Amino acid inspired microscale organization of metallic nanocrystals†
G. S.
Sailaja
ab,
Balagopal N.
Nair
*ac,
Julian D.
Gale
a and
Takeo
Yamaguchi
b
aNanochemistry Research Institute, Department of Chemistry, Curtin University, GPO Box U1987, Perth, WA 6845, Australia
bChemical Resources Laboratory, Tokyo Institute of Technology, Nagatsuta 4259, Midori-ku, Yokohama 226-8503, Japan
cR&D Center, Noritake Company LTD, Miyoshi Higashiyama 300, Miyoshi, Aichi 470-0293, Japan. E-mail: bnair@n.noritake.co.jp
First published on 24th October 2013
Abstract
Amino acid inspired micro-scale organization of platinum and silver nanocrystals and the complementary oligomerization of amino acids is reported. The spatial organization of the microstructures is highly species specific with unique morphologies corresponding to particular combinations of a metal–amino acid system. Alanine, glycine and glutamic acid were used to illustrate the concept. The presence of a poly(amino acid) or stabilizing agent alters the size and configuration of the microscale assembly. The molecular interaction between the Pt and the amino acid and the synthesis conditions play key roles in determining the final shape of these 3D structures. The method offers a very facile strategy for designing diverse molecular building blocks of metallic microstructures for advanced applications by choosing versatile combinations of metal-ions and amino acids. Platinum integrated structures, in addition to their diverse architecture, showed good stability at high temperatures. Electrochemical characterization of samples heat-treated at 400 °C showed a high platinum surface utilization of 41.5%.
Introduction
Fabrication of large-scale microstructures from simple organic building blocks has recently emerged as one of the most important topics of research.1–3 Understanding the mechanism of self-assembly makes it feasible to explore new methods for the fabrication of complex microstructures for diverse applications.4–7 The significance of organic molecule based design of unique and complex self-assembled nanostructures arises not only because of the possibility to use such structures as direct device components for optical, electrical and electronic applications, but also from the fact that such shapes would be difficult to achieve through conventional chemical synthesis techniques alone or in combination with micro-fabrication techniques.8–11 Linear and cyclic peptide building blocks have been previously explored for constructing complex self-assembled nanostructures with unique applications.10–17 Ghadiri et al. originally reported peptide-based preparation of nano-tubular structures of cyclic octapeptides with alternating L- and D-amino acids through self-assembly.18,19 It has also been proven that linear surfactants, such as hepta- and octa-peptides, can self-assemble into a network of open-ended nanotubes.20,21 Even though diphenylalanine-based peptides have been extensively studied due to their superior mechanical properties,10,22 diphenylglycine-based peptides were also found to trigger self-assembly leading to closed cage like nanostructures.17 Nevertheless, selected solvents and/or the presence of external stimuli were prerequisites for obtaining such a preferred orientation.15,16 In the present work, we report the design of different kinds of metallic nanocrystal-molecular building blocks that lead to three-dimensional growth of distinctive micro-assembled hierarchical metallic nanostructures. The size, shape and the stereochemistry of the molecular building blocks are defined primarily by the amino acid–metal ion combination and the inherent properties of the amino acid, its crystallinity and its preferred binding sites. Hence, organization occurs through a complex interplay of molecular interactions between amino acid and metal-ions that ultimately determines the degrees of freedom of such integrated assemblies. Simple amino acids namely D-, L-alanine, glycine and glutamic acid that exhibit differences in inherent crystallinity and basic charge under physiological conditions were investigated with the aim of understanding the characteristic features of micro-assembled structures of platinum nanocrystals. In addition, the effect of a polyamino acid in redefining the configuration chemistry was investigated by synthesizing poly(L-glutamic acid) based Pt-structures and comparing them with those of glutamic acid-based Pt-micro-assembled structures. The role of a stabilizing agent in influencing the reaction kinetics, and thereby altering the assembly characteristics, was studied by performing reduction in both the presence and absence of sodium citrate for a system leading to metallic nanocrystalline structures.It is well known in the literature that the crystallization of alanine can be controlled by altering the chemical environment in terms of solvents, additives, temperature, pH, etc.23 In addition, it is also documented that the active surfaces of a crystal can interact stereo-specifically with additives in the solution and that this may have a dramatic effect in determining its ultimate morphology and growth profile.24 Several reports can be found on the fabrication of interesting morphologies, such as peptide self-assembly, mineralized peptide–amphiphile nanofibres and metallic nanoparticle assembled peptide nanostructures.25–28 However, to the best of our knowledge, amino acids directed metallic nanostructure organization and the complementary oligomerization of amino acid have not been reported so far.
