 Open Access Article
Open Access ArticleNanocarbon-based gas sensors: progress and challenges
Shun
Mao
a,
Ganhua
Lu
b and
Junhong
Chen
*a
aDepartment of Mechanical Engineering, University of Wisconsin-Milwaukee, 3200 N Cramer Street, Milwaukee, WI 53211, USA. E-mail: jhchen@uwm.edu; Fax: +1-414-229-6958; Tel: +1-414-229-2615
bDepartment of Mechanical Engineering, University of Alaska Anchorage, 3100 Providence Drive, Anchorage, Alaska 99508, USA
First published on 7th January 2014
Abstract
Novel materials based on nanocarbons (e.g., carbon nanotubes (CNTs) and graphene) have attracted much attention as sensing elements in miniaturized, low-power consumption, and ubiquitous electronic gas sensors due to their unique structural and electronic properties. This highlight discusses some recent progress in the research on nanocarbon-based electronic gas sensors, including CNTs, graphene, and their composites (i.e., nanocarbon–nanocrystal hybrids), identifies the technological barriers that impair their commercialization, and presents an outlook of the challenges and opportunities for the use of nanocarbon-based materials in next generation gas sensors.
There is a need for simple and reliable gas sensors suitable for trace detection in a wide spectrum of applications ranging from environmental monitoring, industrial, transportation, energy, agriculture, medical diagnosis, and lab-on-a-chip. The last decade has witnessed the explosion of nanomaterials for gas sensing, e.g., metal oxides1–3 and nanocarbon-based materials.4 Among those nanomaterials, carbon nanotubes (CNTs) and graphene5–10 have become two of the most popular sensing materials for gas detection due to their high sensitivity to various gases and low operation temperatures compared with metal oxide gas sensors. The unique structures and outstanding electronic properties of CNTs and graphene, e.g., small size, large specific surface area, high electron mobility, and high sensitivity to electrical perturbations from gas molecules, bring them great promise in miniaturized and high-performance gas sensors and many studies have demonstrated their use in gas and chemical detection. In this highlight, we discuss some recent progress in the research on CNT and graphene-based electronic gas sensors, identify the technological barriers that hinder their commercialization, and give an outlook of the challenges and opportunities for the use of nanocarbon-based materials in next generation gas sensors.
CNT-based gas sensors
CNTs are carbon atom network-based tubular nanomaterials with unique electronic properties owing to their structures.11,12 The electronic properties of CNTs are extremely sensitive to their local chemical environment, making them ideal candidates for gas/chemical sensors. The electrical detection of a gas with CNTs is based on the change in electrical characteristics of CNTs upon their interactions with gas molecules. The study on CNT gas sensors started with two pioneering reports in Science by Dai13 and Zettl14 groups in 2000. Dai and co-workers13 synthesized semiconducting single-walled carbon nanotubes (S-SWCNTs) (p-type transistors) and investigated their conductance change in the presence of NO2 and NH3. They found that upon exposure to gaseous molecules, the electrical conductivity of an S-SWCNT dramatically increased or decreased. The reason for the conductivity changes in the SWCNTs was the carrier (in this case the hole) density changes in the nanotubes due to the gas molecule adsorption. In the other report,14 Zettl et al. showed that the electrical resistance and local density of states of the SWCNTs were dramatically influenced by exposing the tubes to air or oxygen. Their studies demonstrated that SWCNTs could be used as gas sensing elements, and the change in electrical characteristics of SWCNTs could indicate the presence of certain gases.Along with the pioneering studies, people explored the sensing mechanism of SWCNTs and found the gas sensing properties of SWCNTs related to the presence of defects and residual contaminants in the nanotubes. It is believed that the charge transfer between gas species and CNTs is responsible for the conductivity change in the tubes.15 In general, oxidizing gases (e.g., NO2) withdraw electrons from CNTs, whereas reducing gases (e.g., NH3, H2, and CO) donate electrons to CNTs, which could lead to opposite changes in the electrical conductivity of CNTs. Functionalization of CNTs has been proposed to promote the charge transfer between specific gas species and CNTs. For example, decorating CNTs with nanocrystals (NCs) to form CNT–NC hybrids can improve the CNT sensing performance, leading to enhanced sensitivity, faster response/recovery, and better selectivity.16–18
One typical CNT–NC hybrid sensor structure is CNTs decorated with noble metals, e.g., palladium (Pd) or platinum (Pt), which can be used for H2 sensing.17,19–22 Bare CNTs cannot detect H2 effectively at room temperature because of the weak binding energy between the H2 molecule and the CNTs. The hybrid structure of Pd/Pt–CNTs provides a sensing platform by using CNTs as a transducer to convert the chemical reaction between the noble metal and hydrogen into strong electrical signals. In one study, Sun et al. showed a flexible Pd–SWCNT H2 sensor,17 which could detect H2 with a concentration of as low as 30 ppm in air at room temperature. The sensors also exhibited fast response times (typically around 1.5 seconds for 1% H2) and short recovery times (on the order of minutes) when operated at room temperature. Besides Pt and Pd, silver (Ag) NCs have also been used to functionalize CNTs for gas sensing.23,24 One recent study by our group23 demonstrated a fast and selective NH3 sensor using multi-walled CNTs (MWCNTs) decorated with Ag NCs. Ag NCs could significantly enhance the sensitivity, response, and recovery of MWCNT sensors (Fig. 1). The hybrid Ag–MWCNT sensor had a good stability and selectivity, with a detection range from 0.125% to 1% NH3. This study further indicates that the combination of noble metals with CNTs could efficiently improve the sensitivity of CNTs and pave a way to highly sensitive sensors for specific gases.
