO2 to the polyoxometalate. This confirms that the interfacial electron transfer inside the POM layer is very high compared to electron diffusion in the mesoporous titania layer. The unoccupied d orbitals of tungsten can provide a sufficient direction for electron diffusion between POM clusters and increase the photogenerated charge collection in the device. Furthermore, strongly preferential orientation along the (111) direction could reduce the electron transition dead-ends compared to the random orientation of mesoporous anatase TiO2 grains.
4. Conclusions
In the present work, a new efficient dye-sensitized solar cell using a pure thin film layer of a functionalized Keggin-type POM as the photoanode in a cobalt-complex-based electrolyte medium has been reported. A (3-aminopropyl)triethoxysilane linker provides an efficient terminal group for covalent amide binding of D35 on the POM surface. Furthermore, the crystalline structure of Hybrid-POM led to an increase in the electron lifetime and a decrease in the recombination of the electrons with the redox mediator on the surface. This assembled structure not only led to a promising improvement of the photocurrent (Jsc) and IPCE values, but also increased all photovoltaic parameters and the overall efficiency.Acknowledgements
The authors wish to thank the University of Isfahan for financially supporting of this work.References
- M. T. Pope and A. Müller, Angew. Chem., Int. Ed., 1991, 30, 34 CrossRef PubMed.
- M. T. Pope and A. Müller, Polyoxometalate Chemistry From Topology via Self-Assembly to Applications, Kluwer Academic Publishers, Dordrecht, Netherlands, 2001 Search PubMed.
- H. Park and W. Choi, J. Phys. Chem. B, 2003, 107, 3885 CrossRef CAS.
- Z. X. Sun, L. Xu, W. H. Guo, B. B. Xu, S. P. Liu and F. Y. Li, J. Phys. Chem. C, 2010, 114, 5211 CAS.
- N. V. Izarova, M. T. Pope and U. Kortz, Angew. Chem., Int. Ed., 2012, 51, 9492 CrossRef CAS PubMed.
- X. P. Zheng, Y. Lu, H. Zhang, Z. M. Zhang and E. B. Wang, Inorg. Chem. Commun., 2013, 33, 29 CrossRef CAS PubMed.
- Z. Wang, Y. Ma, R. Zhang, A. Peng, Q. Liao, Z. Cao, H. Fu and J. Yao, Adv. Mater., 2009, 21, 1737 CrossRef CAS PubMed.
- L. Wang, L. Xu, Z. Mu, C. Wang and Z. Sun, J. Mater. Chem., 2012, 22, 23627 RSC.
- N. Fu and G. Lu, Chem. Commun., 2009, 3591 RSC.
- A. Dolbecq, E. Dumas, C. R. Mayer and P. Mialane, Chem. Rev., 2010, 110, 6009 CrossRef CAS PubMed.
- (a) T. Tachikawa, S. Tojo, M. Fujitsuka and T. Majima, Chem.–Eur. J., 2006, 12, 3124 CrossRef CAS PubMed; (b) D. Karimian, B. Yadollahi and V. Mirkhani, Dalton Trans., 2015, 1709 RSC.
- X.-J. Sang, J.-S. Li, W.-L. Chen and E.-B. Wang, Mater. Lett., 2012, 87, 39 CrossRef CAS PubMed.
- S.-M. Wang, L. Liu, W.-L. Chen, E.-B. Wang and Z.-M. Su, Dalton Trans., 2013, 42, 2691 RSC.
- J.-S. Li, X.-J. Sang, W. L. Chen, C. Qin, S.-M. Wang, Z.-M. Su and E.-B. Wang, Eur. J. Inorg. Chem., 2013, 2013, 1951 CrossRef CAS PubMed.
- B. O’Regan and M. Grätzel, Nature, 1991, 353, 737 CrossRef PubMed.
- A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo and H. Pettersson, Chem. Rev., 2010, 110, 6595 CrossRef CAS PubMed.
- Q. Zhang, E. Uchaker, S. L. Candelaria and G. Cao, Chem. Soc. Rev., 2013, 42, 3127 RSC.
- M. Nazeeruddin, A. Kay, I. Rodicio, R. Humphry-Baker, E. Muller, P. Liska, N. Vlachopoulos and M. Grätzel, J. Am. Chem. Soc., 1993, 115, 6382 CrossRef CAS.
- S. M. Feldt, E. A. Gibson, E. Gabrielsson, L. Sun, G. Boschloo and A. Hagfeldt, J. Am. Chem. Soc., 2010, 132, 16714 CrossRef CAS PubMed.
- S. Zhang, X. Yang, Y. Numata and L. Han, Energy Environ. Sci., 2013, 6, 1443 CAS.
