Monitoring the interaction of nucleolipoplexes with model membranes†
Costanza
Montis
,
Piero
Baglioni
and
Debora
Berti
*
CSGI and Department of Chemistry, University of Florence, via della Lastruccia 3, 50019, Sesto Fiorentino, Firenze, Italy. E-mail: montis@csgi.unifi.it; baglioni@csgi.unifi.it; berti@csgi.unifi.it; Fax: +390554573036; Fax: +390554573036; Tel: +390554573017 Tel: +390554573033 Tel: +390554573038
First published on 4th October 2013
Abstract
We report on the interaction of nucleolipid/DNA assemblies with model membranes, studied with small angle X-ray scattering, fluorescence microscopy and correlation spectroscopy. The fusion with the membrane can be monitored by following the diffusion properties of a lipid probe, shedding light on the parameters that regulate the internalization of the complexes.
The design and characterization of lipid formulations tailored for drug delivery are a major area of interest of soft matter science. Cationic liposomes have been for instance intensively investigated for the delivery of genes or oligonucleotides to cells, as alternatives to viral vectors. However, the overall positive charge of their complexes with DNA (lipoplexes) causes rapid serum inactivation and cytotoxicity, hampering translation into effective clinical use. In the last decade, zwitterionic and anionic liposomes,1 which bind DNA thanks to Ca2+ electrostatic bridging, have been proposed to amend this drawback. More recently, synthetic nucleolipids of various net charges have been designed and tested for the same purpose;2–4 in this case the complexation of DNA to form nucleolipoplexes includes specific molecular recognition interactions.
The final delivery efficiency in vivo depends on adhesion/fusion of the complex with cell membranes and release of the payload after internalization.5 The formulation of lipid carriers would greatly benefit from the implementation of robust and flexible experimental methods that provide, in simpler model systems, predictive understanding of the biological activity. Surprisingly, for DNA vectors this approach has been only partially addressed in a few recent studies on cationic lipoplexes. This contribution aims to fill this gap, reporting on the interaction of nucleolipid-based DNA carriers with giant unilamellar vesicles (GUVs), investigated through a combination of imaging, static and dynamical techniques, which allow on-line monitoring of the interaction with model membranes.
Liposomes from the nucleolipid POP-Ade (1-palmitoyl-2-oleoyl-phosphatidyladenosine)6 formulated with two different neutral helper lipids, POPC (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine) and DOPE (1,2-dioleoyl-sn-glycero-3-phosphoethanolamine) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 mole ratio (lipid concentration: 0.25 mM), were complexed with DNA 2% w/w to form nucleolipoplexes when 15 mM CaCl2was added.
4 mole ratio (lipid concentration: 0.25 mM), were complexed with DNA 2% w/w to form nucleolipoplexes when 15 mM CaCl2was added.
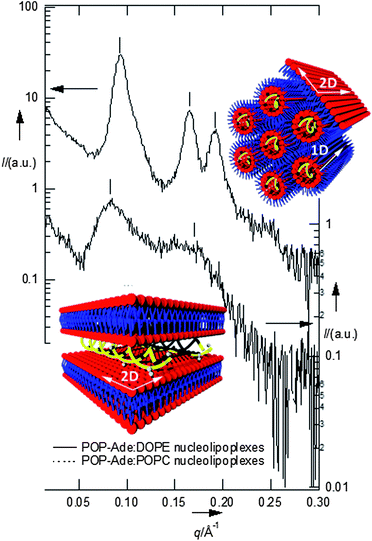
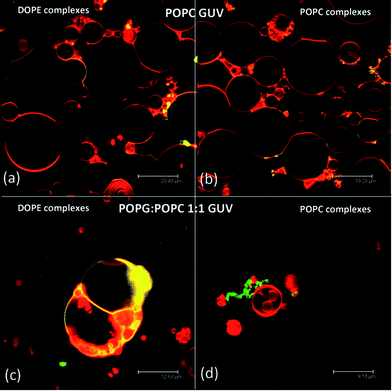
Fig. 1 reports the SAXS (small angle X-ray scattering) patterns of the nucleolipoplexes. The internal liquid crystalline structure is controlled by the spontaneous curvature of the helper lipid: a lamellar phase is formed for POPC, while an inverted hexagonal phase is observed for DOPE. This feature is similar to the behavior of cationic lipoplexes with the same helper lipids, where DNA is sandwiched between the lamellae or confined in the aqueous pool of the micellar rods, respectively;7 interestingly, this structural signature is maintained in nucleolipoplexes, where the DNA double helix is confined in the lipid structure thanks to electrostatic bridging with the polar heads operated by Ca2+. The same complexes have been tagged with a fluorescently labeled lipid and imaged two hours after preparation with Laser Scanning Confocal Microscopy (LSCM), as shown in Fig. 2. The DOPE complexes (2a) show a compact structure on the submicron scale, while for POPC (2b) they appear as a looser cluster of smaller constructs, revealing that the helper lipid affects not only the liquid crystalline structure, but also the morphology of the aggregates at the mesoscale. If a fluorescently tagged DNA is used to form the complex, very similar results are obtained (see ESI Fig. 1†), demonstrating the colocalization of lipids and nucleic acids.