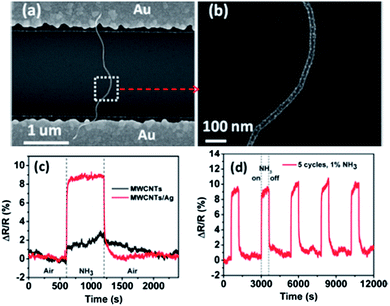 | ||
| Fig. 1 (a) SEM image of a MWCNT decorated with Ag NCs bridging two gold electrode fingers. (b) Magnified SEM image of the Ag–MWCNT structure as marked in (a). (c) The dynamic sensing response (ΔR/R) of the MWCNT to 1% NH3 before and after Ag NCs decoration. (d) Five sensing cycles of the Ag–MWCNT hybrid sensor to 1% NH3. Reprinted with permission from ref. 23. Copyright 2012 American Chemical Society. | ||
Although noble metal–CNT sensors have shown good performance, noble metals are expensive due to their rarity. On the other hand, tin oxide (SnO2), an n-type semiconductor material, is abundantly available and widely used as a gas sensing element. Particularly, SnO2–CNT sensors can work at room temperature, which is significantly lower than typical operation temperatures (200 °C or above) for pure SnO2 sensors.16,25–29 We recently reported an ultrafast hydrogen-sensing platform with SnO2–SWCNT hybrids.30 The sensors were fabricated with 95% sorted semiconducting SWCNTs and SnO2 NCs (Fig. 2). Direct adsorption of H2 onto the SnO2–SWCNT surface induces an electron transfer between gas molecules and SWCNTs, thereby changing the sensor conductivity. The fabricated sensors show an ultrafast response to 1% hydrogen at room temperature with a response time of 2–3 seconds and could fully recover in air within several minutes. To minimize the device-to-device variations, we also fabricated SWCNT films to replace randomly dispersed individual SWCNTs and the results show that the SWCNT film sensors have a much narrower base resistance range, indicating the potential of using SWCNT films for fabricating sensors with repeatable properties. This study shows that with non-precious metal oxides, CNT–NC sensors could deliver performance comparable with that of CNT–precious metal NC sensors.