- S. A. Sapp, C. M. Elliott, C. Contado, S. Caramori and C. A. Bignozzi, J. Am. Chem. Soc., 2002, 124, 11215 CrossRef CAS PubMed.
- M. H. Habibi, M. Mikhak, M. Zendehdel and M. Habibi, Int. J. Electrochem. Sci., 2012, 7, 6787 CAS.
- M. H. Habibi, A. H. Habibi, M. Zendehdel and M. Habibi, Spectrochim. Acta, Part A, 2013, 110, 226 CrossRef CAS PubMed.
- M. H. Habibi, E. Askari, M. Habibi and M. Zendehdel, Spectrochim. Acta, Part A, 2013, 104, 197 CrossRef PubMed.
- G. Jin, S.-M. Wang, W.-L. Chen, C. Qin, Z.-M. Su and E.-B. Wang, J. Mater. Chem. A, 2013, 1, 6727 CAS.
- J.-S. Li, X.-J. Sang, W.-L. Chen, L.-C. Zhang, Z.-M. Sua, C. Qin and E.-B. Wang, Inorg. Chem. Commun., 2013, 38, 78 CrossRef CAS PubMed.
- P. Souchay, Polyanions et Polycations, Gauthier-Villars, Paris, 1963 Search PubMed.
- D. P. Hagberg, X. Jiang, E. Gabrielsson, M. Linder, T. Marinado, T. Brinck, A. Hagfeldt and L. Sun, J. Mater. Chem., 2009, 19, 7232 RSC.
- M. H. Habibi and M. Zendehdel, Curr. Nanosci., 2010, 6, 642 CrossRef CAS.
- M. H. Habibi and M. Zendehdel, J. Inorg. Organomet. Polym., 2011, 21, 634 CrossRef CAS.
- N. Laronze, J. Marrot and G. Hervé, Inorg. Chem., 2003, 42, 5857 CrossRef CAS PubMed.
- M. Sadakane, Y. Limuro, D. Tsukuma, B. S. Bassil, M. H. Dickman, U. Kortz, Y. Zhang, S. Ye and W. Ueda, Dalton Trans., 2008, 47, 6692 RSC.
- J. Gao, S. Cao, Q. Tay, Y. Liu, L. Yu, K. Ye, P. C. S. Mun, Y. Li, G. Rakesh, S. C. J. Loo, Z. Chen, Y. Zhao, C. Xue and Q. Zhang, Sci. Rep., 2013, 3, 1853 Search PubMed.
- H. Lin, C. P. Huang, W. Li, C. Ni, S. I. Shah and Y.-H. Tseng, Appl. Catal. B., 2006, 68, 1 CrossRef CAS PubMed.
- G. Boschloo and D. Fitzmaurice, J. Phys. Chem. B, 1999, 103, 2228 CrossRef CAS.
- A. V. Sankarraj, Electrocatalytic and Antibacterial Application of Sandwich type Polyoxometalates, Auburn University, 2008, p. 89 Search PubMed.
- M. Pazoki, P. W. Lohse, N. Taghavinia, A. Hagfeldt and G. Boschloo, Phys. Chem. Chem. Phys., 2014, 16, 8503 RSC.
- M. Nasr-Esfahani, M. Zendehdel, N. YaghoobiNia, B. Jafari and M. KhosraviBabadi, RSC Adv., 2014, 4, 15961 RSC.
- N. Yaghoobi Nia, P. Farahani, H. Sabzyan, M. Zendehdel and M. Oftadeh, Phys. Chem. Chem. Phys., 2014, 16, 11481 RSC.
- N. A. Jadhav, P. K. Singh, H.-W. Rhee, S. P. Pandey and B. Bhattacharya, Int. J. Electrochem. Sci., 2014, 9, 5377 CAS.
- T. W. Hamann and J. W. Ondersma, Energy Environ. Sci., 2011, 4, 370 CAS.
- B. M. Klahr and T. W. Hamann, J. Phys. Chem. C, 2009, 113, 14040 CAS.
- T. W. Hamann, O. K. Farha and J. T. Hupp, J. Phys. Chem. C, 2008, 112, 19756 CAS.
- Y. Wang and I. A. Weinstock, Chem. Soc. Rev., 2012, 41, 7479 RSC.
- M. H. Habibi, B. Karimi, M. Zendehdel and M. Habibi, J. Ind. Eng. Chem., 2014, 20, 1462 CrossRef CAS PubMed.
- M. H. Habibi, B. Karimi, M. Zendehdel and M. Habibi, Spectrochim. Acta, Part A, 2013, 116, 374 CrossRef CAS PubMed.
- A. Zaban, M. Greenshtein and J. Bisquert, ChemPhysChem, 2003, 4, 859 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra09104g |
| This journal is © The Royal Society of Chemistry 2015 |