Supramolecular chirality induced by a weak thermal force†
Placido
Mineo
*ab,
Valentina
Villari
*b,
Emilio
Scamporrino
a and
Norberto
Micali
b
aDipartimento di Scienze Chimiche and I.N.S.T.M. UdR of Catania, University of Catania, Viale Andrea Doria 6, I-95125 Catania, Italy. E-mail: gmineo@unict.it
bCNR-IPCF Istituto per i Processi Chimico-Fisici, Viale F. Stagno d'Alcontres 37, Messina, Italy. E-mail: villari@me.cnr.it
First published on 1st November 2013
Abstract
We show that the unexpected chirality of aggregated structures based on an uncharged achiral porphyrin system originates from small temperature gradients that act as an asymmetrical physical perturbation; the consequent thermal force gives rise to the thermophoretic motion of the aggregates. We establish that the induced optical activity can be controlled, and even vanished, by minimizing the thermal force.
Spontaneous mirror-symmetry breaking is a very intriguing scientific argument that covers many scientific fields.1,2 In life science, homochirality (single handedness) does not have, up to now, a well known origin as there are several possible external influences that are able to cause enantioselection,3–6 namely, the selection of one of the mirror-image structures.
Some examples of external asymmetric forces able to induce enantioselection are irradiation with circularly polarized light causing asymmetric photosynthesis or photolysis,2,7–9 heterogeneous catalysis,10–12 stirring and vortex motions13–20 and simultaneous acting of external fields.21–23 Once the enantioselection is induced, the amplification of the mirror-asymmetry (chirality) can be realized by additional mechanisms.24–29
From a general point of view, an aggregated structure obtained from an achiral molecule should not exhibit optical activity, as the self-aggregation phenomenon should lead to achiral or, at least, racemic or meso structures;30,31 unless a statistical fluctuation, occurring at an early stage of the aggregation, produces the excess of one enantiomer. In repeated measurements, statistical fluctuations give rise to a random sign of supramolecular chirality because the excess of one or the other enantiomer is generated with the same probability. Then, obtaining a reproducible enantiomeric excess must be the result of an external action.
The formation of optically active aggregates obtained by physical perturbations during the aggregation process of protonated sulfonate porphyrins in water has been reported in recent years.14,15,23,32 Nevertheless, the appearance of circular dichroism (CD) signals for aggregates of achiral porphyrins has been also observed in the absence of any added chiral template or externally applied physical perturbation.33 In these cases, the phenomenon was attributed to a templating effect of chiral biological residuals with opposite charge with respect to the porphyrin ionic groups during the aggregate formation.
In this communication, we show that the chirality of the aggregated structures of an uncharged achiral porphyrin system, synthesized for this purpose (see ESI §1†), is generated by a weak thermal force (through thermophoresis) that acts as a generalized thermodynamic force (Onsager theorem) representing an asymmetrical physical perturbation.34 We prove that the intesity of the CD signal can be controlled (even vanished) through a fine thermostation of the solution. The absence of charge in the investigated porphyrin system results in weak interactions between the molecules in the aggregate, which allows for the application of weak thermal perturbations to generate the enantiomeric enrichment.
The uncharged 5,10,15,20-tetrakis[p(ω-methoxy-polyethyleneoxy)phenyl] porphyrin35–38 (PPeg4, see Fig. 1a), is able to spontaneously form self-assembled structures in water (see Fig. 1b, inset and ESI §2†). The shifted absorption bands (indication of aggregation) with respect to the monomer absorption bands are due to the coupling of the (nearly) degenerate porphyrin dipole transition moments39 differently arranged in the aggregates in a face-to-face (blue-shift) and head-to-tail (red-shift) fashion.40 The formation of PPeg4 aggregates was confirmed by dynamic light scattering experiments (see ESI §2†), which gave a value of the average hydrodynamic radius, RH, of about 200 nm. The mesoscopic structure of these aggregates is fractal-like, as indicated by the scattered intensity profile41 reported in the inset of Fig. 1b. From the fit of the scattering profile, the fractal dimension (∼2.3) and a radius of gyration, Rg, of about 600 nm were obtained. The ratio between Rg and RH is consistent with the tenuous structure of fractals.