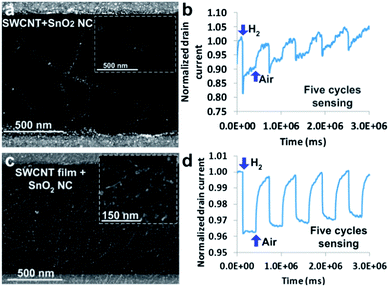 | ||
| Fig. 2 SEM images of (a) individual SWCNTs and (c) an SWCNT film decorated with SnO2 NCs. Insets show the magnified images of the hybrid SWCNT–SnO2 structures. H2 sensing signals from (b) the SnO2–SWCNT and (d) the SnO2–SWCNT film sensor for five cycles of sensing. Reprinted with permission from ref. 30. Copyright 2012 RSC Publishing. | ||
CNTs and functionalized CNTs have shown great promise in gas sensing applications and many reports demonstrate the use of CNTs for the detection of low concentration gas species including NO2, NH3, H2, H2S, SO2, NO, NO2, CO, chemical vapors, etc.6,9,31–33 However, there are several issues preventing the use of CNT sensors in real applications. The first is that the sensor performance, e.g., sensitivity, detection limit, response/recovery time, selectivity, and stability, should be further improved to meet the technical requirements of real applications. It is known from previous reports that semiconducting SWCNTs show much better gas sensing performance, e.g., higher sensitivity and faster response, than metallic SWCNTs and MWCNTs. Therefore, for high quality gas sensors, semiconducting SWCNTs are preferred and low-cost methods should be developed for the synthesis of pure semiconducting SWCNTs or the removal of metallic SWCNTs from the SWCNT bundle.34,35 Surface conditions of the CNTs, e.g., oxygen/water molecules on the CNT surface, may also influence the sensor responses to target gases. One method using ultraviolet (UV) light illumination to remove these contaminants was proposed and this treatment could enhance an SWCNT sensor's performance by orders of magnitude under otherwise identical sensing conditions.31
Another major challenge for the practical application of CNT-based gas sensors is the randomness of CNTs and device-to-device variations, which arise from the sensor fabrication method, i.e., the drop-casting technique. The drop-casting technique is commonly used to prepare nanostructure-based sensors by simply dropping a nanomaterial solution/suspension onto the interdigitated electrodes followed by drying. The drawback of the drop-casting method is that the coverage or distribution of the nanomaterials on the interdigitated electrodes is random and it is very difficult to control the layout of the nanomaterials. With a dielectrophoresis method, a uniform distribution (along the electric field) of the nanomaterials could be achieved in an applied electric field and this method works very well for CNTs.36,37 However, even with the dielectrophoresis method, it is still a great challenge to control the layout of the individual CNTs and make two exactly identical sensors. Fortunately, there are several ways to address this issue. One is using CNT films to replace individual CNTs. With well-controlled film fabrication methods, CNT film sensors could have a similar structure and base resistance, which will result in reproducible sensing responses to target gases. Another way is to use signal processing/data interpretation methods to calibrate the sensor and minimize the device-to-device and run-to-run variations, which will be discussed in the graphene sensor section.
Graphene-based gas sensors
Graphene, a two-dimensional (2D) one-atom-thick free standing carbon layer, has drawn significant attention due to its unique structure and properties.38 Graphene and reduced graphene oxide (RGO)39-based nanostructures have been widely studied for gas sensor applications due to their large specific surface area (2630 m2 g−1)40 and high sensitivity to electrical perturbations from gas molecule adsorption. Because the specific surface area and carrier mobility of graphene are larger than those of CNTs, graphene is believed to be a better sensing element than CNTs. The first graphene gas sensor was reported in 2007 by Novoselov's group,41 which demonstrated that micrometer-size sensors made from graphene are capable of detecting individual gas molecules that attach to or detach from the graphene surface. They showed that the adsorbed molecules change the local carrier concentration in graphene one electron by one electron, which leads to step-like changes in resistance. The gas-induced changes in resistivity had different magnitudes for different gases, and the sign of the change indicated whether the gas was an electron acceptor (e.g., NO2, H2O, iodine) or an electron donor (e.g., NH3, CO, ethanol). This study has opened a door for a new type of gas sensors based on 2D graphene; thereafter, many studies have focused on graphene-based gas sensors and shown that the intrinsic graphene is sensitive to changes in its chemical environment due to its high specific surface area, high carrier mobility, and low electrical noise at room temperature.42–44For certain gases (e.g., NO2, NH3),45–53 intrinsic graphene has high sensitivity under low gas concentrations; however, for those gases, the sensor selectivity is poor,23 which limits its use in practical applications. Similar to CNT gas sensors, functionalizing graphene with NCs is an effective method to tune the sensor selectivity.54 We reported a selective gas-sensing platform with RGO decorated with SnO2 NCs.55 This hybrid SnO2–RGO platform showed an enhanced NO2 but weakened NH3 sensing compared with bare RGO, showing promise in tuning the sensitivity and selectivity of RGO-based gas sensors (Fig. 3). The experimental results suggest that the reason for the different sensitivity changes in NO2 and NH3 sensing is the p–n junction structure formed between p-type RGO and n-type SnO2 NCs, and it is anticipated that this structure will modify the gas-sensing performance of the RGO sensor.