The design of unique metal–organic assembled structures directed towards specific applications is of importance in several fields and numerous investigations in the past have focused on fabricating such structures.12–17 However, many of these techniques require complex procedures or specific reaction conditions in order to achieve the desired outcome. Using two of the most versatile metals, platinum and silver, as examples, this study presents the development of micro-scale assembled structures of metal nanocrystals mediated by different amino acids through a facile method at room temperature in aqueous solution. Our results suggest that the mechanism associated with the morphological diversity observed in this microscale organization could be species-specific molecular interactions between metal-ions and the amino acid triggering the 3D organization of the nanocrystalline building blocks. This broad understanding of the mechanism of molecular interactions between amino acids and the metal-ions can provide new insights into the design of unique microstructures for potential applications. Promising results observed on the electrochemical characterization of our platinum nanostructures indeed validate this prospect.
Experimental
2.1. Materials
11.5 wt% platinum solution (Noritake Company Ltd, Japan) and silver nitrate (Sigma-Aldrich, USA) were used as the sources of platinum and silver, respectively. D,L-Alanine (Acros Organics, USA), glycine (Sigma-Aldrich, USA), glutamic acid (Sigma-Aldrich, USA), poly(glutamic acid) sodium salt, Mw 750–5000 (Sigma-Aldrich, USA), tri-sodium citrate (Ajax Finechem, Australia) and sodium borohydride (Asia Pacific Specialty Chemicals Ltd, Australia) were all of analytical grade and used without further purification.2.2. Synthesis
Water purified by reverse osmosis using a Milli-Q (MQ) system was used throughout the study. In a typical synthesis of Pt–amino acid micro-assembled structures, standard H2PtCl6 solution and amino acid in the ratio 0.1 wt% of Pt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mole of amino acid were allowed to undergo complexation at room temperature for 72 hours. Pt–amino acid complexes of D,L-alanine, glycine and glutamic acid were prepared. Pt–amino acid complexes were reduced by adding freshly prepared 112 mM sodium borohydride (NaBH4) solution. NaBH4 was added drop-wise under very gentle stirring; the mixture was then allowed to stay undisturbed overnight. The black assembled structures settled at the bottom of the reaction vessel were carefully collected, and then washed several times with MQ water. These structures were vacuum dried at 50 °C and characterized. In a separate reaction, the Pt–alanine complex was reduced by adding freshly prepared 112 mM sodium borohydride (NaBH4) very slowly, in the absence of stirring and was kept undisturbed for 24 hours. The product was collected, washed and dried, after which morphological evaluation was performed.
1 mole of amino acid were allowed to undergo complexation at room temperature for 72 hours. Pt–amino acid complexes of D,L-alanine, glycine and glutamic acid were prepared. Pt–amino acid complexes were reduced by adding freshly prepared 112 mM sodium borohydride (NaBH4) solution. NaBH4 was added drop-wise under very gentle stirring; the mixture was then allowed to stay undisturbed overnight. The black assembled structures settled at the bottom of the reaction vessel were carefully collected, and then washed several times with MQ water. These structures were vacuum dried at 50 °C and characterized. In a separate reaction, the Pt–alanine complex was reduced by adding freshly prepared 112 mM sodium borohydride (NaBH4) very slowly, in the absence of stirring and was kept undisturbed for 24 hours. The product was collected, washed and dried, after which morphological evaluation was performed.
In the case of Pt microstructures with poly(glutamic acid), 0.1 wt% of Pt solution was complexed with 0.1 M poly(glutamic acid) sodium salt solution, keeping the Pt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) poly(glutamic acid) ratio at 1
poly(glutamic acid) ratio at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. The reaction was carried out at room temperature and the mixture was aged for 72 hours. The reduction of the Pt–poly(glutamic acid) complex was performed in a similar manner to that of other Pt–amino acid complexes. The formation of black particles was instantaneous in this case and the nanoparticle solution was very stable for weeks. The nanoparticles were collected by vacuum filtration and washed several times with MQ water, followed by drying at 50 °C.
