Open Access Article
Open Access ArticleElectronic and transport properties of carbon and boron-nitride ferrocene nanopeapods
Guiling
Zhang
a,
Sun
Peng
a,
Yan
Shang
a,
Zhao-Di
Yang
a and
Xiao Cheng
Zeng
*b
aInnovative Research Team of Green Chemical Technology in University of Heilongjiang Province, College of Chemical and Environmental Engineering, Harbin University of Science and Technology, Harbin 150040, China
bDepartment of Chemistry, University of Nebraska-Lincoln, Lincoln, NE 68588, USA. E-mail: xzeng1@unl.edu
First published on 29th September 2014
Abstract
Electronic and transport properties of novel ferrocene based carbon nanotube (CNT) and boron-nitride nanotube (BNNT) nanopeapods, including Fe(Cp)2@CNT, Fe2(Cp)3@CNT, Fe(Cp)2@BNNT, and Fe2(Cp)3@BNNT (where Cp refers as cyclopentadiene), are investigated using the density functional theory and non-equilibrium Green's function methods. Computed electronic structures of the Fe(Cp)2@CNT and Fe2(Cp)3@CNT nanopeapods suggest that their electric conductivity is primarily contributed by the CNT π channel while the electron hopping from the core Fe(Cp)2 or Fe2(Cp)3 to the sheath CNT may have some contribution to the transport properties. Encapsulating Fe(Cp)2 into BNNT is more favorable for the electron conduction, owing to the splitting of the BNNT bandgap by the Fe(Cp)2 state. In contrast, introducing Fe2(Cp)3 into the BNNT is not beneficial to the conduction due to intramolecular electron transfer within the core Fe2(Cp)3 which can cause a trap effect. Because the transport channels can be changed by the applied bias voltage, the transport properties cannot be solely predicted from the electronic structures of infinite systems alone. For computing transport properties, we use two-probe device model systems with a finite-sized nanopeapod sandwiched between two CNT electrodes. Again, we find that encapsulating either Fe(Cp)2 or Fe2(Cp)3 into CNTs has little effect on the conductivity owing to the strong metallic character of the CNT sheath. Encapsulating Fe(Cp)2 into BNNTs can notably enhance electron conducting due to electron hopping from the core Fe(Cp)2 to the sheath BNNT. Encapsulating Fe2(Cp)3 into BNNTs, however, has little effect on the electron conductivity of BNNT nanopeapods due to the trap effect of the longer guest molecules. Hence, the length of guest molecules can effectively tune electronic and transport properties of the BNNT nanopeapods.
1 Introduction
Carbon nanotubes are capable of encapsulating guest atoms or molecules into their inner cylindrical space, thus forming a quasi-one-dimensional core–sheath structure of X@CNT, where X denotes the core atoms or molecules. Thus far, a large number of organic and inorganic species, such as fullerenes,1–6 single elements,7,8 multimeric thiophene oligomers,9,10 methyl chloride,11 oxides,12etc. have already been introduced into CNT's cylindrical inner space as guest molecules, thereby open exciting possibility of creating new materials that can be tailored to a particular electronic functionality. For example, the iron-fullerene complexes (η5-Cp)FeC60R5 (R = Me and Ph; Cp = C5H5) have been successfully encapsulated into CNTs by Nakamura et al.13 Li et al. demonstrated “ferrocene nanopeapods” by cramming ferrocene molecules into the CNTs.14Boron nitride nanotubes (BNNTs)15,16 are another prevailing tubular nanomaterials which have also been the subject of extensive studies over the past decade. Like CNTs, BNNTs also possess many intriguing properties such as strong hardness, high thermal conductivity and chemical inertness.17–19 Indeed, BNNTs are known to be more chemically and thermally stable than CNTs.20 As such, BNNTs are especially suitable for the task of shielding and protecting guest molecules from external chemical attack.21 Many previous studies have reported that BNNTs can be used to encapsulate metal nanowires (Ni, Co, Fe, and Cu),22–26 oxides (α-Al2O3 and Al18B4O33),27,28 SiC carbides,29,30 GaN nitrides,31,32 potassium halide nanowires,33 or fullerenes.34
Recently, the potential to change electron transport properties of CNTs and BNNTs by encapsulating suitable atoms or molecules has attracted increasing attention from researchers in many fields. However, the specific role of guest molecules inside nanotubes on the electronic and transport properties can be highly system-dependent. For example, a previous study shows that the electric conduction pathway within Fe@CNT is actually through CNT percolation35 while the encapsulated iron nanoparticles have little influence on the CNT conductivity. However, Hsu and coworkers found that the Fe core in CNTs can generate an electromagnetic inductive phase, suggesting some participation in electron transport.36 Therefore, a theoretical study of the one-dimensional (1D) core–sheath structures X@CNT and X@BNNT will be informative for understanding special effects of the core on the electronic and transport properties of endohedral X@CNT and X@BNNT materials.