 | ||
| Fig. 3 (a) Schematic of the novel gas-sensing platform of a RGO sheet decorated with SnO2 NCs. (b) Gas sensing signals of NO2 and NH3 from RGO sensors with and without SnO2 NCs. The sensing signal is normalized by the measured sensor current in air (base line, Ig/Ia = 1). Reprinted with permission from ref. 55. Copyright 2012 RSC Publishing. | ||
Intrinsic graphene has very high sensitivity to NO2 and NH3; however, the sp2 carbon–carbon bonds in graphene are chemically stable, which leads to a relatively weak interaction between graphene and many other gas molecules (e.g., H2 and CO). To enhance the sensitivity of graphene to these gas species, chemical or physical functionalization of graphene with noble metals has been widely studied.56,57 In particular, Pd NCs on RGO sheets could make the RGO sheets sensitive to hydrogen. In one report,58 Pd-functionalized graphene nanoribbons were fabricated and used for H2 sensing. Intrinsic graphene is a semimetal with zero bandgap; however, by engineering the graphene structure into a graphene nanoribbon (quasi-one dimensional structures with narrow widths (<10 nm) and atomically smooth edges), the graphene could exhibit bandgaps useful for room temperature transistor operations.59,60 This nanoribbon structure greatly enhances the semiconducting properties of graphene; analogous to semiconducting SWCNTs, these graphene nanoribbons are suggested to have enhanced gas sensor sensitivity compared with pristine graphene sheets. For this sensor, Pd-functionalized graphene nanoribbons show high sensitivity to H2 at room temperature (ΔR/R ∼55% for 40 ppm H2) with fast response and recovery. This work shows the possibility of using semiconducting graphene in a wide range of gas sensing applications, especially for gases that have weak interactions with graphene.
Noble metals and metal oxides, e.g., Pt, Au, SnO2, Cu2O, and ZnO,19,61–66 have been demonstrated to have synergistic effect with graphene for improved gas sensing performance. At the same time, ternary systems with graphene and two distinct NCs have also been proposed and they show further improved sensing properties compared with pure graphene and graphene–NC binary systems. For example, Russo et al. demonstrated a ternary system for hydrogen sensing with RGO functionalized with SnO2 and Pt NCs.67 The ternary system showed hydrogen sensing performance superior to the corresponding pure and binary graphene systems (RGO–SnO2 and RGO–Pt) for H2 detection. They attributed the outstanding performance of the ternary system to the hetero-junction formed between the n-type SnO2 and the p-type RGO and the catalytic effect of Pt NCs for dissociating H2. Similar to the ternary system, doped NCs, e.g., doped SnO2 NCs, are also used for graphene sensors. The idea of the doped SnO2 NCs is that the carrier concentration of the semiconducting SnO2 can be dramatically increased by dopants, which facilitates the electron transfer during the interactions with gases. In addition, dopants can generate a large amount of oxygen vacancies and chemisorbed oxygen species, which can further enhance the interaction between SnO2 NCs and gases. Recently, we reported a NO2 sensor with In-doped SnO2 NCs distributed on the RGO (RGO–IDTO) (Fig. 4).68 Excellent NO2 sensing performance with high sensitivity and selectivity was exhibited by the RGO–IDTO sensors. The addition of indium to SnO2 significantly enhanced the sensor sensitivity to NO2 and a detection limit of 0.3 ppm was found. The RGO–IDTO sensors also show excellent selectivity toward NO2 in the presence of other gases, including H2S, CO, H2, and NH3, which can be understood as a “superposition effect”. This study shows that the sensitivity and selectivity of graphene–NC sensors can be greatly improved by suitable dopants in the NCs.
