Co- and distinct existence of Tris-NTA and biotin functionalities on individual and adjacent micropatterned surfaces generated by photo-destruction†
Atanu
Biswas‡
,
Abhijit
Saha‡
,
Dhruba
Ghosh
,
Batakrishna
Jana
and
Surajit
Ghosh
*
Chemistry Division, CSIR-Indian Institute of Chemical Biology, 4 Raja S. C. Mullick Road, Jadavpur, Kolkata-700032, West Bengal, India. E-mail: sghosh@iicb.res.in; Fax: +91-33-2473-5197/0284; Tel: +91-33-2499-5872
First published on 10th December 2013
Abstract
Micropatterned surfaces with Tris-NTA and biotin functionalities both in the same micropattern as well as individually in adjacent micropatterns are generated by UV light illumination through photo-masks. These surfaces are extremely useful for the immobilization of oligohistidine and biotin tagged multiple biomolecules/proteins.
Chemically functionalised, micropatterned surfaces have huge importance for the development of various biotechnological and biomedical devices such as information storage, microfluidics, microelectronics, photonics, bioreactors, microarrays, smart materials, etc.1,2 Recently, many techniques such as photolithography, microcontact printing and imprint lithography have been reported for generating micropatterned surfaces to functionally immobilize proteins and cells on micropatterned areas.3–6 A key challenge for studying protein–protein, antibody–antigen and DNA–protein interactions on micropatterned surfaces is retaining their functionality under the immobilized conditions because most proteins are sensitive to the surface and denature upon contact with it. For this reason, maintaining the biocompatibility of the surface is crucial, but this is easily achievable by chemical modification of the surface through covalent conjugation with polymers such as polyethylene glycol. In biochemistry, important functionalities are extremely useful for immobilizing proteins or any other biomolecules onto surfaces to study protein–protein or protein–DNA interactions. Two examples of such functionalities are nitrilotriacetic acid (NTA) and tris-(nitrilotris-acetic acid) (Tris-NTA) with multivalent head groups, which contain a chelator for Ni2+ and have a very high affinity for oligohistidine-tagged proteins and biotin, which in turn has a very high affinity for avidin.7 Recent developments in using various modified, micropatterned surfaces with these two functionalities for the local immobilization of biomolecules onto the micropatterned surfaces and the nanoparticle-based delivery of multiple biomolecules into cells have proved the importance of designing new and advanced platforms for surface patterning for a wide range of applications.8 Our previous micropatterned surface design was based on an individual functionality, such as either Tris-NTA or biotin, for the immobilization of either oligohistidine or biotin tagged biomolecules.8b,f The limitations of previous techniques are that we cannot immobilize both oligohistidine and biotin tagged biomolecules or proteins onto the same micropattern or immobilize individual oligohistidine and biotin tagged biomolecules or proteins on adjacent micropatterns. Now, our first question was, could we design a micropatterned surface with both Tris-NTA and biotin on the same pattern so that we can immobilize both oligohistidine and biotin tagged biomolecules or proteins onto the same micropatterned surface? Our next question was, could we design side by side micropatterns, one with Tris-NTA and the adjacent one with biotin so that we can immobilize oligohistidine tagged biomolecules on one micropattern and biotin tagged biomolecules on the adjacent one? To address these questions, in this manuscript, for the first time, we have demonstrated the fabrication of a micropatterned surface with both Tris-NTA and biotin functionalities on the same micropattern (termed hereafter “TBSMP”) as well as a surface with Tris-NTA and biotin on adjacent micropatterns (termed hereafter “TBAMP”) by UV light illumination through different types of photo-mask.
For simplicity, we have separated the two methods and discussed them separately. First, a schematic view of the generation of the “TBSMP” surface is illustrated in Scheme 1. A brief description for the generation of the “TBSMP” surface is discussed in this section. After functionalisation with both Tris-NTA and biotin, the glass surface was illuminated with UV light through a positive photo-mask (chromium coated features on quartz glass) in the presence of Co2+ over various time scales and the micropatterned surface was washed with 100 mM HCl and wash buffer prior to the protein binding experiments (Scheme 1). To test the binding capacity of an oligohistidine tagged protein and a biotin tagged biomolecule, we incubated the glass surface with a Ni2+ solution and constructed a flow chamber. This was followed by the sequential incubation with NeutrAvidin, an oligohistidine tagged protein, and a biotin tagged dye, and periodic washing to remove excess or unbound molecules. Next, the “TBSMP” surface was observed under a total internal reflection fluorescence (TIRF) microscope, which revealed co-localisation of green and red micropatterned areas (Fig. 1a and b).