It is known that CNTs can be either metallic or semiconducting, depending on their diameter and chirality, while the BNNTs are always insulating with bandgaps of 4–5 eV.37–40 Such a striking difference in electronic properties between CNTs and BNNTs will inevitably lead to different physical properties of the corresponding endohedral X@CNT and X@BNNT. For example, density-functional theory (DFT) calculation suggests that the (η6-C60-V)@CNT nanopeapod is metallic with characteristics of multiple carriers contributed from the CNT, C60, and V, while the (η6-C60-V)@BNNT nanopeapod is predicted to be semiconducting with a narrow bandgap, and its charge carriers are contributed only by the C60V chain.41
In the laboratory, many multidecker organometallic sandwich clusters have been synthesized.42,43 It has been shown that the 1D sandwich compounds can exhibit semiconducting or even conducting properties.44,45 The Fen(Cp)n+1 nanowires have attracted much attention as they are promising components for nanoelectronic devices, especially in high density storage and quantum computing.46 The Fen(Cp)n+1 sandwich clusters have been synthesized in the gas phase and characterized by mass spectroscopy.42 Note that ferrocene molecules have already been encapsulated into CNTs to create a class of self-assembled hybrid structures named as “ferrocene nanopeapods”.14 Thus, we expect that the Fen(Cp)n+1 clusters may also be encapsulated into nanotubes to form “Fen(Cp)n+1 nanopeapods”. In this study, we investigate the electronic and transport properties of Fen(Cp)n+1 nanopeapods and their differences from pure nanotubes. Specifically we select Fe(Cp)2@CNT (1a), Fe2(Cp)3@CNT (1b), Fe(Cp)2@BNNT (2a), and Fe2(Cp)3@BNNT (2b) as prototype nanopeapod systems to compute their electronic structures and transport properties using DFT and non-equilibrium Green's function (NEGF) methods. We find that the length of the core per supercell of CNTs, i.e., Fe(Cp)2versus Fe2(Cp)3, can have notable effects on the electronic and transport properties of the CNT and BNNT nanopeapods.
2 Models and computational methods
Fig. 1 shows optimized structures of nanopeapods 1a, 1b, 2a, and 2b. Here, metallic CNTs (6, 6) and insulating BNNTs (6, 6) are selected as the host nanotubes. For computing electronic structures, the infinite nanotube systems of 1a, 1b, 2a, and 2b are modeled via using the periodic conditions in the axial direction. For computing transport properties, the two-probe devices (a unit cell of the nanopeapod sandwiched between two CNT (6, 6) electrodes) are adopted. For the purpose of the benchmark test, we have computed electronic properties using infinite nanotube systems and transport properties using the two-probe devices for pure CNTs (6, 6) and BNNTs (6, 6). | ||
| Fig. 1 Optimized structures of 1a, 1b, 2a, and 2b. 1a and 2a also illustrate the two-probe devices for electron transport computation. | ||
For the periodic systems, the supercell contains 120 atoms for the nanotubes and one Fe(Cp)2 or Fe2(Cp)3 unit inside the nanotube. The Cps of Fe(Cp)2/Fe2(Cp)3 are packed along the axial direction as shown in Fig. 1. The supercell length L is about 12.3 Å in the axial direction (z direction), long enough to neglect intermolecular interactions among core molecules Fe(Cp)2 or Fe2(Cp)3. The nanopeapods are separated by 20.0 Å in two other directions to neglect inter-tube interactions. All the periodic systems are fully optimized until the maximum absolute force is less than 0.02 eV Å−1.
For the two-probe systems, we carve out a supercell for each nanopeapod system as the central scatter region which is sandwiched between two identical CNT (6, 6) electrodes (cf.1a and 2a in Fig. 1). A reason for choosing CNTs (6, 6) as the electrodes is its known metallic character and good matching with the scatter region of peapods. The entire two-probe devices are denoted as CNT/peapod/CNT, i.e., CNT/1a/CNT (CNT/Fe(Cp)2@CNT/CNT), CNT/1b/CNT (CNT/Fe2(Cp)3@CNT/CNT), CNT/2a/CNT (CNT/Fe(Cp)2@BNNT/CNT), and CNT/2b/CNT (CNT/Fe2(Cp)3@BNNT/CNT). The entire system including two electrodes and a scatter region is fully optimized until the maximum absolute force is less than 0.02 eV Å−1. Transport current is computed by changing the applied bias in the step of 0.2 V in the range of −1.0 to 1.0 V.
All the computations for the infinitely long and two-probe systems are performed using an ab initio code package, Atomistix ToolKit (ATK), which is based on the combination of DFT and the NEGF methods.47–50 A generalized gradient approximation (GGA) within the Perdew–Burke–Ernzerhof (PBE) formalism is employed to describe the exchange correlations between electrons. A double-ζ basis functional with polarization (DZP) is used for all atoms. A (1 × 1 × 150) k-point in the string Brillouin zone (x, y, and z directions, respectively) is used. 150 Ry cutoff energy is applied to describe the periodic wave function.