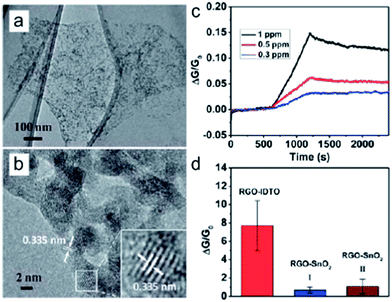 | ||
| Fig. 4 (a and b) TEM and high-resolution TEM images of RGO–IDTO nanohybrids. (c) Dynamic sensing responses of RGO–IDTO toward different NO2 concentrations. (d) Sensitivity comparison of RGO–IDTO and RGO–SnO2 nanohybrids to 100 ppm NO2. Reprinted with permission from ref. 68. Copyright 2013 RSC Publishing. | ||
Although graphene and graphene–NC hybrids have been widely studied for gas sensing applications and many promising sensor platforms have been reported, the graphene gas sensors have inherent limitations arising from the electronic properties of graphene. As we discussed, intrinsic graphene has a zero bandgap and exhibits a low on–off current ratio when used as the conducting channel in a field-effect transistor. Therefore, the sensitivity of the graphene sensor is limited by its low on–off ratio/small bandgap even if the graphene has a high specific surface area and high carrier mobility. Fortunately, there are methods to convert graphene from a semimetal to a semiconductor. In particular, a sizable energy gap can be opened up in graphene through the quantum confinement effect by engineering graphene in the form of a graphene nanoribbon or a graphene nanomesh, or chemical modification of the graphene by doping, e.g., nitrogen and silica,69,70 or surface functionalization.59 Until now, studies on graphene sensors with increased graphene semiconducting properties are limited,69,71–74 and we believe that the improvement in graphene semiconducting properties by structure engineering or chemical modification will be an appealing direction for the future development of graphene-based gas sensors. On the other hand, the use of RGO in graphene sensors is likely to be an issue with respect to reproducibility and extensive processing; the oxygen groups in RGO may impact the electronic properties of the sensor and may also react with the gas molecules during the sensing. Recent developments in a shearing process of graphite exfoliation in organic solvents to prepare defect-free graphene rather than chemical processing may assist fabrication of graphene sensors with a simple synthesis procedure and better sensing performance.75
Similar to CNT gas sensors, the device-to-device variations and run-to-run variations caused by incomplete recovery of graphene devices could also hinder the use of graphene-based gas sensors for real-world applications. To address this issue, we previously proposed a signal processing/data interpretation method (i) to circumvent the run-to-run variations in sensing performance caused by insufficient recovery of individual RGO sensors and (ii) to deal with common variations among RGO devices due to various fabrication factors, such as differences in contact resistances, amount of RGO sheets, and RGO configurations on sensor electrodes.76 This signal processing method for RGO sensors has been derived for a sensing mechanism based on adsorption/desorption-induced charge transfer; therefore, the method could be useful for similar sensors (e.g., CNT sensors) operating with the same mechanism. This study shows a promising route to addressing the device-to-device and run-to-run variations among the graphene and CNT gas sensors and proposes a general method for the gas sensor calibration.
Conclusions and outlook
Nanocarbons, e.g., CNTs and graphene, have been widely studied as gas sensing platforms due to their unique structures and electronic properties. Nanocarbon-based gas sensors bear tremendous advantages, i.e., high sensitivity and selectivity, fast response/recovery, low operation temperature, low power consumption, easy operation with simple instruments, and relatively low cost, and could be ideal candidates for the next generation gas/chemical sensors. However, nanocarbon-based commercial gas sensors are yet to come and there is still a need for breakthroughs in sensor research either for improving the sensor performance or lowering the sensor cost/developing more adoptable sensor fabrication methods.In general, engineering the CNT/graphene structure with physical/chemical modifications shows great promise in the sensor performance enhancement. Some reported sensor performances in laboratory demonstrations could meet or even exceed the standards for practical applications. However, when the sensors are fabricated in a large scale, degradation in the sensor performance/quality is very common. Therefore, the quality of nanocarbon-based sensors, e.g., reproducibility, long-term stability, and false control, represents a great challenge for commercialization of sensors. In addition, advancements in the fabrication of sensors are highly needed for their commercialization and research is needed to develop cost-effective and scalable production methods that can retain essential properties of such materials. With a chemical vapor deposition (CVD) method, low-cost production of high quality CNTs is possible; however, the cost of synthesizing pure semiconducting SWCNTs is still high, which is a barrier for large-scale manufacturing. For graphene sensors, chemical production of GO could be an efficient way to reduce the cost of the CVD-grown graphene; however, the quality and reduction of GO may be an issue for large-scale manufacturing. In general, the material cost of the sensors may not be a big issue for their commercialization since the amounts of nanomaterials, e.g., CNTs, graphene, and nanocrystals, used in the sensors are very small. However, the fabrication cost, e.g., for controlled assembly of nanomaterials on the sensor electrodes, may be an issue since large scale and low cost fabrication of the sensors with precisely controlled structures and high performance is still a challenge. Nevertheless, with the development of new manufacturing methods and declined cost of CNTs and graphene, nanocarbon-based sensors are believed to have great potential to replace existing electrochemical, metal oxide, and catalytic bead/pellistor gas sensors in the future.
Acknowledgements
Chen gratefully acknowledges financial support from the National Science Foundation (IIP-1128158 and CMMI-0900509) and the U.S. Department of Energy (DE-EE-0003208). Lu thanks UAA for a Faculty Development Grant and ANSEP for financial support.Notes and references
- E. Comini, G. Faglia, G. Sberveglieri, Z. W. Pan and Z. L. Wang, Appl. Phys. Lett., 2002, 81, 1869 CrossRef CAS PubMed.