10. The reaction was carried out at room temperature and the mixture was aged for 72 hours. The reduction of the Pt–poly(glutamic acid) complex was performed in a similar manner to that of other Pt–amino acid complexes. The formation of black particles was instantaneous in this case and the nanoparticle solution was very stable for weeks. The nanoparticles were collected by vacuum filtration and washed several times with MQ water, followed by drying at 50 °C.
In a typical synthesis of silver micro-assembled structures, AgNO3 solution and D,L-alanine (molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) were reacted overnight at room temperature both in the presence and absence of 38.8 mM tri-sodium citrate (TSC). The black silver–alanine micro-assembled structures were formed upon controlled reduction using freshly prepared 112 mM NaBH4 solution that was added drop-wise as in the case of platinum. The product was allowed to stabilize for over 5 hours. The products, being fragile, were washed very carefully with MQ water until the wash solution showed neutral pH and dried at 50 °C under vacuum before being characterized.
10) were reacted overnight at room temperature both in the presence and absence of 38.8 mM tri-sodium citrate (TSC). The black silver–alanine micro-assembled structures were formed upon controlled reduction using freshly prepared 112 mM NaBH4 solution that was added drop-wise as in the case of platinum. The product was allowed to stabilize for over 5 hours. The products, being fragile, were washed very carefully with MQ water until the wash solution showed neutral pH and dried at 50 °C under vacuum before being characterized.
2.3. Characterization
The morphological evaluation of the microstructures was performed using a field emission scanning electron microscope (FESEM, Hitachi S-4800) attached to an Oxford Energy dispersive X-ray machine. The X-ray diffraction (XRD) patterns were recorded using a Siemens Kristalloflex diffractometer D500. Samples were mounted on a sample holder and XRD patterns were collected using Cu Kα radiation (2θ ranging from 2 to 90 degrees). The transmission electron microscopy analysis was performed using a HRTEM, with a JEOL-2010 200 kV transmission electron microscope. FT-IR spectra of the samples were obtained via the KBr pellet method using a Nicolet 5700 system.2.4. Electrochemical measurements
The measurement of electrochemical properties of the porous Pt–alanine nanostructures was performed using rotating disc cyclic voltammetry (RD-CV). The catalyst ink was prepared by mixing Pt–alanine superstructures (heat treated at 400 °C) with carbon and Nafion solution. The catalyst ink was then transferred onto a polished glassy carbon disk surface and dried to form the ink layer. The measurement was performed at 25 °C using a freshly prepared 0.1 M HClO4 solution. Standard measurement techniques and calculation procedures were followed to calculate the catalytically active surface area of platinum in the ink.Results and discussion
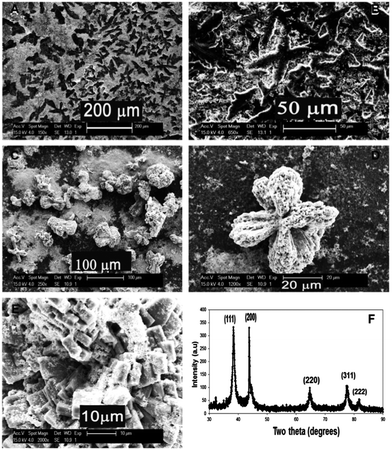
Morphological features of the micro-scale assembly of Pt-nanocrystals and the corresponding individual molecular building blocks based on different kinds of amino acids reduced under stirring conditions are provided in Fig. 1. The SEM micrographs (Fig. 1(A)–(C)) illustrate the alanine directed Pt-nanocrystal assembly, while the glycine directed micro-assembly is shown in Fig. 1(D)–(F). Although these organized structures show some resemblance to each other in their low magnification images, the fundamental building block units are explicitly different, as evident from images C and F, which should definitely reflect on their physico-chemical properties. The basic repeat units of alanine-based Pt-structures appeared as discs, while those for glycine-based structures seemed more or less spherical with needle like protrusions. It is possible to envisage that even though alanine and glycine are both simple amino acids and are neutral with respect to side chain charges, alanine differs due to its inherent crystalline property and hence has a strong effect on binding/organization.The SEM micrographs of glutamic acid and poly(glutamic acid) directed Pt-nanocrystal assemblies are shown in Fig. 1(G–I) and (J–L), respectively. Here, though the monomer units of amino acids are the same, the chain lengths