3 Results and discussion
First, we investigate geometrics and band structures of the infinitely long nanopeapods of 1a, 2a, 1b, and 2b, followed by computing transport properties based on the two-probe devices of CNT/nanopeapod/CNT with the metallic CNT (6, 6) electrodes.3.1 Geometric structures and electronic properties
| Species | Total energies (in unit of eV) |
|---|---|
| 1a Fe(Cp)2@CNT | −21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 307.81607 307.81607 |
| 1b Fe2(Cp)3@CNT (FM) | −22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 925.03092 925.03092 |
| 1b Fe2(Cp)3@CNT (AFM) | −22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 924.98010 924.98010 |
| 2a (Cp)2@BNNT | −23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 612.79319 612.79319 |
| 2b Fe2(Cp)3@BNNT (FM) | −25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 267.18630 267.18630 |
| 2b Fe2(Cp)3@BNNT (AFM) | −25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 267.11294 267.11294 |
Chemical stability for encapsulating either Fe(Cp)2 or Fe2(Cp)3 into nanotubes is evaluated by computing the reaction energy per supercell for the net reaction [nanotube (NT) + Fe(Cp)2 or Fe2(Cp)3 → peapod − ΔEr]. Here, the computed reaction energies ΔEr are −3.27, −7.18, −1.89, and −2.93 eV for 1a, 1b, 2a, and 2b, respectively. The negative values indicate exothermic energies. Hence, incorporation of Fe(Cp)2 or Fe2(Cp)3 into either CNTs or BNNTs is energetically favorable.
The optimized supercell length in the axial direction (L), the radii of the nanotube (R), the face-to-face distances between adjacent Cps (r1), the average C–C bond lengths in Cp (r2), and the distances between the H atom of Cp and the nearest atom of the nanotube (r3) for 1a, 1b, 2a and 2b are given in Table 2. Incorporation of Fe(Cp)2 or Fe2(Cp)3 into nanotubes induces a slight expansion of the host nanotube as reflected from the larger values of R compared to the pristine nanotube. The distances r1 are within the range of 3.188–3.280 Å, very close to the Cp-to-Cp separation in the Fe(Cp)2 molecule (3.32 Å)51–53 but slightly shorter than that (3.40 Å) in the ferrocene dimer.54 The computed C–C bond lengths in Cp r2 are in the range of 1.447–1.463 Å for both CNT and BNNT nanopeapods, very close to the experimentally measured bond lengths for ferrocene (1.440 Å).55 The shortest distances between the H atom of Cp and the nearest atom of the nanotube (r3) are in the range of 1.998–2.094 Å. Similar to the ferrocene, the H atoms on the terminal Cp rings tilt inward (toward the Fe atoms) by 6–9°.
| Species | L | R | r 1 | r 2 | r 3 |
|---|---|---|---|---|---|
| Pure CNT | 12.310 | 8.298 | |||
| 1a Fe(Cp)2@CNT | 12.302 | 8.352 | 3.280 | 1.447 | 2.016 |
| 1b Fe2(Cp)3@CNT | 12.313 | 8.328 | 3.258 | 1.458 | 1.998 |
| Pure BNNT | 12.315 | 8.147 | |||
| 2a Fe(Cp)2@BNNT | 12.304 | 8.452 | 3.188 | 1.452 | 2.094 |
| 2b Fe2(Cp)3@BNNT | 12.313 | 8.489 | 3.248 | 1.463 | 2.078 |
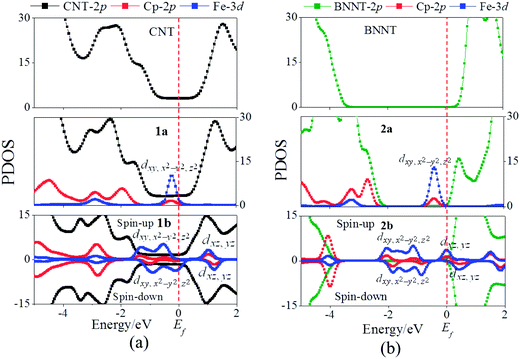
Fig. 2 displays the band structures of the CNT nanopeapods 1a and 1b, as well as the Kohn–Sham orbitals near the Fermi level (Ef). For pure CNTs, the valence band I (red line in Fig. 2(a)) and the conduction band II (blue line in Fig. 2(a)) cross at Ef with large dispersion, suggesting a typical metallic character. In 1a, two nearly degenerate and flat levels, the Fe dxy and dx2−y2 bands (cyan and wine lines in Fig. 2(b)), are located just below Ef. The Kohn–Sham orbital shows that the Fe dz2 state is coupled with the Cp π state (denoted as a dz2(Fe)-π(Cp) state). Notably, this dz2(Fe)-π(Cp) state hybridizes with the CNT π state, giving rise to a band just below Ef with modest dispersion (dark yellow line in Fig. 2(b)). Hence, the core–sheath interaction occurs when Fe(Cp)2 is encapsulated into the CNT. The CNT band I (red line in Fig. 2(b)) is located just below the dz2(Fe)-π(Cp) state and crosses with the dz2(Fe)-π(Cp) state. The unoccupied Fe dxz and dyz states are far away from Ef and hence not displayed in Fig. 2(b). The conduction band still includes the CNT band II in 1a (blue line in Fig. 2(b)). Thus, there may be two conducting pathways in 1a: one through the CNT and another through electron hopping from the core Fe(Cp)2 to the sheath CNT.