- Q. Wan, Q. H. Li, Y. J. Chen, T. H. Wang, X. L. He, J. P. Li and C. L. Lin, Appl. Phys. Lett., 2004, 84, 3654 CrossRef CAS PubMed.
- G. Eranna, B. C. Joshi, D. P. Runthala and R. P. Gupta, Crit. Rev. Solid State Mater. Sci., 2004, 29, 111 CrossRef CAS.
- E. Llobet, Sens. Actuators, B, 2013, 179, 32 CrossRef PubMed.
- S. Basu and P. Bhattacharyya, Sens. Actuators, B, 2012, 173, 1 CrossRef PubMed.
- P. Bondavalli, P. Legagneux and D. Pribat, Sens. Actuators, B, 2009, 140, 304 CrossRef CAS PubMed.
- Q. He, S. Wu, Z. Yin and H. Zhang, Chem. Sci., 2012, 3, 1764 RSC.
- K. R. Ratinac, W. Yang, S. P. Ringer and F. Braet, Environ. Sci. Technol., 2010, 44, 1167 CrossRef PubMed.
- A. Star, V. Joshi, S. Skarupo, D. Thomas and J.-C. P. Gabriel, J. Phys. Chem. B, 2006, 110, 21014 CrossRef PubMed.
- F. Yavari and N. Koratkar, J. Phys. Chem. Lett., 2012, 3, 1746 CrossRef CAS.
- R. H. Baughman, A. A. Zakhidov and W. A. de Heer, Science, 2002, 297, 787 CrossRef CAS PubMed.
- S. Iijima and T. Ichihashi, Nature, 1993, 363, 603 CrossRef CAS.
- J. Kong, N. R. Franklin, C. W. Zhou, M. G. Chapline, S. Peng, K. J. Cho and H. J. Dai, Science, 2000, 287, 622 CrossRef CAS.
- P. G. Collins, K. Bradley, M. Ishigami and A. Zettl, Science, 2000, 287, 1801 CrossRef CAS.
- A. Goldoni, R. Larciprete, L. Petaccia and S. Lizzit, J. Am. Chem. Soc., 2003, 125, 11329 CrossRef CAS PubMed.
- G. Lu, L. E. Ocola and J. Chen, Adv. Mater., 2009, 21, 2487 CrossRef CAS.
- Y. Sun and H. H. Wang, Adv. Mater., 2007, 19, 2818 CrossRef CAS.
- M. Zhang, H. C. Su, Y. Rheem, C. M. Hangarter and N. V. Myung, J. Phys. Chem. C, 2012, 116, 20067 CAS.
- A. Kaniyoor, R. I. Jafri, T. Arockiadoss and S. Ramaprabhu, Nanoscale, 2009, 1, 382 RSC.
- M. K. Kumar and S. Ramaprabhu, J. Phys. Chem. B, 2006, 110, 11291 CrossRef CAS PubMed.
- S. Mubeen, T. Zhang, B. Yoo, M. A. Deshusses and N. V. Myung, J. Phys. Chem. C, 2007, 111, 6321 CAS.
- M. Penza, R. Rossi, M. Alvisi and E. Serra, Nanotechnology, 2010, 21, 105501 CrossRef CAS PubMed.
- S. Cui, H. Pu, G. Lu, Z. Wen, E. C. Mattson, C. Hirschmugl, M. Gajdardziska-Josifovska, M. Weinert and J. Chen, ACS Appl. Mater. Interfaces, 2012, 4, 4898 CAS.
- D. W. H. Fam, A. I. Y. Tok, A. Palaniappan, P. Nopphawan, A. Lohani and S. G. Mhaisalkar, Sens. Actuators, B, 2009, 138, 189 CrossRef CAS PubMed.
- N. Du, H. Zhang, B. Chen, X. Ma, Z. Liu, J. Wu and D. Yang, Adv. Mater., 2007, 19, 1641 CrossRef CAS.
- Y. X. Liang, Y. J. Chen and T. H. Wang, Appl. Phys. Lett., 2004, 85, 666 CrossRef CAS PubMed.
- Y. L. Liu, H. F. Yang, Y. Yang, Z. M. Liu, G. L. Shen and R. Q. Yu, Thin Solid Films, 2006, 497, 355 CrossRef CAS PubMed.