are different. Hence the availability of functional groups and the binding preferences are amplified, leading to a huge difference in the microstructure of the resulting products. Pt-structures directed by glutamic acid have triangular shaped individual building blocks as viewed in Fig. 1(I) which assembled to form more or less helical superstructures as shown in Fig. 1(G) and (H). In the case of poly(glutamic acid) directed Pt-assembly, large multi-branched leaf-like structures of several micrometers (Fig. 1(J) and (K)) were formed. A closer examination of these structures showed interesting ordered microstructural arrangements shown in Fig. 1(L). Amino acids under physiological conditions exist in the zwitterionic state, while for peptides, the end groups are condensed to form peptide bonds and therefore exist as a different chemical structure. Polyamino acids, due to their increased molecular weight and associated increased chain length, as well as their altered configuration, influence the structure and size of the building block. Hence a completely dissimilar and much larger assembly is formed in the case of poly(glutamic acid) in contrast to glutamic acid.
Fig. 2 evidences the morphological differences of Pt–alanine complexes reduced in the absence of stirring. The SEM micrographs of Pt–alanine superstructures at low-magnification (Fig. 2(A)) showed wire-like carbon containing structures that are tens to hundreds of micrometers long decorated by platinum nanocrystals. From the magnified view (Fig. 2(B) & (C)) it is apparent that the structure is typically composed of a porous nanocrystal assembly along a tubular skeleton. SEM images of the samples heat-treated at 400 °C as shown in Fig. 2(D) & (E) evidence the stability of the integrated superstructures.
Fig. 2(C) shows the image of a tubular structure with cracks that partially reveal the inside surface. Fig. S1 (ESI†) shows the image of a piece of the sample with deeper cracks in it, and further confirms the porous nature of the structure. Although it has been reported that alanine crystals can be made into hollow rectangular structures,29 the geometry is cylindrically tubular in our samples. The relatively shorter tubes visible in the micrographs are likely to be the fragments of longer structures that have broken up during washing or during processing for characterization. Usually, the length of the tube is of the order of tens of micrometers, as evidenced by the micrograph in Fig. 2(A), yet the structures are apparently discrete compared to peptide nanotubes reported elsewhere.10,13,15
It is interesting to note that the carbon skeleton decorated with platinum nanocrystals retained its structural integrity even after heat treatment at 400 °C for 3 hours (Fig. 2(D) and (E)). Fig. S2† (ESI) shows the image of a slice of the tube where the carbon-skeleton was removed. The connectivity of the porous structure shown should be good enough to make hollow tubes of platinum from the superstructure under controlled heat-treatment conditions, which Fig. S3 (ESI†) indeed supports. The micrographs in Fig. 2(D) further demonstrate that the wire-like morphology is consistently maintained over length scales of the order of tens of micrometers. This indicates the possibility of the application of these supported structures as supported catalysts, for example in fuel cells.
The X-ray diffraction patterns of the as-synthesized Pt–amino acid micro-assembled structures from different amino acids are given in Fig. 3 while the XRD patterns of Pt–alanine superstructures before and after heat-treatment are shown in Fig. S4† (ESI). The XRD pattern showed peaks at 39.6, 46.4, 67.7, 81.8 (2θ degree) assigned to the diffraction from (111), (200), (220), and (311) planes of the FCC lattice of Pt (JCPDS no. 04-0802).
The primary Pt nanoparticle size calculated from the XRD patterns in Fig. 3 using the Scherrer formula was in the range of 3–6 nm for all of the present samples. The crystallite size of the Pt–alanine based nanoparticles heat-treated at 400 °C was calculated to be 12.5 nm using the Scherrer equation from the spectra shown in the ESI.† The energy dispersive X-ray spectra and smart maps of Pt–alanine superstructures before and after heat treatment (Fig. S5(a) and (b)† respectively) revealed the presence of both platinum and carbon as part of the structure (ESI†).