For nanopeapod 1b, the spin-up state clearly shows different features from the spin-down state, which results in a magnetic moment of 0.81 μB per supercell (with two Fe atoms), close to 1.0 μB per supercell for the Fen(Cp)n+1 nanowire.56 The Kohn–Sham orbitals plotted in Fig. 2(c) and (d) show the strong core–sheath interaction in 1b as in 1a. More bands, originated mainly from the Fe dxy, dx2−y2, and dz2 states, are introduced below Ef in both spin-up and spin-down states. Again, the unoccupied Fe dxz and dyz orbitals are far away from Ef and thus not plotted in Fig. 2(c) and (d). Compared to pure CNTs, one can see that the CNT bands II and III (blue and green lines in Fig. 2(c)) in the spin-up state of 1b downshift considerably, and both bestride Ef. As such, the CNT π state in the spin-up state behaves like half-filled and dominates the electron transport. In the spin-down state, the CNT band II (blue line in Fig. 2(d)) crosses Ef and the CNT band III (green line in Fig. 2(d)) is located just above Ef. Therefore, the CNT π state in the spin-down state still serves as a major transport pathway. One occupied band originated mainly from the Fe d orbital of the spin-down state upshifts across Ef, turned into a half-filled band, which suggests that electrons could transfer to the core Fe2(Cp)3. Hence, the core Fe2(Cp)3 may also serve as a localized trap-state in the process of electron transport, which is not beneficial to the conducting. Overall, the conductivity of 1b is likely contributed from multiple factors: (1) the CNT π channel dominates the conductivity; (2) electron hopping from core Fe2(Cp)3 to CNTs may have appreciable effects on the transport properties; and (3) Fe2(Cp)3 may entails a trap effect on the electron transport.
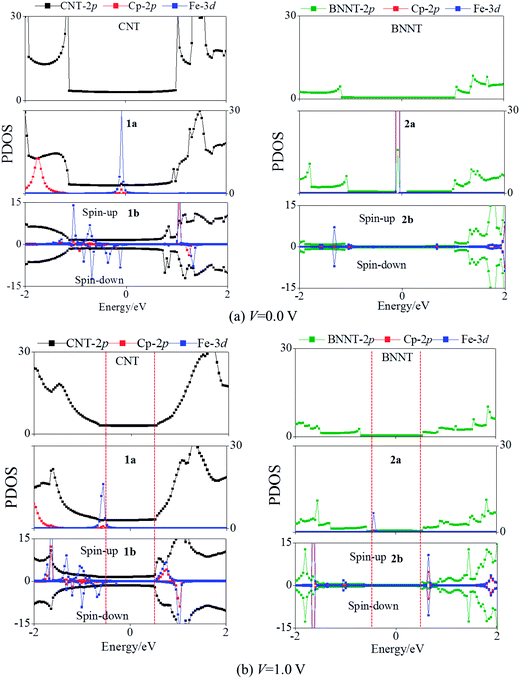
Fig. 3(a) plots the PDOS of pure CNTs, 1a, and 1b. Evidently, the CNT π states of pure CNTs, 1a, and 1b exhibit similar PDOS peaks and broad valley feature around Ef. Thus, the CNT π states are still the main transport channels even with the core Fe(Cp)2 or Fe2(Cp)3. In 1a, the PDOS peaks of Fe dxy, dx2−y2, and dz2 states are located below Ef, hybridized with the CNT π state. The unoccupied Fe dxz and dyz PDOS peaks do not arise even up to 2.0 eV (Fig. 3(a)). The conduction band of 1a is dominated by the CNT π state. The core Fe(Cp)2 may participate in the electron tunneling through electron hopping to the CNT. In the case of 1b, a similar Fe2(Cp)3–CNT hopping channel also arises in the spin-up state. Compared to 1a, the PDOS peak of the unoccupied Fe dxz and dyz states moves to the lower energy region, but is still located at about 1.0 eV. The splitting of the spin states of 1b results in an asymmetric PDOS distribution for the spin-up and spin-down states. The Fe dxy, dx2−y2, and dz2 orbitals in the spin-down state are shifted toward the higher energy region compared to the spin-up state, so that the Fe d orbital of the spin-down state crosses Ef with a half-filled character, again a manifestation of the trap effect due to the core Fe2(Cp)3.
 | ||
| Fig. 3 Computed projected density of states (PDOS) of 1a, 1b, 2a, and 2b nanopeapod systems. For comparison, PDOS of the pure CNT and BNNT systems are also presented. | ||
In summary, electronic structures of the 1a and 1b systems suggest that the electric conductivity is primarily contributed by the CNT π channel, while electron hopping from the core Fe(Cp)2 or Fe2(Cp)3 to the sheath CNT may have some contribution to the transport properties. On the other hand, the core Fe2(Cp)3 may also have a trap effect on the electron transport. Comparison with transport properties obtained based on the two-probe devices of CNT/1a/CNT and CNT/1b/CNT will be discussed in Section 3.2.
In Fig. 4, computed band structures of the pure BNNT, 2a, and 2b are plotted, so are the Kohn–Sham orbitals near Ef. For pure BNNTs, as expected, the valence band I (red line in Fig. 4(a)) and the conduction band II (green line in Fig 4(a)) are separated by a large bandgap of 4.52 eV. In 2a, the core Fe(Cp)2 introduces three flat bands (cyan, wine, and dark yellow lines in Fig. 4(b)) in the range of −0.37 to −0.43 eV and these bands are mainly contributed from the occupied Fe dxy, dx2−y2, and dz2 orbitals. The unoccupied orbitals stem mainly from Fe dxz and dyz are far away from Ef and thus not shown in Fig. 4(b). Unlike 1a which entails strong core–sheath interactions, in 2a little FeCp2–BNNT interaction occurs as reflected from the Kohn–Sham orbitals and the flat feature of the Fe(Cp)2 bands in Fig. 4(b). The three nearly degenerate Fe(Cp)2 bands split the native BNNT band gap into two subgaps. As a result, the Fe(Cp)2 state turns into the valence band and the BNNT band II is the conduction band. As such, the bandgap is reduced to 0.73 eV for 2a from 4.52 eV of the pristine BNNT. This notable band reduction indicates that electron hopping from the core Fe(Cp)2 to the sheath BNNT is significant in 2a.
For 2b, more orbitals originated from the core Fe2(Cp)3 appear in the bandgap region of BNNTs. Clearly, the spin-up and the spin-down states are different, leading to a magnetic moment of 0.98 μB per supercell. In the spin-up state, the Fe dxz and dyz orbitals, due to coupling with the Cp π orbital, are downshifted and across Ef (cyan and wine lines in Fig. 4(c)), showing a half-filled character and a trap-state. This case differs from 1a, 1b, and 2a for which the Fe dxz and dyz orbitals are located far away from Ef. For the spin-down state, the conduction band is contributed by Fe dxz and dyz orbitals coupled with certain BNNT π orbitals as can be seen from the Kohn–Sham orbitals shown in Fig. 4(d) (cyan and wine lines). The valence band (dark yellow and dark cyan lines in Fig. 4(d)) stems only from Fe dxy and dx2−y2 orbitals. Hence, the core Fe2(Cp)3 can also have a trap effect on the electron transport in the spin-down state.
Fig. 3(b) shows computed PDOS of the pure BNNT, 2a, and 2b. The BNNT retains the insulating properties in 2a and 2b, as the BNNT π PDOS are largely separated above and below Ef. In 2a, the Fe dxy, dx2−y2, and dz2 orbitals contribute a peak below Ef, resulting in a valence state, while the BNNT π orbitals also contribute to the conduction state. Thus, electrons can be transported by hopping from the core Fe(Cp)2 to the sheath BNNT, consistent with the conclusion based on computed band structures. Like in 1a, the unoccupied Fe dxz and dyz PDOS are far away from Ef (Fig. 3(b)). No PDOS hybridization between the BNNT and the FeCp2 is seen near Ef, suggesting little Fe(Cp)2–BNNT interaction in 2a. This result is consistent with the Kohn–Sham orbital diagram in Fig. 4(b). In 2b, the Fe dxz and dyz orbitals, coupled with the Cp π orbital, are downshifted less substantially compared with 1a, 1b, and 2a. As a result, the Fe dxz and dyz PDOS peaks appear just across Ef in the spin-up state, showing a half-filled feature and a trap state. Overall, the Fe d PDOS in the spin-up and spin-down states are asymmetric. In the spin-down state, the Fe dxz and dyz orbitals are located just above Ef while hybridized with the BNNT π state, resulting in the conduction band; the valence band originates from the Fe dxy, dx2−y2, and dz2 orbitals. Therefore, the trap effect could also exist in the spin-down state. These results are in line with the band structure analysis.
From the above analysis for 2a and 2b, it seems that encapsulating Fe(Cp)2 is more favorable for the electron conduction, owing to the splitting of the BNNT bandgap by the Fe(Cp)2 state. Electrons could transport through hopping from the core Fe(Cp)2 to the sheath BNNT. In contrast, introducing Fe2(Cp)3 into the BNNT is not beneficial to the conduction due to intramolecular electron transfer within the core Fe2(Cp)3 which can cause a trap effect. More related discussions are given in Section 3.2.