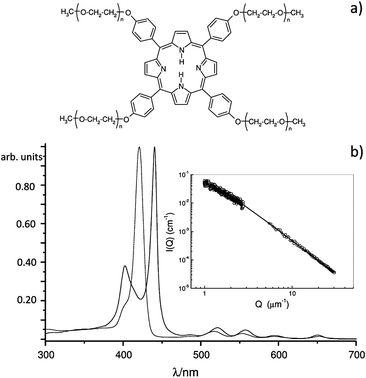 | ||
| Fig. 1 (a) Structure of PPeg4: the four polyethylene glycol chains, with a polymerization degree n ∼ 9, are the peripheral substituents of the porphyrin. (b) Absorption spectra of the monomer in THF (band at 421 nm) and the aggregates in water (bands at 400 and 440 nm). The concentration of PPeg4 is 4 μM. In the inset of (b), the scattering profile in water is the typical for fractals; the continuous line represents the fitting curve according to eqn (1) in ESI §2.† | ||
Unlike the self-assembly of charged porphyrin systems (e.g. porphyrin tetrasulfonate), in which strong electrostatic forces are present, the aggregation of PPeg4 occurs through weak interactions (hydrogen bonds, π–π or hydrophobic interactions) between the porphyrin molecules.42 This allows us to use a weak physical perturbation to induce symmetry breaking in the already aggregated structures.
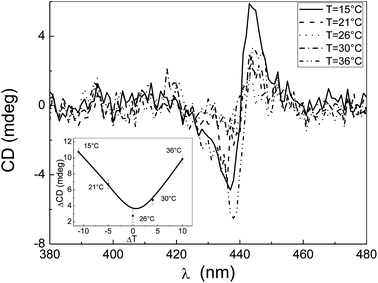
The monomeric form of PPeg4 (in the organic solvent THF) is achiral and does not show any CD signals. The self-aggregation of an achiral molecule is expected to give rise to achiral or racemic structures, which will not display circular dichroism (CD) signals. However, as observed in Fig. 2, the CD spectrum of a PPeg4 aqueous solution shows a signal with an intensity that increases with the temperature. Also, the CD band features (shape and intensity) are strongly dependent on the experimental procedure according to which the temperature value is reached (see ESI §3).†
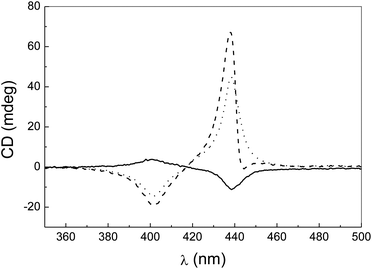 | ||
| Fig. 2 Circular dichroism spectra at T = 26 °C (full line), T = 30 °C (dotted line) and T = 36 °C (dashed line) by thermostating the PPeg4 aqueous solution with a standard Jasco thermostat. | ||
In order to ensure that the CD signal variations are not due to aggregate structure modifications43 at a mesoscopic scale, we performed light scattering measurements at different temperatures (see ESI §4†), which did not give any indication of structural rearrangements. Therefore, the observed strongly thermal dependence can be explained by considering the effect of uncontrolled temperature gradients originated by different temperatures at the cuvette walls (see ESI §1).†
In order to de-couple the effect of convective motions44 (flows in the solution as in stirring) from that of the unprecedented observed thermophoresis on the supramolecular chirality, a new experiment was carried out in another spectropolarimeter apparatus equipped with a fully home-made accurate thermostat, designed for dynamic light scattering studies of phase transitions. The thermostat ensures a small temperature gradient (thermostation better than ±0.05 °C) and hence a weak thermal force. The absence of convection was checked by using the dynamic light scattering technique in heterodyne detection, useful to obtain particle drift velocities under convective flows.45,46 No oscillation in the scattered electric field correlation function due to a Doppler shift was observed when this home-made thermostat was used.