Rotating disc cyclic voltammetry tests were performed on these samples to test if the entire platinum surface is electrochemically active. The electrochemical surface area (ECSA) was measured to be 9.3 m2 g−1-Pt for the sample heat-treated at 400 °C. Although this ECSA value is lower than that of commercial grade catalysts (ECSA in the range 50–100 m2 g−1-Pt), because of the larger size of the platinum nanoparticles in this study the results were not unexpected. Based on the size of the heat-treated platinum nanoparticle being 12.5 nm, as calculated from XRD spectra, the platinum surface utilization for catalytic activity could be calculated as 41.5%. This surface usage value indicates that a significant portion of the nanoparticles was accessible for the electrochemical reaction.
The geometrical differences in unit building blocks of each of the Pt–amino acid combinations and the resulting configurational variations of their assembly are well evidenced from the transmission electron micrographs (Fig. 4). The geometry of the unit building blocks of Pt-structures based on alanine (Fig. 4(A) and (B)), glycine (Fig. 4(C) and (D)) and glutamic acid (Fig. 4(E) and (F)) is unambiguously different and is apparent from the growing ends of these structures (Fig. 4(B), (D) and (F)). The growing edge of Pt–alanine superstructures resembled the pyramidal growth of thin discs (Fig. 4(B)) that constitute the repeat unit of the alpha helical structures. For glycine based structures, it could be implied from the TEM image (Fig. 4(D)) that the sharp needle like protruding structures that appeared in the SEMs are basically composed of diagonally oriented Pt unit structures. More interestingly, the growing edges of glutamic acid directed Pt-structures revealed its elongated nanotubular morphology (Fig. 4(F)). The stacking of these nanotubes should have given the triangular look to the building blocks when viewed in SEM (Fig. 1(H) & (I)). It can be seen that each of these glutamic acid based structures are individually composed of Pt-lattices arranged like stacked concentrated circles. The nanoparticle size visible in the TEM micrographs is also in the order of 3–6 nm, similar to the values calculated from XRD plots.
 | ||
| Fig. 4 TEM images of different types of Pt–amino acid molecular building blocks formed under stirring conditions. (A and B) Pt–alanine; (C and D) glycine; and (E and F) glutamic acid. | ||
The unique morphologies and physicochemical properties of self-assembled hierarchical nanostructures of noble metals have attracted considerable interest due to their multifaceted and advanced functional properties.29,30 Remarkable features of silver nanostructure assemblies formed under ambient conditions offer fascinating opportunities to be used as catalysts in antimicrobial and imaging applications.31,32 Hence we have attempted to explore the conceptual extension of the amino acid inspired microscale growth of platinum nanocrystals towards other noble metals, by performing the same reaction with silver. The role of surfactant in the reaction was also evaluated by using tri-sodium citrate (TSC) along with Ag–amino acid systems. In the case of silver nanocrystal assembly, dendritic structures were observed in the absence of TSC in the reaction medium (Fig. 5(A) and (B)), while the presence of TSC changed the growth pattern of the nanocrystals into flower-like superstructures (Fig. 5(C) and (D)). The high magnification image (Fig. 5(E)) imparts insights into the multistage assembly of silver nanocrystals that are initially grown as a solid rectangular block-like superstructure before undergoing a secondary organization to transform into the flower-like morphology. The X-ray diffraction pattern of the as-synthesized silver structures (Fig. 5(F)) was found to match with its face-centered cubic crystal structure.