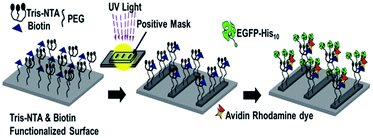 | ||
| Scheme 1 The Tris-NTA and biotin dual functionalised micropatterned surface (TBSMP) generated by photo destruction through a positive photo-mask. | ||
The photo destruction efficiencies of Tris-NTA and biotin functionalised surfaces were measured by time dependent UV light illumination. The dual functionalised surfaces were illuminated with UV light through a positive photo-mask for various time periods (20, 30, 45, 60, 90 and 120 min) to establish the optimum illumination time required for producing a high quality “TBSMP” surface. They were then characterized by the immobilization of a decahistidine tagged EGFP protein and avidin rhodamine dye, followed by microscopic imaging, which revealed co-localisation of green and red micropatterned surfaces (Fig. 1a and b). The observed green and red fluorescence signals in the background/non-patterned areas gradually diminished with longer exposure times (20–120 min; Fig. 1a and b), whereas the green and red fluorescence signals were almost unchanged in the micropatterned areas. Next, we quantitatively measured the fluorescence signals from the micropatterned and non-patterned areas for both the fluorophores after different illumination times and it was observed that the intensities of both the green and red fluorescence on the micropatterned areas were similar. Slight differences in the measured intensities for both the fluorophores are attributed to uneven densities of Tris-NTA and biotin on the micropatterned surfaces (Fig. 1c and d, black points). The error bars represent the standard deviation of the measured fluorescence intensities from various micropatterned and non-patterned areas for both fluorophores. The green and red fluorescence signals from the non-patterned areas gradually diminished. In the case of Tris-NTA, the destruction process was faster and we observed a significant change after 20 min but in the case of biotin we did not observe significant destruction after 20 min of illumination (Fig. 1d, blue point). Therefore it is obvious that the biotin destruction process is slower than the Tris-NTA destruction process, and we observed destruction upto 60 min. After that there were no significant changes in the destruction (Fig. 1c and d, red points). These results confirmed that both Tris-NTA and biotin remained in the non-illuminated areas but were destroyed by UV light in the illuminated areas. However, the destruction depended on the illumination time and the power of the UV light. The results also showed that the optimum illumination time required for generating a high quality dual micropattern was 60 min.
We have shown further application of the “TBSMP” surface by immobilizing molecular motors along with atto488 biotin dye, and confirmed whether or not the “TBSMP” surface supports the transport of microtubule filaments in the presence of adenosine triphosphate (ATP). This experiment was designed following our previously described method.8b In brief, we co-immobilized a recombinant dimeric kinesin with a C-terminal decahistidine tag (kinesin-H10) and atto488 biotin onto the “TBSMP” surface (Fig. 2b and c). It was found that an Alexa568-labelled microtubule landed selectively on the kinesin/biotin Atto488 immobilized line pattern and was transported from one point to another point on the same pattern (Fig. 2a, Movie S1†), which confirmed the successful immobilization of functional proteins onto the “TBSMP” surface.