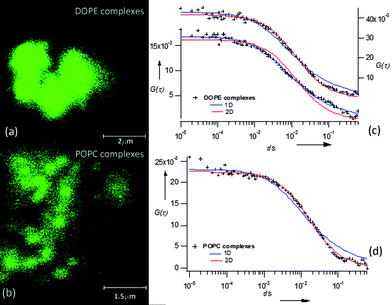 | ||
| Fig. 2 Left panel: LSCM images of DOPE (a) and POPC (b) nucleolipoplexes sedimented on the chamber bottom two hours after preparation; right panel: ACFs of DOPE (c) and POPC (d) nucleolipoplexes; the continuous lines are 1D (eqn (1)) and 2D (eqn (2)) curve fits. | ||
Fluorescence correlation spectroscopy was employed to monitor the lipid diffusion within nucleolipoplexes, introducing a fluorescent lipid probe, Liss Rhod PE, with the same net charge as the nucleolipids. The temporal fluctuations of the emission intensity, due to the diffusion of the probe inside–outside the detection volume, are analyzed in terms of their autocorrelation function (ACF), G(τ).8 The main information gathered is the characteristic decay time (τD) of the ACF (inversely proportional to the diffusion coefficient of the fluorescent species), and the functional form of the curve, which depends on the diffusion mode of the probe. Fig. 2 shows three examples of FCS curves, measured on aggregates sedimented on the chamber bottom. In this case the center-of-mass translational contribution of the whole complex is eliminated and any observed diffusion is ascribed to internal motions of the lipid probe within the liquid crystal. For POPC complexes, the ACF can be analyzed according to a 2D normal diffusion model (eqn (1), α = 1, Fig. 2d).
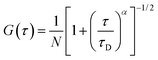 | (1) |
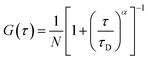 | (2) |
Very few FCS studies on 1D confined diffusion have been presented so far, namely on diffusion in cylindrical pores of a nanoporous alumina,10 and hexagonally packed rodlike micelles.11 Here for the first time this method is applied to lipid–DNA complexes and allows differentiation between different internal liquid crystalline phases.
The observed mode is consistent with lipids that slide along the tubular micelles describing a 1D motion, as represented in Fig. 1 (upper panel). Alternatively, if FCS is monitored in the interfacial regions of the complexes, the ACFs can be described according to a 2D diffusion (Fig. 2c). In these cases we are also illuminating the planar monolayer which is known to wrap the inverted hexagonal phase, also represented in Fig. 1. 1D or 2D diffusion prevails, depending on the balance of the internal hexagonal or of the external monolayer regions in the excitation volume. This picture is fully consistent with SAXS analysis, but provides additional information, thanks to the spatial resolution accessible with confocal microscopy. The possibility of monitoring diffusion with spatial resolution on the submicron scale provides an ideal alternative to ensemble averaged techniques (DLS or PGSE-NMR) and a unique tool to unravel complex behavior in microheterogeneous fluids.
Table 1 compares the τD values of the complexes with those of free-standing lipid bilayers, i.e. giant unilamellar vesicles (GUVs from POPC or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 POPC
1 POPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) POPG (1-palmitoyl-2-oleoyl-sn-phosphatidyl-glycerol, ESI Fig. 1†)). The motion of the lipid probes in GUVs is planar, since the diameter of the vesicles is significantly higher than the excitation volume, and the bilayer curvature can be approximated to zero. Calibration-dependent methodologies and calibration-free techniques, such as Z-scan,9 have been applied to obtain the diffusion coefficients (see ESI Fig. 1 and 2†). The τD values in GUVs agree with the literature for similar systems.12 Interestingly, they are one order of magnitude lower than those observed for lamellar nucleolipoplexes, despite the identical local structure, and significantly lower than those obtained for DOPE–DNA assemblies. The slower diffusional dynamics in nucleolipoplexes indicates a local stiffening of the bilayer, clearly related to DNA complexation and possibly also due to Ca2+, which bridges the phosphate groups on the lipid polar heads and on the DNA backbone.