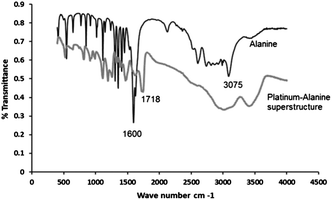
Investigation of the role of the metal–amino acid interaction has been achieved by examining the Pt–alanine superstructure using local structural probes based on Fourier-Transform infrared (FT-IR) spectroscopy. The likely coordination of metal (in this case platinum) by the carboxylate group of alanine could be inferred from the FT-IR spectra of alanine and the Pt–alanine superstructure (Fig. 6). It can be seen that the relatively sharp N–H stretching peak of alanine at 3075 cm−1 has been transformed into a much broader peak in the range 3320–3450 cm−1 for the Pt–alanine superstructure. Further, there is a major shift of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching band of alanine from 1600 cm−1 to 1718 cm−1. Published reports suggest that Pt(II) and (IV) are capable of forming coordination complexes with α-amino acids, including alanine.34–36 The Pt(II) coordinated complexes showed a prominent peak shift for both N–H and C
O stretching band of alanine from 1600 cm−1 to 1718 cm−1. Published reports suggest that Pt(II) and (IV) are capable of forming coordination complexes with α-amino acids, including alanine.34–36 The Pt(II) coordinated complexes showed a prominent peak shift for both N–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations. Hence it is proposed that the peak shift associated with the Pt–alanine superstructures is due to the formation of a bonding interaction between platinum and alanine.35,37 In addition, it could be seen from the spectra that the reduction process has a significant effect on the fundamental chemical structure of alanine. This has occurred as a result of the strong pH change that is essential for reducing platinum ions. Hence, a mechanism for the formation of metallic superstructures described in this study could be proposed based on the complementary interaction of metal-ions with alanine and other amino acids. We suggest that such a complementary interaction, that takes place parallel to the formation of micro-assembled structures, leading to the oligomerization of amino acid itself, occurs through the prior existence of a weak platinum–amino acid complex (non-covalent type interaction) in the initial phase of the reaction. This inspires and directs the configuration and growth of such metal–amino acid superstructures upon reduction.
O stretching vibrations. Hence it is proposed that the peak shift associated with the Pt–alanine superstructures is due to the formation of a bonding interaction between platinum and alanine.35,37 In addition, it could be seen from the spectra that the reduction process has a significant effect on the fundamental chemical structure of alanine. This has occurred as a result of the strong pH change that is essential for reducing platinum ions. Hence, a mechanism for the formation of metallic superstructures described in this study could be proposed based on the complementary interaction of metal-ions with alanine and other amino acids. We suggest that such a complementary interaction, that takes place parallel to the formation of micro-assembled structures, leading to the oligomerization of amino acid itself, occurs through the prior existence of a weak platinum–amino acid complex (non-covalent type interaction) in the initial phase of the reaction. This inspires and directs the configuration and growth of such metal–amino acid superstructures upon reduction.
It has been documented that the c-axis of D,L-alanine is dipolar in nature; the (001) face being positively charged due to the abundance of NH3+ proton donors while the (00![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) basal plane is negatively charged due to the exposed carboxylate groups. In aqueous solution, the COO− ends grow and dissolve faster than the amino ends, facilitating the extension of the alanine molecular dipole along the c-axis of alanine.33,35 Further, the growth kinetics of specific planes is highly dependent on the pH, solvent–surface interactions and surface binding energy. Hence when the active surfaces of a crystal interact stereo-specifically with additives, such as metal ions in the solution, the ultimate morphology and growth profile are affected.24,37 Similarly, depending on the functional group of the surfactant/polymer and its ability to lower the interfacial energy, alanine molecules may interact with their preferred faces (slow or fast growing faces) leading to interesting architectures as in the case of platinum and silver nanostructures reported in this study. Scheme 1, for e.g., is a representation of the formation of an alanine-directed Pt-nanocrystal superstructure.
) basal plane is negatively charged due to the exposed carboxylate groups. In aqueous solution, the COO− ends grow and dissolve faster than the amino ends, facilitating the extension of the alanine molecular dipole along the c-axis of alanine.33,35 Further, the growth kinetics of specific planes is highly dependent on the pH, solvent–surface interactions and surface binding energy. Hence when the active surfaces of a crystal interact stereo-specifically with additives, such as metal ions in the solution, the ultimate morphology and growth profile are affected.24,37 Similarly, depending on the functional group of the surfactant/polymer and its ability to lower the interfacial energy, alanine molecules may interact with their preferred faces (slow or fast growing faces) leading to interesting architectures as in the case of platinum and silver nanostructures reported in this study. Scheme 1, for e.g., is a representation of the formation of an alanine-directed Pt-nanocrystal superstructure.
In addition to the molecular interactions between metal ions and alanine, the high surface energy of the in situ formed metallic nanoparticles may also play a key role in imparting the specific organization of the superstructure. D,L-Alanine, being polar in the aqueous phase, can easily undergo a greater degree of specific interaction with polar additives (Pt–Ag ions) that may eventually influence its specific orientation. Such a specificity of the interactions between D,L-alanine and selected metal ions is illustrated by the entirely different morphologies obtained for platinum and silver superstructures in this work. These observations therefore provide broader insights into the interactions that may exist between alanine and other crystalline amino acids, with ions of noble metals.
Attempts to understand the mechanism of formation of Pt-nanostructures inspired by amino acids provide strong evidence of the influence of metal cations on the oligomerization of the amino acids, glycine and alanine.38 Lawless and Levi in 1979 reported on the role of metal cations, Cu and Zn on the polymerization of alanine and glycine (percentage of polymerization by Zn is 1.6% and for Cu 6.7%); a similar reaction could be assumed in the present case as well. It is suggested that the reduction of the metal ions and oligomerization of the amino acid occurred in parallel as a result of the complex interplay between the structure, stereochemistry and crystallinity of the amino acid and thereby defines the geometry of the unit building block and the ultimate morphology of the resulting superstructures.
Conclusions
In summary, the amino acid directed micro-scale organization of metallic nanocrystal structures occurs via the formation of unique molecular building blocks corresponding to each of the amino acid–metal ion combinations. The geometry of the unit molecular building block is defined by the inherent characteristics of the amino acid, crystallinity, metal–amino acid interactions and preferences in binding sites. The degree of organization and the configuration chemistry can be altered by polyamino acid/stabilizing agents. Most significantly, this approach indicates the feasibility of exploring these specific interactions and binding affinities of different combinations of metal ion–amino acid systems for fabricating diverse metallic nanocrystal assemblies for specific applications in catalysis, electronics and biomedical fields.Acknowledgements
We would like to acknowledge our colleagues in Noritake Company Ltd. and Curtin University for their support and guidance. Thanks to Dr G. M. Anil Kumar and Ms K. Kato of Noritake Company Ltd. for the electrochemical measurements. J.D.G. thanks the ARC for funding through the Discovery program.References
- H. Liu, J. Xu, Y. Li and Y. Li, Acc. Chem. Res., 2010, 43(12), 1496 CrossRef CAS PubMed
.
- X. D. Xu, J. Zhang, L. J. Chen, X. L. Zhao, D. X. Wang and H. B. Yang, Chem.–Eur. J., 2012, 18, 1659 CrossRef CAS PubMed
.
- B. Zhou, Z. Sun, D. Li, T. Zhang, L. Deng and Y. N. Liu, Nanoscale, 2013, 5, 2669 RSC
.
- G. M. Whitesides, J. P. Mathias and C. T. Seto, Science, 1991, 254, 1312 CAS
.
- M. Sarikaya, C. Tamerler, A. K. Jen, K. Schulten and F. Baneyx, Nat. Mater., 2003, 2, 577 CrossRef CAS PubMed
.
- M. Antonietti and G. A. Ozin, Chem.–Eur. J., 2004, 10(1), 28 CrossRef CAS PubMed
.
- S. Mann, Nature, 1993, 365, 499 CrossRef CAS
.
- D. D. Y. Ryu and D. H. Nam, Biotechnol. Prog., 2000, 16, 2 CrossRef CAS PubMed
.
- N. C. Seeman and A. M. Belcher, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 6452 CrossRef PubMed
.
- M. Reches and E. Gazit, Science, 2003, 300, 625 CrossRef CAS PubMed
.
- J. Fang, H. You, P. Kong, Y. Yi, X. Song and B. Ding, Cryst. Growth Des., 2007, 7(5), 864 CAS
.
- N. Kol, L. Adler-Abramovich, D. Barlam, R. Z. Shneck, E. Gazit and I. Rousso, Nano Lett., 2005, 5, 1343 CrossRef CAS
.
- A. Aggeli, M. Bell, N. Boden, J. N. Keen, P. F. Knowles, T. C. B. McLeish, M. Pitkeathly and S. E. Radford, Nature, 1997, 386, 259 CrossRef CAS PubMed
.
- N. Hendler, N. Sidelman, M. Reches, E. Gazit, Y. Rosenberg and S. Richter, Adv. Mater., 2007, 19, 1485 CrossRef CAS
.
- X. Y. Gao and H. Matsui, Adv. Mater., 2005, 17, 2037 CrossRef CAS
.
- M. Reches and E. Gazit, Nat. Nanotechnol., 2006, 1, 195 CrossRef CAS PubMed
.
- M. Reches and E. Gazit, Nano Lett., 2004, 4(4), 581 CrossRef CAS
.
- M. R. Ghadiri, J. R. Granja, R. A. Milligan, D. E. McRee and N. Khazanovich, Nature, 1993, 366, 324 CrossRef CAS PubMed
.
- J. D. Hartgerink, J. R. Granja, R. A. Milligan and M. R. Ghadiri, J. Am. Chem. Soc., 1996, 118, 43 CrossRef CAS
.
- S. Vauthey, S. Santoso, H. Gong, N. Watson and S. Zhang, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 5355 CrossRef CAS PubMed
.
- S. Zhang, D. M. Marini, W. Hwang and S. Santoso, Curr. Opin. Chem. Biol., 2002, 6, 865 CrossRef CAS
.
- L. Adler-Abramovich, M. Reches, V. L. Sedman, S. Allen, S. J. B. Tendler and E. Gazit, Langmuir, 2006, 22, 1313 CrossRef CAS PubMed
.
- L. J. W. Shimon, M. Vaida, L. Addadi, M. Lahav and L. Leiserowitz, J. Am. Chem. Soc., 1990, 112, 6215 CrossRef CAS
.
- I. Weissbuch, R. Popovitz-biro, M. Lahav and L. Leiserowltz, Acta Crystallogr., Sect. B: Struct. Sci., 1995, 51, 115 CrossRef
.
- M. Yemini, M. Reches, J. Rishpon and E. Gazit, Nano Lett., 2005, 5, 183 CrossRef CAS PubMed
.
- X. Fu, Y. Wang, L. Huang, Y. Sha, L. Gui, L. Lai and Y. Tang, Adv. Mater., 2003, 15(11), 902 CrossRef CAS
.
- O. Carny, D. E. Shalev and E. Gazit, Nano Lett., 2006, 6(8), 1594 CrossRef CAS PubMed
.
- J. D. Hartgerink, E. Beniash and S. I. Stupp, Science, 2001, 294, 1684 CrossRef CAS PubMed
.
- J. Xiao, Y. Xie, R. Tang, M. Chen and X. Tian, Adv. Mater., 2001, 13, 1887 CrossRef CAS
.
- B. Zhang, P. Xu, X. Xie, H. Wei, Z. Li, N. H. Mack, X. Han, H. Xu and H. Wang, J. Mater. Chem., 2011, 21, 2495 RSC
.
- B. Wiley, Y. Sun and Y. Xia, Acc. Chem. Res., 2007, 40, 1067 CrossRef CAS PubMed
.
- Z. Huang, X. Jiang, D. Guo and N. Gu, J. Nanosci. Nanotechnol., 2011, 11(11), 9395 CrossRef CAS PubMed
.
- D. D. Medina and Y. Mastai, Cryst. Growth Des., 2008, 8(10), 3646 CAS
.
- S. Wohlrab, N. Pinna, M. Antonietti and H. Colfen, Chem.–Eur. J., 2005, 11, 2903 CrossRef CAS PubMed
.
- P. K. Siu, D. L. Ma and C. M. Che, Chem. Commun., 2005, 1025 Search PubMed
.
- L. F. Krylova, L. M. Kovtunova and G. V. Romanenko, Bioinorg. Chem. Appl., 2008, 1 CrossRef PubMed
.
- E. Kasotakis, E. Mossou, L. Adler-Abramovich, E. P. Mitchell, V. T. Forsyth, E. Gazit and A. Mitraki, Biopolymers, 2009, 92, 164 CrossRef CAS PubMed
.
- J. G. Lawless and N. Levi, J. Mol. Evol., 1979, 13, 281 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ta13540c |
| This journal is © The Royal Society of Chemistry 2014 |