The remaining thermophoretic force (with circular and vertical components), despite its small magnitude, gives rise to a vortical migration of the aggregates from the hottest to the coldest zones (see ESI §1†). Unlike the chirality caused by flows of the whole solution, the chiral motion generated by thermophoresis involves the aggregated particles only.
In the CD experiments (see Fig. 3) carried out by using such a thermostat (the room temperature was kept constant at 26 ± 0.5 °C), it can be observed that the intensity of the CD signal decreases when the difference between the sample temperature and the room temperature decreases.
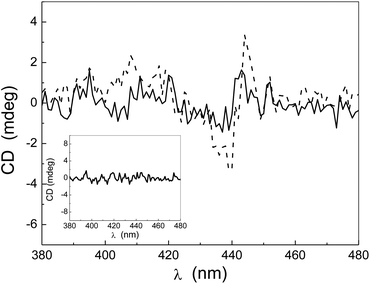
In addition, comparison of two measurements carried out at the same sample temperature but at different room temperatures (see Fig. 4) confirms that the CD intensity decreases with the decreasing temperature difference with respect to the laboratory temperature.
As a result of the use of this cell holder that minimizes the temperature gradient, the measured signals are much smaller than those observed by using the standard thermostated sample holder and reported in Fig. 2. Nevertheless, despite being smaller, a residual CD signal is still present. By using a two-stage thermostat set at 36 °C that limits the temperature fluctuations with an accuracy of ±0.01 °C, and hence removes the gradient, no CD signals were detected (see the inset of Fig. 4).
From the measurement of the contribution from the linear dichroism47,48 (LD), i.e. the alignment of the species, on the CD under a temperature gradient and in its absence, we could calculate that there is negligible cross-talk between the LD and the CD and therefore, no artifacts are present (see ESI §5).†
Conclusions
The data indicate that the supramolecular chirality can also be induced on a self-assembled achiral porphyrin by a weak thermal force which, through thermophoresis, generates the chiral motion of the aggregates only and not of the solvent. Such a thermal force can be controlled by tuning the temperature gradient between the sample and the surroundings. When the gradient, and consequently the thermal force, is cancelled, the supramolecular chirality vanishes. Then, the chiral motion of the aggregates generated by thermophoresis seems to be able to produce local chiral deformations.These results provide new evidence on the influence of weak asymmetric perturbations in determining the enantiomeric selection and suggest that some uncontrolled asymmetric external force is acting when unexpected circular dichroism signals are present.
Acknowledgements
We thank the Ministero Istruzione Università e Ricerca (MIUR, Roma) for partial financial support -PON 2010, project PON01-00074. We also thank Dr Fabiola Spitaleri for her assistance on MALDI-TOF and NMR measurements.Notes and references
- L. D. Barron, Chirality, 2012, 24, 879 CrossRef CAS PubMed
.
- B. L. Feringa and R. A. van Delden, Angew. Chem., Int. Ed., 1999, 38, 3418 CrossRef
.
-
G. H. Wagnière, On Chirality and the Universal Asymmetry: Reflections on Image and Mirror Image, Wiley-VCH, Weinheim, 2007 Search PubMed
.
-
P. L. Luisi, The Emergence of Life: From Chemical Origins to Synthetic Biology, Cambridge Univ. Press, Cambridge, 1st edn, 2006 Search PubMed
.
- S. F. Mason, Nature, 1984, 311, 19 CrossRef CAS
.
- C. Grande and N. H. Patel, Nature, 2009, 457, 1007 CrossRef CAS PubMed
.