- N. Van Hieu, L. T. B. Thuy and N. D. Chien, Sens. Actuators, B, 2008, 129, 888 CrossRef CAS PubMed.
- C. Wongchoosuk, A. Wisitsoraat, A. Tuantranont and T. Kerdcharoen, Sens. Actuators, B, 2010, 147, 392 CrossRef CAS PubMed.
- S. Mao, S. Cui, K. Yu, Z. Wen, G. Lu and J. Chen, Nanoscale, 2012, 4, 1275 RSC.
- G. Chen, T. M. Paronyan, E. M. Pigos and A. R. Harutyunyan, Sci. Rep., 2012, 2, 343 Search PubMed.
- J. P. Novak, E. S. Snow, E. J. Houser, D. Park, J. L. Stepnowski and R. A. McGill, Appl. Phys. Lett., 2003, 83, 4026 CrossRef CAS PubMed.
- Y. Zhang, S. Li, J. Zhang, Z. Pan, D. Min, X. Li, X. Song and J. Liu, Sci. Rep., 2013, 3, 1267 CAS.
- M. S. Arnold, A. A. Green, J. F. Hulvat, S. I. Stupp and M. C. Hersam, Nat. Nanotechnol., 2006, 1, 60 CrossRef CAS PubMed.
- A. A. Green, M. C. Duch and M. C. Hersam, Nano Res., 2009, 2, 69 Search PubMed.
- M. C. Hersam, Nat. Nanotechnol., 2008, 3, 387 CrossRef CAS PubMed.
- Y. Zhang, S. Cui, J. Chang, L. E. Ocola and J. Chen, Nanotechnology, 2013, 24, 025503 CrossRef PubMed.
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183 CrossRef CAS PubMed.
- S. Mao, H. Pu and J. Chen, RSC Adv., 2012, 2, 2643 RSC.
- C. N. R. Rao, A. K. Sood, K. S. Subrahmanyam and A. Govindaraj, Angew. Chem., Int. Ed., 2009, 48, 7752 CrossRef CAS PubMed.
- F. Schedin, A. K. Geim, S. V. Morozov, E. W. Hill, P. Blake, M. I. Katsnelson and K. S. Novoselov, Nat. Mater., 2007, 6, 652 CrossRef CAS PubMed.
- J. T. Robinson, F. K. Perkins, E. S. Snow, Z. Wei and P. E. Sheehan, Nano Lett., 2008, 8, 3137 CrossRef CAS PubMed.
- F. Shen, D. Wang, R. Liu, X. Pei, T. Zhang and J. Jin, Nanoscale, 2013, 5, 537 RSC.
- S. Some, Y. Xu, Y. Kim, Y. Yoon, H. Qin, A. Kulkarni, T. Kim and H. Lee, Sci. Rep., 2013, 3, 1868 Search PubMed.
- Y. Dan, Y. Lu, N. J. Kybert, Z. Luo and A. T. C. Johnson, Nano Lett., 2009, 9, 1472 CrossRef CAS PubMed.
- J. D. Fowler, M. J. Allen, V. C. Tung, Y. Yang, R. B. Kaner and B. H. Weiller, ACS Nano, 2009, 3, 301 CrossRef CAS PubMed.
- G. Lu, L. E. Ocola and J. Chen, Appl. Phys. Lett., 2009, 94, 083111 CrossRef PubMed.
- G. Lu, L. E. Ocola and J. Chen, Nanotechnology, 2009, 20, 445502 CrossRef PubMed.
- G. Lu, K. Yu, L. E. Ocola and J. Chen, Chem. Commun., 2011, 47, 7761 RSC.
- F. Yavari, E. Castillo, H. Gullapalli, P. M. Ajayan and N. Koratkar, Appl. Phys. Lett., 2012, 100, 203120 CrossRef PubMed.
- F. Yavari, Z. Chen, A. V. Thomas, W. Ren, H.-M. Cheng and N. Koratkar, Sci. Rep., 2011, 1, 166 Search PubMed.
- K. Yu, Z. Bo, G. Lu, S. Mao, S. Cui, Y. Zhu, X. Chen, R. S. Ruoff and J. Chen, Nanoscale Res. Lett., 2011, 6, 202 CrossRef PubMed.
- K. Yu, P. Wang, G. Lu, K.-H. Chen, Z. Bo and J. Chen, J. Phys. Chem. Lett., 2011, 2, 537 CrossRef CAS.
- S. Cui, S. Mao, G. Lu and J. Chen, J. Phys. Chem. Lett., 2013, 4, 2441 CrossRef CAS.
- S. Mao, S. Cui, G. Lu, K. Yu, Z. Wen and J. Chen, J. Mater. Chem., 2012, 22, 11009 RSC.
- F. Zhang, F. Md Yasin, X. Chen, J. Mo, C. L. Raston and H. Zhang, RSC Adv., 2013, 3, 25166 RSC.
- J. Zou, L. J. Hubble, K. S. Iyer and C. L. Raston, Sens. Actuators, B, 2010, 150, 291 CrossRef CAS PubMed.
- J. L. Johnson, A. Behnam, S. J. Pearton and A. Ural, Adv. Mater., 2010, 22, 4877 CrossRef CAS PubMed.
- G. Lu, K. Yu, Z. Wen and J. Chen, Nanoscale, 2013, 5, 1353 RSC.
- X. Li, X. Wang, L. Zhang, S. Lee and H. Dai, Science, 2008, 319, 1229 CrossRef CAS PubMed.
- S. Deng, V. Tjoa, H. M. Fan, H. R. Tan, D. C. Sayle, M. Olivo, S. Mhaisalkar, J. Wei and C. H. Sow, J. Am. Chem. Soc., 2012, 134, 4905 CrossRef CAS PubMed.
- A. Gutes, B. Hsia, A. Sussman, W. Mickelson, A. Zettl, C. Carraro and R. Maboudian, Nanoscale, 2012, 4, 438 RSC.
- M. Shafiei, P. G. Spizzirri, R. Arsat, J. Yu, J. du Plessis, S. Dubin, R. B. Kaner, K. Kalantar-Zadeh and W. Wlodarski, J. Phys. Chem. C, 2010, 114, 13796 CAS.
- J. Yi, J. M. Lee and W. Il Park, Sens. Actuators, B, 2011, 155, 264 CrossRef CAS PubMed.
- Z. Zhang, R. Zou, G. Song, L. Yu, Z. Chen and J. Hu, J. Mater. Chem., 2011, 21, 17360 RSC.
- L. Zhou, F. Shen, X. Tian, D. Wang, T. Zhang and W. Chen, Nanoscale, 2013, 5, 1564 RSC.
- P. A. Russo, N. Donato, S. G. Leonardi, S. Baek, D. E. Conte, G. Neri and N. Pinna, Angew. Chem., Int. Ed., 2012, 51, 11053 CrossRef CAS PubMed.
- S. Cui, Z. Wen, E. C. Mattson, S. Mao, J. Chang, M. Weinert, C. J. Hirschmugl, M. Gajdardziska-Josifovska and J. Chen, J. Mater. Chem. A, 2013, 1, 4462 CAS.
- F. Niu, J.-M. Liu, L.-M. Tao, W. Wang and W.-G. Song, J. Mater. Chem. A, 2013, 1, 6130 CAS.
- X. Wang, X. Li, L. Zhang, Y. Yoon, P. K. Weber, H. Wang, J. Guo and H. Dai, Science, 2009, 324, 768 CrossRef CAS PubMed.
- J. L. Johnson, A. Behnam, Y. An, S. J. Pearton and A. Ural, J. Appl. Phys., 2011, 109, 124301 CrossRef PubMed.
- B. H. Kim, S. J. Hong, S. J. Baek, H. Y. Jeong, N. Park, M. Lee, S. W. Lee, M. Park, S. W. Chu, H. S. Shin, J. Lim, J. C. Lee, Y. Jun and Y. W. Park, Sci. Rep., 2012, 2, 690 Search PubMed.
- R. Lv, Q. Li, A. R. Botello-Mendez, T. Hayashi, B. Wang, A. Berkdemir, Q. Hao, A. L. Elias, R. Cruz-Silva, H. R. Gutierrez, Y. A. Kim, H. Muramatsu, J. Zhu, M. Endo, H. Terrones, J.-C. Charlier, M. Pan and M. Terrones, Sci. Rep., 2012, 2, 586 Search PubMed.
- Y.-H. Zhang, Y.-B. Chen, K.-G. Zhou, C.-H. Liu, J. Zeng, H.-L. Zhang and Y. Peng, Nanotechnology, 2009, 20, 185504 CrossRef PubMed.
- X. Chen, J. F. Dobson and C. L. Raston, Chem. Commun., 2012, 48, 3703 RSC.
- G. Lu, S. Park, K. Yu, R. S. Ruoff, L. E. Ocola, D. Rosenmann and J. Chen, ACS Nano, 2011, 5, 1154 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |