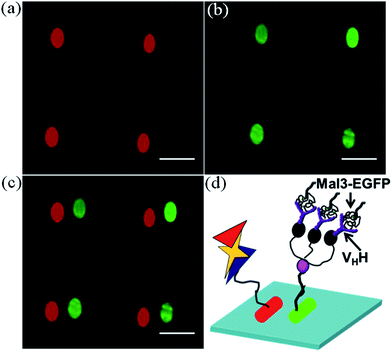
Here, we describe the method and application of the “TBAMP” surface. The glass surface was functionalised with 6-nitroveratryloxycarbonyl protected PEG2000-amine, which is a photo-cleavable amino-protecting group.9 The glass surface was then illuminated with UV light through a negative photo-mask (the features were not coated with chromium, but the quartz glass was coated with chromium) for 30 min to generate free amino functional groups on the UV light illuminated areas (Scheme 2). Here, we have marked the position of the mask with a diamond marker. Then the free amino functional groups were reacted with tertiary butyl protected Tris-NTA in the presence of DIC inside a dark room at room temperature for 4 hours. After that the glass surface was washed with plenty of chloroform and dried under a nitrogen flow inside a dark room. Then the tertiary butyl protected Tris-NTA functionalised micropatterned surface was further illuminated with UV light for 30 min through a negative photo-mask, which was shifted slightly compared to the previous position. The free amino functional groups were reacted with Biotin-NHS for 2 hours and after that the glass surface was washed with plenty of DMF and water, and dried under a nitrogen stream. Finally, this surface was treated with trifluoroacetic acid for the deprotection of the tertiary butyl groups from Tris-NTA. After that it was washed with plenty of water and dried under a nitrogen stream. Next, the “TBAMP” surfaces were characterized by the immobilization of a decahistidine tagged EGFP protein and avidin rhodamine dye following the previously described method. Microscopic images were captured under a TIRF microscope, which revealed the side by side generation of oval shaped green and red micropatterned areas (Fig. 3a–c). The generation of “TBAMP” surfaces follows different surface chemistry and we observed that 30 min was the optimum illumination time required for generating a high quality micropatterned surface. A cartoon diagram representing the immobilization of different proteins on two neighbouring micropatterns is shown in Fig. 3d. After the optimization of the “TBAMP” patterning, we showed the application of this type of micropatterned surface and its capturing ability for multiple proteins. For this purpose, we immobilized avidin rhodamine dye onto the biotin micropatterned surface. On the Tris-NTA micropatterned surface, we first immobilized the decahistidine tagged anti-GFP VHH–His6 antibody, followed by the immobilization of the Mal3–EGFP protein (Mal3 is a fission yeast homolog of EB1 which binds with the microtubule plus-end and lattice). TIRF microscopic images revealed that there were neighbouring green and red micropatterned surfaces (Fig. 4a–c), clearly indicating the immobilization of the non-His tagged Mal3–EGFP through the VHH–His6 antibody. These results indicate that Mal3–EGFP binds with the immobilized VHH–His6 antibody on the “TBAMP” surfaces. A cartoon representing the probable immobilization of the avidin rhodamine dye and the Mal3–EGFP protein on a “TBAMP” surface is shown in Fig. 4d.
 | ||
| Scheme 2 A micropattern with Tris-NTA in one area and biotin in a neighbouring area (TBAMP) is generated by photo destruction through a negative photo-mask. | ||
There are reports about the immobilization of biomolecules/proteins on micropatterned surfaces in the literature.10 However, none of these methods are applicable for immobilizing both oligohistidine and biotin tagged multiple biomolecules or proteins simultaneously on the same micropatterned surface or on adjacent micropatterned surfaces individually. The key advantage of this type of micropatterned surface is that the approach is very simple, flexible and reusable for oligohistidine proteins compared to other methods in the literature. The only limitation in the surface chemistry is the occasional inhomogeneous density of functionalities on the surface due to a lack of control during the chemical reaction step. The mechanistic aspect of the photo destruction step for the “TBSMP” surface is the combination of a photochemical fenton reaction in the case of Tris-NTA8b and a simple UV light mediated destruction for biotin.8f The “TBAMP” surface was generated by the UV light mediated photo-destruction of the 6-nitroveratryloxycarbonyl group (Fig. S1†).