- J. J. Flores, W. A. Bonner and G. A. Massey, J. Am. Chem. Soc., 1977, 99, 3622 CrossRef CAS
.
- J. Bailey, A. Chrysostomou, J. H. Hough, T. M. Gledhill, A. McCall, S. Clark, F. Ménard and M. Tamura, Science, 1998, 281, 672 CrossRef CAS
.
- W. L. Noorduin, A. A. C. Bode, M. van der Meijden, H. Meekes, A. F. van Ettegervan, W. J. P. Enckevort, P. C. M. Christianen, B. Kaptein, R. M. Kellogg, T. Rasing and E. Vlieg, Nat. Chem., 2009, 1, 729 CrossRef CAS PubMed
.
- A. Kuhn and P. Fischer, Angew. Chem., Int. Ed., 2009, 48, 6857 CrossRef CAS PubMed
.
- T. Kawasaki, S. Kamimura, A. Amihara, K. Suzuki and K. Soai, Angew. Chem., Int. Ed., 2011, 50, 6796 CrossRef CAS PubMed
.
- T. Mai, M. Branca, D. Gori, R. Guillot, C. Kouklovsky and V. Alezra, Angew. Chem., Int. Ed., 2012, 51, 4981 CrossRef CAS PubMed
.
- V. Aquilanti and G. S. Maciel, Origins Life Evol. Biospheres, 2006, 36, 435 CAS
.
- J. M. Ribò, J. Crusats, F. Sagues, J. Claret and R. Rubires, Science, 2001, 292, 2063 CrossRef PubMed
.
- A. D'Urso, R. Randazzo, L. Lo Taro and R. Purrello, Angew. Chem., Int. Ed., 2010, 49, 108 CrossRef CAS PubMed
.
- C. Escudero, J. Crusats, I. Dìez-Pèrez, Z. El-Hachemi and J. M. Ribò, Angew. Chem., Int. Ed., 2006, 45, 8032 CrossRef CAS PubMed
.
- O. Arteaga, A. Canillas, J. Crusats, Z. El-Hachemi, J. Llorens, E. Sacristan and J. M. Ribò, ChemPhysChem, 2010, 11, 3511 CrossRef CAS PubMed
.
- M. Kostur, M. Schindler, P. Talkner and P. Hanggi, Phys. Rev. Lett., 2006, 96, 014502 CrossRef
.
- A. Raudino and M. Pannuzzo, J. Chem. Phys., 2012, 137, 134902 CrossRef PubMed
.
- O. Ohno, Y. Kaizu and H. Kobayashi, J. Chem. Phys., 1993, 99, 4128 CrossRef CAS
.
- G. L. J. A. Rikken and E. Raupach, Nature, 2000, 405, 932 CrossRef CAS PubMed
.
- M. Avalos, R. Babiano, P. Cintas, J. L. Jiménez, J. C. Palacios and L. D. Barron, Chem. Rev., 1998, 98, 2391 CrossRef CAS PubMed
.
- N. Micali, H. Engelkamp, P. G. van Rhee, P. C. M. Christianen, L. M. Scolaro and J. C. Maan, Nat. Chem., 2012, 4, 201 CrossRef CAS PubMed
.
- C. Viedma, Phys. Rev. Lett., 2005, 94, 065504 CrossRef
.
- A. R. A. Palmans and E. W. Meijer, Angew. Chem., Int. Ed., 2007, 46, 8948 CrossRef CAS PubMed
.
- R. Randazzo, A. Mammana, A. D'Urso, R. Lauceri and R. Purrello, Angew. Chem., Int. Ed., 2008, 47, 9879 CrossRef CAS PubMed
.
- R. Lauceri, A. Raudino, L. M. Scolaro, N. Micali and R. Purrello, J. Am. Chem. Soc., 2002, 124, 894 CrossRef CAS PubMed
.
- H. Onouchi, T. Miyagawa, K. Morino and E. Yashima, Angew. Chem., Int. Ed., 2006, 45, 2381 CrossRef CAS PubMed
.