POPG (1-palmitoyl-2-oleoyl-sn-phosphatidyl-glycerol, ESI Fig. 1†)). The motion of the lipid probes in GUVs is planar, since the diameter of the vesicles is significantly higher than the excitation volume, and the bilayer curvature can be approximated to zero. Calibration-dependent methodologies and calibration-free techniques, such as Z-scan,9 have been applied to obtain the diffusion coefficients (see ESI Fig. 1 and 2†). The τD values in GUVs agree with the literature for similar systems.12 Interestingly, they are one order of magnitude lower than those observed for lamellar nucleolipoplexes, despite the identical local structure, and significantly lower than those obtained for DOPE–DNA assemblies. The slower diffusional dynamics in nucleolipoplexes indicates a local stiffening of the bilayer, clearly related to DNA complexation and possibly also due to Ca2+, which bridges the phosphate groups on the lipid polar heads and on the DNA backbone.
| Liss Rhod PE diffusing medium | τ D(1D) (s) | τ D(2D) (s) |
|---|---|---|
| a Z-scan. b Calibration dependent methodology. | ||
| POPC nucleolipoplexes | — | 1.21 × 10−2 ± 5 × 10−3 |
| DOPE nucleolipoplexes | 6.8 × 10−3 ± 3 × 10−3 | 1.40 × 10−2 ± 4 × 10−3 |
| POPC giant unilamellar vesicles | — | 1.32 × 10−3 ± 8 × 10−5a |
| 1.26 × 10−3 ± 4 × 10−5b | ||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 POPG 1 POPG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) POPC giant unilamellar vesicles POPC giant unilamellar vesicles |
— | 1.75 × 10−3 ± 9 × 10−5a |
| 1.80 × 10−3 ± 1 × 10−4b | ||
The interaction of nucleolipoplexes with biological membranes is of primary importance for their efficiency in therapy. Therefore, the evaluation of the structural aspects that determine the interaction properties in model systems is particularly relevant for their applications in this area. To address this point, we have studied the interaction between nucleolipoplexes and GUVs, intended as models of cell bilayers through LSCM and FCS. Fig. 3 shows POPC and DOPE nucleolipoplexes, labeled with β-Bodipy (green), incubated for two hours with POPC and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 POPG
1 POPG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) POPC GUVs stained with Liss-Rhod PE (red). For POPC target membranes (Fig. 3, upper panel) we observe a cross-migration of the lipid probes, irrespectively of the liquid crystalline phase of the nucleolipoplexes, which results in a colocalization of the originally separated probes (Fig. 3a and b, yellow). Conversely, when negatively charged GUVs are considered, the cross-migration is observed only for DOPE vectors (Fig. 3c), while lipid mixing does not occur for lamellar nucleolipoplexes (Fig. 3d), even if several contact regions appear.
POPC GUVs stained with Liss-Rhod PE (red). For POPC target membranes (Fig. 3, upper panel) we observe a cross-migration of the lipid probes, irrespectively of the liquid crystalline phase of the nucleolipoplexes, which results in a colocalization of the originally separated probes (Fig. 3a and b, yellow). Conversely, when negatively charged GUVs are considered, the cross-migration is observed only for DOPE vectors (Fig. 3c), while lipid mixing does not occur for lamellar nucleolipoplexes (Fig. 3d), even if several contact regions appear.
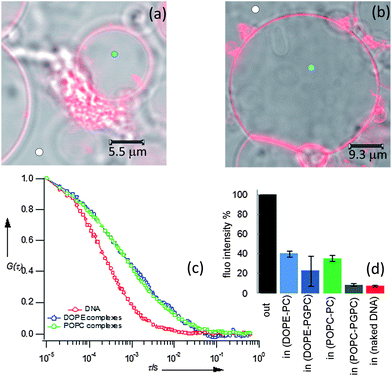
It is known that a close proximity of opposing layers is the prerequisite to trigger fusion and lipid exchange. We can hypothesize that a local excess of Ca2+ promotes the initial docking of nucleolipoplexes on GUVs, irrespectively of their lipid composition. After this initial contact, the occurrence of fusion and lipid exchange is controlled by an energy barrier, which clearly depends on the surface charge of the target membrane. For negative model bilayers, which more closely mimic cell membranes,13 only hexagonal complexes can overcome this activation barrier. These results correlate with the generally observed higher efficiency in vitro of hexagonal lipoplexes, whose curvature favors the formation of stalk-like structures,14 but remarkably they also show that the whole process depends on the charge of the target membrane. To further investigate this point, labeled GUVs were incubated with unstained nucleolipoplexes (LSCM images (a) and (b)) and the lipid exchange was monitored through FCS. Fig. 4c and d display some representative ACFs acquired in the nucleolipoplex–GUV interaction regions. The diffusion of the lipid probe cannot be described by a simple combination of the diffusion in the two separated environments (GUVs and nucleolipoplexes) (two-component curve fits of the ACFs reported in the ESI†); therefore, the lipid mixing imaged with LSCM occurs through formation of a contact region with different structural properties.
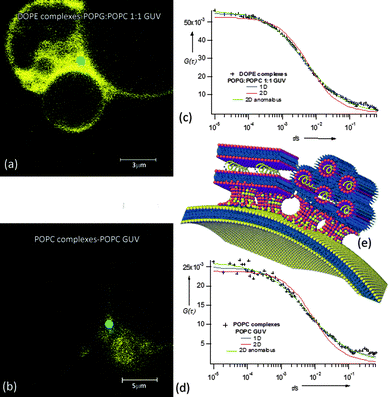 | ||
Fig. 4 LSCM images of DOPE (a) and POPC (b) nucleolipoplexes incubated with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 POPG 1 POPG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) POPC (a) and POPC (b) GUVs fluorescently labeled with Liss Rhod PE. ACFs acquired in the interaction regions (light blue rounds), 1D normal (eqn (1)) and 2D normal and anomalous (eqn (2)) diffusion fit. (e) Cartoon sketching POPC and DOPE nucleolipoplex–GUV fusion intermediates' hypothesized structure. POPC (a) and POPC (b) GUVs fluorescently labeled with Liss Rhod PE. ACFs acquired in the interaction regions (light blue rounds), 1D normal (eqn (1)) and 2D normal and anomalous (eqn (2)) diffusion fit. (e) Cartoon sketching POPC and DOPE nucleolipoplex–GUV fusion intermediates' hypothesized structure. | ||
The ACFs can be well described either according to a 1D (eqn (2), α = 1) or a 2D anomalous (eqn (1), α ≠ 1) diffusion model, both for POPC and DOPE nucleolipoplexes, with decay time values (τD) intermediate between nucleolipoplexes and GUVs (ESI Table 4†).
Membrane fusion is generally described as proceeding through formation of a negatively curved stalk-like structure from opposing lipid membranes brought in close proximity, where the initial fusion of the external layers is followed by the mixing of the internal layers.15 Our FCS data can be consistently interpreted with this model, hypothesizing a fusion intermediate as represented in Fig. 4e: several point-like contact regions between nucleolipoplexes and GUVs evolve in stable stalk-like intermediates and lipid diffusion along the GUV plane is either hindered by the presence of several fusion regions (2D anomalous diffusion), or detected as a 1D diffusion along the tubular stalk-like structures, with the coexistence of domains of different fluidities, originally part of nucleolipoplexes and GUVs. Interestingly, no dependence on the initial liquid-crystalline phase of the complexes is observed at this stage.
Finally, to investigate DNA uptake and release after the fusion between nucleolipoplexes and GUVs, FCS was employed to track a 50-mer fluorescently labeled ds-DNA. The free 50-mer had a diffusion coefficient of 46.3 ± 2 μm2 s−1, in agreement with the literature data.16 When added to unstained GUVs in the absence of a lipid formulation, it diffuses outside the vesicles, unperturbed; after several hours of incubation, the fluorescence intensity inside the GUVs is negligible, indicating no penetration through the bilayer of the free nucleic acid, as expected. Fig. 5 displays some representative confocal images and FCS curves when nucleolipoplexes with DOPE (5a) and POPC (5b) are added to the unstained GUVs. The ACFs outside the GUVs, monitored 20 μm up the chamber bottom (Fig. 5c), yield lower diffusion coefficients (D ≈ 2.6 μm2 s−1) with respect to the naked DNA, consistently with the formation of nucleolipoplexes. Since the DNA is the only fluorescent species, some meaningful information can be gathered from the time-averaged fluorescence intensity in the confocal volume, monitored inside and outside the giant vesicles. For DOPE–DNA complexes, after two hours of incubation, a meaningful fluorescence intensity, around 30% of the external value, is detectable for both GUV types, indicating a corresponding partition of DNA, which has therefore crossed the membrane barrier. Conversely, for POPC nucleolipoplexes, only for POPC GUVs we can observe a similar DNA passage. This is in striking agreement with the results reported in Fig. 3, demonstrating that, even in this coarse model, lipid mixing is the prerequisite to internalization. The ACFs for DNA, measured inside the GUVs after incubation, are consistent with partially free and complexed oligomers (see ESI Fig. 5†). Interestingly, despite the initially very similar size of the complexes, there is a striking dependence on the type of the target bilayer (ESI Table 3†).
The accepted view on cell internalization of lipoplexes is through an endocytosis mediated process; however this paradigm has been recently challenged by a study showing that siRNA delivered by cationic lipoplexes can enter cells also when the endocytic pathways are fully inhibited.17 This result unambiguously highlighted that lipoplex–plasma membrane fusion is a possible pathway for the direct uptake of nucleic acids. Our data reproduce this in vitro observation in a highly simplified model system, where the target of the complexes is a simple bilayer. Moreover, we have been able to identify the molecular determinants that promote this mechanism, i.e. the liquid crystalline nature of the complexes and, unexpectedly, the surface charge of the target membrane.
In the present study, we have monitored the interaction of DNA–lipid complexes with model membranes, to gain insight into the structural features that eventually regulate the efficiency of DNA transfection for therapeutic purposes. Through observation of the motion of a lipid probe inside the DNA–lipid complexes or in the target membrane, it is possible to follow each step of this multistage process and to characterize the structural intermediates that form upon contact, fusion and release. Our model system captures some of the salient features observed in cellular systems, showing DNA release that would explain a recently observed non-endocytic pathway for internalization.
Acknowledgements
The authors acknowledge financial support from MIUR for funding under the projects PRIN 2010-2011(2010BJ23MN) and FIRB RBPR05JH2P_007 ITALNANONET.Notes and references
- S. D. Patil, D. G. Rhodes and D. J. Burgess, AAPS J., 2005, 7, E61–E77 CrossRef CAS PubMed.
- D. Berti, Curr. Opin. Colloid Interface Sci., 2006, 11, 74–78 CrossRef CAS PubMed.
- D. Berti, C. Montis and P. Baglioni, Soft Matter, 2011, 7, 7150–7158 RSC.
- C. M. LaManna, H. Lusic, M. Camplo, T. J. McIntosh, P. Barthelemy and M. W. Grinstaff, Acc. Chem. Res., 2012, 45, 1026–1038 CrossRef CAS PubMed.
- J. E. Gagner, S. Shrivastava, X. Qian, J. S. Dordick and R. W. Siegel, J. Phys. Chem. Lett., 2012, 3, 3149–3158 CrossRef CAS.
- C. Montis, S. Milani, D. Berti and P. Baglioni, J. Colloid Interface Sci., 2012, 373, 57–68 CrossRef CAS PubMed.
- C. R. Safinya, Curr. Opin. Struct. Biol., 2001, 11, 440–448 CrossRef CAS.
- J. Ries and P. Schwille, BioEssays, 2012, 34, 361–368 CrossRef PubMed.
- R. Machan and M. Hof, Int. J. Mol. Sci., 2010, 11, 427–457 CrossRef CAS PubMed.
- J. Hohlbein, M. Steinhart, C. Schiene-Fischer, A. Benda, M. Hof and C. G. Hubner, Small, 2007, 3, 380–385 CrossRef CAS PubMed.
- A. W. Kirkeminde, T. Torres, T. Ito and D. A. Higgins, J. Phys. Chem. B, 2011, 115, 12736–12743 CrossRef CAS PubMed.
- L. Guo, J. Y. Har, J. Sankaran, Y. M. Hong, B. Kannan and T. Wohland, ChemPhysChem, 2008, 9, 721–728 CrossRef CAS PubMed.
- G. Lei and R. C. MacDonald, J. Membr. Biol., 2008, 221, 97–106 CrossRef CAS PubMed.
- J. Heuvingh, F. Pincet and S. Cribier, Eur. Phys. J. E, 2004, 14, 269–276 CrossRef CAS PubMed.
- W. Wickner and R. Schekman, Nat. Struct. Mol. Biol., 2008, 15, 658–664 CAS.
- S. P. Gordon, S. Berezhna, D. Scherfeld, N. Kahya and P. Schwille, Biophys. J., 2005, 88, 305–316 CrossRef CAS PubMed.
- J. J. Lu, R. Langer and J. Z. Chen, Mol. Pharmaceutics, 2009, 6, 763–771 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: All the experimental details, the complete list of FCS fitting equations, additional FCS data. DOI: 10.1039/c3sm52254g |
| This journal is © The Royal Society of Chemistry 2014 |