3.2 Two-probe devices
To obtain more quantitative transport properties of the nanopeapods and analyze the effect of core Fe(Cp)2 or Fe2(Cp)3 on the transport properties, we construct a model system such that a supercell of 1a, 1b, 2a, or 2b is sandwiched between two CNT (6, 6) electrodes to form two-probe devices (denoted as CNT/nanopeapod/CNT). Calculation results based on this model system suggest that electric conductivities of the two-probe devices are mostly consistent with the electronic structures of the corresponding infinitely long nanopeapod systems, although the computed electronic structures can be influenced by applied external bias voltage.The computed I–V curves based on the two-probe devices are given in Fig. 5. It can be seen that pure CNTs, 1a, and 1b show a metallic feature and give nearly the same I–V curves (Fig. 5(a)), suggesting that the conductivity is entirely contributed by the CNT (π state) in the two-probe devices, consistent with the conclusion based on the computed electronic structures. Moreover, conductivities of the CNT/1b/CNT and CNT/2b/CNT devices appear independent of the spin state within the considered bias range of −1.0 to 1.0 V, although the band structures and PDOS distributions are dependent on the spin state. Fig. 5(b) shows that the pure BNNT, 2a, and 2b exhibit features of insulators. Their conductivities follow the sequence 2a > pure BNNT > 2b, again consistent with the predication based on computed electronic structures, that is, encapsulation of Fe(Cp)2 into BNNTs would enhance conductivity while encapsulating Fe2(Cp)3 into BNNTs would lower the conductivity.
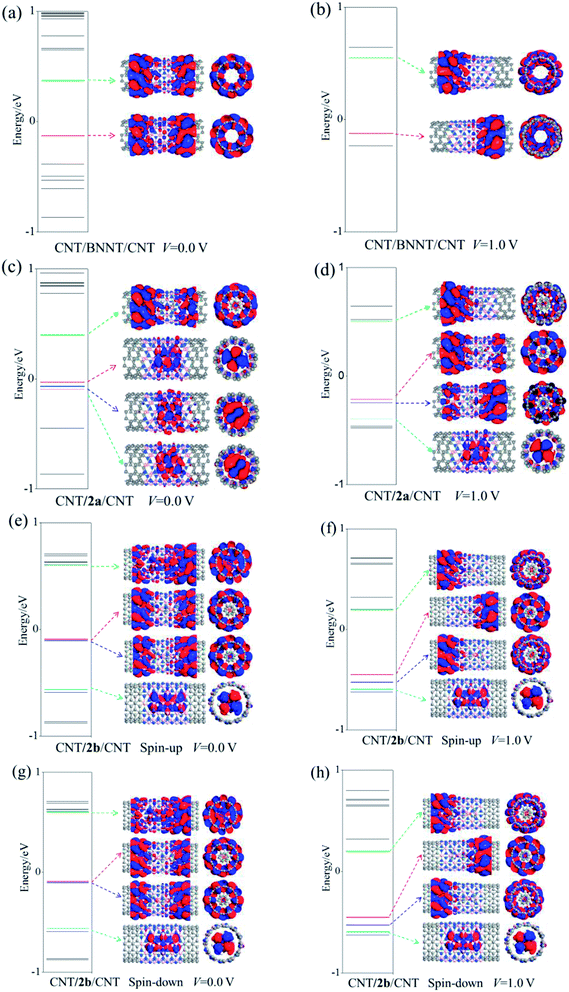
In general, computed electric conductivity is dependent on multiple factors, including, for examples, energy spectra, the molecular projected self-consistent Hamiltonian (MPSH) states, the PDOS, the transmission spectra (TS), and electrostatic potentials, among others. Next, we analyze the extent to which these factors affect the transport properties of each two-probe CNT/nanopeapod/CNT devices. Results are depicted in Fig. 6–10, from which one can see that the applied external bias voltage can alter the transport channel.
4 Conclusions
We have investigated the electronic and transport properties of a novel form of Fe(Cp)2@CNT, Fe2(Cp)3@CNT, Fe(Cp)2@BNNT, and Fe2(Cp)3@BNNT by means of DFT and NEGF methods. We have found that endohedral encapsulation of Fe(Cp)2/Fe2(Cp)3 into CNTs (6, 6) or BNNTs (6, 6) is energetically favorable. In Fe2(Cp)3@CNT and Fe2(Cp)3@BNNT nanopeapods the spin of Fe is coupled ferromagnetically. Conductivities of Fe2(Cp)3@CNT and Fe2(Cp)3@BNNT nanopeapods appear to be independent of the spin state within the bias range of −1.0 to 1.0 V, even though the band structures and PDOS distributions of the spin-up state and the spin-down state of the infinite structures are different. Characteristics of computed electric conductivities based on the two-probe devices are qualitatively consistent with the computed electronic structures of the corresponding infinite nanopeapod systems.Encapsulating either Fe(Cp)2 or Fe2(Cp)3 into the CNT has little effect on the conductivity owing to the strong metallic character of the CNT sheath. Encapsulating Fe(Cp)2 into the BNNT however can notably enhance electron conduction due to added electron hopping from the core Fe(Cp)2 to the sheath BNNT. Encapsulating Fe2(Cp)3 into the BNNT does not assist electron conduction due to the trap effect. Hence, the electronic and transport properties of the BNNT nanopeapods are very sensitive to the length of guest molecules while those of the CNT nanopeapods are insensitive to the length of guest molecules. These properties of BNNT and CNT nanopeapods can be exploited for nanoelectronic and sensor applications.
Acknowledgements
XCZ is supported by grants from the NSF (EPS-1010674) and ARL (W911NF1020099), and by the University of Nebraska Holland Computing Center. GLZ is supported by grants from the NSFC (51073048 and 51473042), the NSF of Heilongjiang Province of China (B201102), the SF for leaders in the academy of Harbin City of China (2013RFXXJ024), and foundation for Scientist Leaders of Heilongjiang Provincial Talent Echelon.References
- B. W. Smith, M. Monthioux and D. E. Luzzi, Nature, 1998, 396, 323 CrossRef CAS PubMed.
- A. Burteaux, B. Claye, W. Smith, M. Monthoioux, D. E. Luzzi and J. E. Fischer, Chem. Phys. Lett., 1999, 310, 21 CrossRef.
- J. Sloan, R. E. Dunin-Borkowski, J. L. Hutchison, K. S. Coleman, V. C. Williams, J. B. Claridge, A. P. E. York, C. Xu, S. R. Bailey, G. Brown, S. Friedrichs and M. L. H. Green, Chem. Phys. Lett., 2000, 316, 191 CrossRef CAS.
- Y. Kataura, T. Maniwa, K. Kodama, K. Kikuchi, K. Hirahara, S. Suenaga, S. Iijima, Y. Suzuki, Y. Achiba and W. Krätschmer, Synth. Met., 2001, 121, 1195 CrossRef.
- K. Hirahara, S. Bandow, K. Suenaga, H. Kato, T. Okazaki, H. Shinohara and S. Iijima, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 64, 115420 CrossRef.
- K. Masatoshi, K. Haruka, S. Takuya, I. Toru, S. Hironori, O. Toshiya, K. Hiromichi, M. Yutaka and Y. J. Kazuhiro, J. Am. Chem. Soc., 2012, 134, 9545 CrossRef PubMed.
- P. M. Ajayan and S. Iijima, Nature, 1993, 361, 333 CrossRef CAS.
- R. S. Lee, H. J. Kim, J. E. Fischer, A. Thess and R. E. Smalley, Nature, 1997, 388, 255 CrossRef CAS PubMed.
- M. Milko, P. Puschnig, P. Blondeau, E. Menna, J. Gao, M. A. Loi and C. Draxl, J. Phys. Chem. Lett., 2013, 4, 2664 CrossRef CAS PubMed.
- Y. Takashi and Y. Hiroki, J. Phys. Chem. C, 2014, 118, 5510 Search PubMed.
- G. Pietro, B. Andrea, C. Matteo and Z. Francesco, J. Phys. Chem. C, 2014, 118, 5032 Search PubMed.
- H. Jiang, Y. Hu, S. Guo, C. Yan, P. S. Lee and C. Li, ACS Nano, 2014, 8, 6038 CrossRef CAS PubMed.
- E. Nakamura, M. Koshino, T. Saito, Y. Niimi, K. Suenaga and Y. Matsuo, J. Am. Chem. Soc., 2011, 133, 14151 CrossRef CAS PubMed.
- N. Sun, L. Guan, Z. Shi, N. Li, Z. Gu, Z. Zhu, M. Li and Y. Shao, Anal. Chem., 2006, 78, 6050 CrossRef CAS PubMed.
- A. Rubio, J. L. Corkill and M. L. Cohen, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 49, 5081 CrossRef CAS.
- N. G. Chopra, R. J. Luyken, K. Cherrey, V. H. Crespi, M. L. Cohen, S. G. Louie and A. Zettl, Science, 1995, 269, 966 CrossRef CAS PubMed.
- E. Hernandez, C. Goze, P. Bernier and A. Rubio, Phys. Rev. Lett., 1998, 80, 4502 CrossRef CAS.
- C. W. Chang, A. M. Fennimore, A. Afanasiev, D. Okawa, T. Ikuno, H. Garcia, L. Deyu, A. Majumdar and A. Zettl, Phys. Rev. Lett., 2006, 97, 085901 CrossRef CAS.
- D. Golberg, X. D. Bai, M. Mitome, C. C. Tang, C. Y. Zhi and Y. Bando, Acta Mater., 2007, 55, 1293 CrossRef CAS PubMed.
- Y. Chen, J. Zhou, S. J. Campell and G. L. Caer, Appl. Phys. Lett., 2004, 84, 2430 CrossRef CAS PubMed.
- P. Ravinder and V. Subramanian, J. Phys. Chem. C, 2013, 117, 5095 CAS.
- K. B. Shelimov and M. Moskovits, Chem. Mater., 2000, 12, 250 CrossRef CAS.
- D. Golberg, Y. Bando, K. Kurashima and T. Sato, J. Nanosci. Nanotechnol., 2001, 1, 49 CrossRef CAS PubMed.
- C. C. Tang, Y. Bando, D. Golberg, X. X. Ding and S. R. Qi, J. Phys. Chem. B, 2003, 107, 6539 CrossRef CAS.
- R. Z. Ma, Y. Bando and T. Sato, Chem. Phys. Lett., 2004, 350, 1 CrossRef.
- A. L. M. Reddy, B. K. Gupta, T. N. Narayanan, A. A. Martí, P. M. Ajayan and G. C. Walker, J. Phys. Chem. C, 2012, 116, 12803 CAS.
- R. Z. Ma, Y. Bando and T. Sato, Adv. Mater., 2002, 14, 366 CrossRef CAS.
- H. S. Song, J. Zhang, J. Lin, S. J. Liu, J. J. Lou, Y. Huang, E. M. Elssfah, A. Elsanousi, X. X. Ding, J. M. Gao and C. Tang, J. Phys. Chem. C, 2007, 111, 1136 CAS.
- C. C. Tang, Y. Bando, T. Sato and K. Kurashima, J. Mater. Chem., 2002, 12, 1910 RSC.
- Y. B. Li, P. S. Dorozhkin, Y. Bando and D. Golberg, Adv. Mater., 2005, 17, 545 CrossRef CAS.
- W. Q. Han and A. Zettl, Appl. Phys. Lett., 2002, 81, 5051 CrossRef CAS PubMed.
- J. Zhang, L. D. Zhang, F. H. Jiang and Z. H. Dai, Chem. Phys. Lett., 2004, 383, 423 CrossRef CAS PubMed.
- W. Q. Han, C. W. Chang and A. Zettl, Nano Lett., 2004, 4, 1355 CrossRef CAS.
- W. Mickelson, S. Aloni, W. Q. Han, J. Cumings and A. Zettl, Science, 2003, 300, 467 CrossRef CAS PubMed.
- P. C. P. Watts, W. K. Hsu, G. Z. Chen, D. J. Fray, H. W. Kroto and D. R. M. Walton, J. Mater. Chem., 2001, 11, 2482 RSC.
- P. C. P. Watts, W. K. Hsu, D. P. Randall, V. Kotzeva and G. Z. Chen, Chem. Mater., 2002, 14, 4505 CrossRef CAS.
- A. Rubio, J. L. Corkill and M. L. Cohen, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 49, 5081 CrossRef CAS.
- X. Blase, A. Rubio, S. G. Louie and M. L. Cohen, Europhys. Lett., 1994, 28, 335 CrossRef CAS.
- J. Cumings and A. Zettl, Chem. Phys. Lett., 2000, 316, 211 CrossRef CAS.
- W. Q. Han, W. Mickelson, J. Cumings and A. Zettl, Appl. Phys. Lett., 2002, 81, 1110 CrossRef CAS PubMed.
- G. Zhang, R. Zhou and X. C. Zeng, J. Mater. Chem. C, 2013, 1, 4518 RSC.
- S. Nagao, A. Kato and A. Nakajima, J. Am. Chem. Soc., 2000, 122, 4221 CrossRef CAS.
- P. Kruse, E. R. Johnson, G. A. DiLabio and R. A. Wolkow, Nano Lett., 2002, 2, 807 CrossRef CAS.
- T. Kuhlmann, S. Roth, J. Rozière and W. Siebert, Angew. Chem., Int. Ed. Engl., 1986, 25, 105 CrossRef.
- M. Y. Lavrentiev, H. Köppel and M. C. Böhm, Chem. Phys., 1993, 169, 85 CrossRef CAS.
- N. J. Long, Metallocenes, Blackwell Science Press, Oxford, 1998 Search PubMed.
- J. Taylor, H. Guo and J. Wang, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 63, 245407 CrossRef.
- M. Brandbyge, J. L. Mozos, P. Ordejón, J. Taylor and K. Stokbro, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 65, 165401 CrossRef.
- J. M. Soler, E. Artacho, J. D. Gale, A. Garcia, J. Junquera, P. Ordejon and D. Sanchez-Portal, J. Phys.: Condens. Matter, 2002, 14, 2745 CrossRef CAS.
- ATK, Version 13.8, atomistix a/s, 2013, http://www.quantumwise.com Search PubMed.
- J. C. Wu, X. F. Wang, L. P. Zhou, H. X. Da, K. H. Lim, S. W. Yang and Z. Y. Li, J. Phys. Chem. C, 2009, 113, 7913 CAS.
- H. X. Da, H. M. Jin, S. W. Yang and K. H. Lim, J. Phys. Chem. C, 2009, 113, 21422 CAS.
- J. F. Yang, L. P. Zhou, Q. Han and X. F. Wang, J. Phys. Chem. C, 2012, 116, 19996 CAS.
- K. Peter, R. J. Erin, A. D. Gino and A. W. Robert, Nano Lett., 2002, 2, 807 CrossRef.
- M. Lein, J. Frunzke, A. Timoshkin and G. Frenking, Chem.–Eur. J., 2001, 7, 4155 CrossRef CAS.
- L. Shen, S. Yang, M. Ng, V. Ligatchev, L. Zhou and Y. J. Feng, J. Am. Chem. Soc., 2008, 130, 13956 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |