Preparation of a novel magnetic cellulose nanocrystal and its efficient use for enzyme immobilization†
Abstract
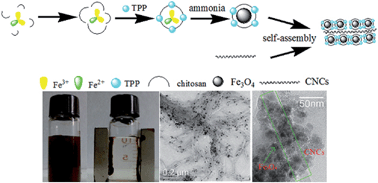
A novel biocompatible magnetic cellulose nanocrystal (MCNC) composite was in situ prepared via a simple co-precipitation-electrostatic-self-assembly technique and was structurally characterized. The results showed that the anionic cellulose nanocrystals (CNCs) were successfully composited with cationic chitosan-coated Fe3O4 by self-assembly technology. The electrostatic interaction between CNCs and chitosan, and that between chitosan and Fe3O4, were the key driving forces for the formation of the composite. Papain, a widely used protease, could be successfully immobilized on the activated MCNCs with formaldehyde. The immobilized papain exhibited higher thermal stability than the free enzyme, with the relative activity being higher than 80% after incubation at 40 °C for 7 h while that of free papain was less than 30%. Also, the pH stability of immobilized papain was superior to that of free papain. Moreover, the immobilized papain showed significantly better tolerance to the three solvents tested compared with its free counterpart. The optimum range of pH for immobilized papain (pH 5–10) was remarkably wider than that of free enzyme (pH 5–7). The relative activities of immobilized papain at 50–70 °C were more than 90%, which significantly surpassed those of free papain. The immobilized papain also manifested excellent storage stability, with relative activity being as high as 93.6% after 16 days of storage at 4 °C. Furthermore, the obtained kinetic constant values showed that papain immobilized on the MCNCs had relatively high catalytic efficiency. Additionally, the immobilized papain could be easily separated and recycled from the reaction system through magnetic forces. Obviously, the prepared MCNCs as novel supports are promising and competitive for enzyme immobilization.

 Please wait while we load your content...
Please wait while we load your content...