In summary, we have developed two different types of micropatterned surfaces with two different functional groups for the successful immobilization of multiple proteins/biomolecules. These patterning methods are highly biocompatible in nature as we observed that the functional proteins remained active after being immobilized on the surface. We have also shown the transportation of microtubules and antibody–antigen interactions on the “TBSMP” and “TBAMP” surfaces, respectively. The generation of these types of micropatterned surfaces has a wide range of applications for the reconstitution of complex biological events. For example, “TBSMP” is extremely useful and provides flexibility for biochemists who need to immobilize multiple biomolecules onto a local environment. “TBAMP” offers the reconstitution of centrosome and chromosome surfaces by immobilizing the Xenopus Microtubule Associated Protein (XMAP215–His6) onto the Tris-NTA micropattern, which can nucleate microtubules selectively from the micropatterned areas,10e and at the same time the biotin micropattern can be used for mimicking the chromosome surface by immobilizing biotin tagged-DNA in an Xenopus Egg extract environment.8d
Acknowledgements
The authors wish to thank Mr D. Sarkar for microscopy, and Dr Thomas Surrey, London Cancer Research Institute, UK for providing plasmids of all the proteins used in this manuscript, and both the positive and negative photo-masks; AB is thankful to UGC; AS and BJ thank CSIR, India and DG thanks the CSIR-Network project (BSC0113) for their fellowship. SG kindly acknowledges the CSIR-IICB Network project (BSC0113) and the Department of Science and Technology, India (Project Number SR/S0/BB/0102/2012) for financial support. We thank the anonymous referees for their nice comments to improve the quality of this manuscript.Notes and references
-
(a) M. G. van den Heuvel and C. Dekker, Science, 2007, 317, 333 CrossRef CAS PubMed
; (b) A. Boker, J. He, T. Emrick and T. P. Russell, Soft Matter, 2007, 3, 1231 RSC
; (c) D. C. Turner, C. Chang, K. Fang, S. L. Brandow and D. B. Murphy, Biophys. J., 1995, 69, 2782 CrossRef CAS
; (d) H. Sato and T. Homma, J. Nanosci. Nanotechnol., 2007, 7, 225 CrossRef CAS PubMed
; (e) L. Ionov, N. Houbenov, A. Sidorenko, M. Stamm and S. Minko, Adv. Funct. Mater., 2006, 16, 1153 CrossRef CAS
; (f) A. Sidorenko, T. Krupenkin, A. Taylor, P. Fratzl and J. Aizenberg, Science, 2007, 315, 487 CrossRef CAS PubMed
; (g) M. J. Dalby, N. Gadegaard, R. Tare, A. Andar, M. O. Riehle, P. Herzyk, C. D. W. Wilkinson and R. O. C. Oreffo, Nat. Mater., 2007, 6, 997 CrossRef CAS PubMed
; (h) L. Ionov, V. Bocharovab and S. Dieza, Soft Matter, 2009, 5, 67 RSC
; (i) A. S. Blawas and W. M. Reichert, Biomaterials, 1998, 19, 595 CrossRef CAS
; (j) S. T. Plummer, Q. Wang, P. W. Bohn, R. Stockton and M. A. Schwartz, Langmuir, 2003, 19, 7528 CrossRef CAS
; (k) H. Kim, J. Doh, D. J. Irvine, R. E. Cohen and P. T. Hammond, Biomacromolecules, 2004, 5, 822 CrossRef CAS PubMed
; (l) S. P. Fodor, J. L. Read, M. C. Pirrung, L. Stryer, A. T. Lu and D. Solas, Science, 1991, 251, 767 CAS
; (m) F. J. Xu, H. Z. Li, J. Li, Y. H. Eri Teo, C. X. Zhu, E. T. Kang and K. G. Neoh, Biosens. Bioelectron., 2008, 24, 779 CAS
; (n) H. Zhu, M. Bilgin, R. Bangham, D. Hall, A. Casamayor, P. Bertone, N. Lan, R. Jansen, S. Bidlingmaier, T. Houfek, T. Mitchell, P. Miller, R. A. Dean, M. Gerstein and M. Snyder, Science, 2001, 293, 2101 CrossRef CAS PubMed
; (o) S. H. Gan, P. Yang and W. T. Yang, Biomacromolecules, 2009, 10, 1238 CrossRef CAS PubMed
.
-
(a) J. Fink, M. Théry, A. Azioune, R. Dupont, F. Chatelain, M. Bornens and M. Piel, Lab Chip, 2007, 7, 672 RSC
; (b) C. A. Goubko and X. Cao, Mater. Sci. Eng., C, 2009, 29, 1855 CrossRef CAS PubMed
; (c) Z. Liu, J. L. Tan, D. M. Cohen, M. T. Yang, N. J. Sniadecki, S. A. Ruiz, C. M. Nelson and C. S. Chen, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 9944 CrossRef CAS PubMed
; (d) S. Raghavan, C. J. Shen, R. A. Desai, N. J. Sniadecki, C. M. Nelson and C. S. Chen, J. Cell Sci., 2010, 123, 2877 CrossRef CAS PubMed
.
-
(a) B. Lom, K. E. Healy and P. E. Hockberger, J. Neurosci. Methods, 1993, 50, 385 CrossRef CAS
; (b) A. Kumar and G. M. Whitesides, Appl. Phys. Lett., 1993, 63, 2002 CrossRef CAS PubMed
; (c) S. Y. Chou, P. R. Krauss and P. J. Renstrom, Science, 1996, 272, 85 CAS
.
-
(a) J. Hyun, Y. J. Zhu, A. Liebmann-Vinson, T. P. Beebe and A. Chilkoti, Langmuir, 2001, 17, 6358 CrossRef CAS
; (b) J. P. Park, S. J. Lee, T. J. Park, K. B. Lee, I. S. Choi, S. Y. Lee, M. G. Kim and B. H. Chung, Biotechnol. Bioprocess Eng., 2004, 9, 137 CrossRef CAS
; (c) Y. M. Huang, M. Uppalapati, W. O. Hancock and T. N. Jackson, Lab Chip, 2008, 8, 1745 RSC
; (d) J. M. Alonso, A. Reichel, J. Piehler and A. del Campo, Langmuir, 2008, 24, 448 CrossRef CAS PubMed
; (e) P. Jonkheijm, D. Weinrich, M. Kohn, H. Engelkamp, P. C. Christianen, J. Kuhlmann, J. C. Maan, D. Nusse, H. Schroeder, R. Wacker, R. Breinbauer, C. M. Niemeyer and H. Waldmann, Angew. Chem., Int. Ed., 2008, 47, 4421 CrossRef CAS PubMed
; (f) N. P. Reynolds, J. D. Tucker, P. A. Davison, J. A. Timney, C. N. Hunter and G. J. Leggett, J. Am. Chem. Soc., 2009, 131, 896 CrossRef CAS PubMed
; (g) T. Korten, A. Månsson and S. Diez, Curr. Opin. Biotechnol., 2010, 21, 477 CrossRef CAS PubMed
.
-
(a) D. J. Pritchard, H. Morgan and J. M. Cooper, Angew. Chem., Int. Ed. Engl., 1995, 34, 91 CrossRef CAS
; (b) P. S. Petrou, M. Chatzichristidi, A. M. Douvas, P. Argitis, K. Misiakos and S. E. Kakabakos, Biosens. Bioelectron., 2007, 22, 1994 CrossRef CAS PubMed
; (c) P. Jonkheijm, D. Weinrich, M. Köhn, H. Engelkamp, P. C. M. Christianen, J. Kuhlmann, J. C. Maan, D. Nüsse, H. Schroeder, R. Wacker, R. Breinbauer, C. M. Niemeyer and H. Waldmann, Angew. Chem., Int. Ed., 2008, 47, 4421 CrossRef CAS PubMed
; (d) Y. K. Kim, S. R. Ryoo, S. J. Kwack and D. H. Min, Angew. Chem., Int. Ed., 2009, 48, 3507 CrossRef CAS PubMed
; (e) K. Jang, K. Sato, K. Mawatari, T. Konno, K. Ishihara and T. Kitamori, Biomaterials, 2009, 30, 1413 CrossRef CAS PubMed
; (f) K. Jang, Y. Xu, Y. Tanaka, K. Sato, K. Mawatari, T. Konno, K. Ishihara and T. Kitamori, Biomicrofluidics, 2010, 4, 032208 CrossRef PubMed
; (g) D. S. Shin, J. H. Seo, J. L. Sutcliffe and A. Revzin, Chem. Commun., 2011, 47, 11942 RSC
.
- C. You, M. Bhagawati, A. Brecht and J. Piehler, Anal. Bioanal. Chem., 2009, 393, 1563 CrossRef CAS PubMed
.
-
(a) S. Lata, A. Reichel, R. Brock, R. Tamp and J. Piehler, J. Am. Chem. Soc., 2005, 127, 10205 CrossRef CAS PubMed
; (b) S. Lata, M. Gavutis and J. Piehler, J. Am. Chem. Soc., 2006, 128, 6 CrossRef CAS PubMed
; (c) A. Tinazli, J. Tang, R. Valiokas, S. Picuric, S. Lata, J. Piehler, B. Liedberg and R. Tampe, Chem.–Eur. J., 2005, 11, 5249 CrossRef CAS PubMed
; (d) S. Lata and J. Piehler, Anal. Chem., 2005, 77, 1096 CrossRef CAS
.
-
(a) P. Bieling, I. A. Telley, J. Piehler and T. Surrey, EMBO Rep., 2008, 9, 1121 CrossRef CAS PubMed
; (b) M. Bhagawati, S. Ghosh, A. Reichel, K. Froehner, T. Surrey and J. Piehler, Angew. Chem., Int. Ed., 2009, 48, 9188 CrossRef CAS PubMed
; (c) P. Bieling, I. A. Telley and T. Surrey, Cell, 2010, 142, 420 CrossRef CAS PubMed
; (d) R. Heald, R. Tournebize, T. Blank, R. Sandaltzopoulos, P. Becker, A. Hyman and E. Karsenti, Nature, 1996, 382, 420 CrossRef CAS PubMed
; (e) A. Dinarina, C. Pugieux, M. M. Corral, M. Loose, J. Spatz, E. Karsenti and F. Nedelec, Cell, 2009, 138, 502 CrossRef CAS PubMed
; (f) A. Biswas, A. Saha, B. Jana, P. Kurkute, G. Mondal and S. Ghosh, ChemBioChem, 2013, 14, 689 CrossRef CAS PubMed
; (g) B. Jana, G. Mondal, A. Biswas, I. Chakraborty, A. Saha, P. Kurkute and S. Ghosh, Macromol. Biosci., 2013, 13, 1478 CrossRef CAS PubMed
; (h) B. Jana, G. Mondal, A. Biswas, I. Chakraborty and S. Ghosh, RSC Adv., 2013, 3, 8215 RSC
; (i) A. Saha, I. Chakraborty, C. Kraft, S. Bhushan and S. Ghosh, RSC Adv., 2013, 3, 7688 RSC
.
-
(a) A. Patchornik, B. Amit and R. Woodward, J. Am. Chem. Soc., 1970, 92, 6333 CrossRef CAS
; (b) J. A. McCray and D. R. Trentham, Annu. Rev. Biophys. Biophys. Chem., 1989, 18, 239 CrossRef CAS PubMed
; (c) S. A. Sundberg, R. W. Barrett, M. Pirrung, A. L. Lu, B. Kiangsoontra and C. P. Holmes, J. Am. Chem. Soc., 1995, 117, 12050 CrossRef CAS
.
-
(a) M. Gropeanu, M. Bhagawati, R. A. Gropeanu, G. M. R. Muñiz, S. Sundaram, J. Piehler and A. d. Campo, Small, 2013, 9, 838 CrossRef CAS PubMed
; (b) S. Waichman, C. You, O. Beutel, M. Bhagawati and J. Piehler, Anal. Chem., 2011, 83, 501 CrossRef CAS PubMed
; (c) S. Waichman, M. Bhagawati, Y. Podoplelova, A. Reichel, A. Brunk, D. Paterok and J. Piehler, Anal. Chem., 2010, 82, 1478 CrossRef CAS PubMed
; (d) S. WonHan, S. Lee, J. Hong, E. Jang, T. Lee and W. G. Koh, Biosens. Bioelectron., 2013, 45, 129 CrossRef PubMed
; (e) S. Ghosh, C. Hentrich and T. Surrey, ACS Chem. Biol., 2013, 8, 673 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3sm53000k |
| ‡ Authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2014 |