- E. Bellacchio, R. Lauceri, S. Gurrieri, L. M. Scolaro, A. Romeo and R. Purrello, J. Am. Chem. Soc., 1998, 120, 12353 CrossRef CAS
.
- L. D. Barron, J. Am. Chem. Soc., 1986, 108, 5539 CrossRef CAS
.
- L. D. Barron, Space Sci. Rev., 2008, 135, 187 CrossRef CAS
.
- N. Petit-Garrido, J. Claret, J. Ignés-Mullol and F. Sagués, Nat. Commun., 2012, 3, 1001 CrossRef PubMed
.
- Z. El-Hachemi, C. Escudero, O. Arteaga, A. Canillas, J. Crusats, G. Mancini, R. Purrello, A. Sorrenti, A. D'Urso and J. M. Ribò, Chirality, 2009, 21, 408 CrossRef CAS PubMed
.
- The analysis of thermophoresis in nanoparticle suspensions is still a debated topic and the calculation of the thermal gradients and the resulting vortexes would require the development of a transport model and a fluid dynamic approach which are beyond the aim of the paper.
- E. Scamporrino, D. Vitalini and P. Mineo, Macromolecules, 1996, 29, 5520 CrossRef CAS
.
- P. Mineo, E. Scamporrino, D. Vitalini, R. Alicata and S. Bazzano, Rapid Commun. Mass Spectrom., 2005, 19, 2773 CrossRef CAS PubMed
.
- P. Mineo, D. Vitalini and E. Scamporrino, Macromol. Rapid Commun., 2002, 23, 681 CrossRef CAS
.
- N. Micali, V. Villari, P. Mineo, D. Vitalini, E. Scamporrino, V. Crupi, D. Majolino, P. Migliardo and V. Venuti, J. Phys. Chem. B, 2003, 107, 5095 CrossRef CAS
.
- G. Pescitelli, S. Gabriel, Y. Wang, J. Fleischhauer, R. Woody and N. Berova, J. Am. Chem. Soc., 2003, 125, 7613 CrossRef CAS PubMed
.
- M. Kasha, Radiat. Res., 1963, 20, 55 CrossRef CAS
.
- V. Villari and N. Micali, J. Pharm. Sci., 2008, 97, 1703 CrossRef CAS PubMed
.
- V. Villari, P. Mineo, E. Scamporrino and N. Micali, RSC Adv., 2012, 2, 12989 RSC
.
- M. A. Castriciano, A. Romeo, G. De Luca, V. Villari, L. M. Scolaro and N. Micali, J. Am. Chem. Soc., 2011, 133, 765 CrossRef CAS PubMed
.
- E. Bodenschatz, W. Pesch and G. Ahlers, Annu. Rev. Fluid Mech., 2000, 32, 709 CrossRef
.
- H. Miike, Y. Kuriyama, Y. Itou, H. Hashimoto and Y. Ebina, Phys. Rev. A: At., Mol., Opt. Phys., 1985, 31, 2756 CrossRef CAS
.
-
W. Schärtl, Light Scattering from polymer solutions and nanoparticle dispersions, Springer-Verlag, Berlin, Heidelberg, 2007 Search PubMed
.
- A. Tsuda, M. A. Alam, T. Harada, T. Yamaguchi, N. Ishii and T. Aida, Angew. Chem., Int. Ed., 2007, 46, 8198 CrossRef CAS PubMed
.
- M. Wolffs, S. J. George, Ž. Tomović, S. C. J. Meskers, A. P. H. J. Schenning and E. W. Meijer, Angew. Chem., Int. Ed., 2007, 46, 8203 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods, light scattering and circular dichroism data at different temperature values, evaluation of the linear dichroism contribution. See DOI: 10.1039/c3sm52322e |
| This journal is © The Royal Society of Chemistry 2014 |