Both protein adsorption and aggregation contribute to shear yielding and viscosity increase in protein solutions†
Maria Monica
Castellanos
a,
Jai A.
Pathak
*b and
Ralph H.
Colby
*a
aDepartment of Materials Science and Engineering, The Pennsylvania State University, University Park, PA 16802, USA. E-mail: rhc@plmsc.psu.edu
bFormulation Sciences Department, MedImmune, 1 MedImmune Way, Gaithersburg, MD 20878, USA. E-mail: PathakJ@medimmune.com
First published on 13th November 2013
Abstract
A combination of sensitive rotational rheometry and surface rheometry with a double-wall ring were used to identify the origins of the viscosity increase at low shear rates in protein solutions. The rheology of two high molecular weight proteins is discussed: Bovine Serum Albumin (BSA) in a Phosphate Buffered Saline solution and an IgG1 monoclonal antibody (mAb) in a formulation buffer containing small quantities of a non-ionic surfactant. For surfactant-free BSA solutions, the interfacial viscosity dominates the low shear viscosity measured in rotational rheometers, while the surfactant-laden mAb solution has an interfacial viscosity that is small compared to that from aggregation in the bulk. A viscoelastic film forms at the air/water interface in the absence of surfactant, contributing to an apparent yield stress (thus a low shear viscosity increase) in conventional bulk rheology measurements. Addition of surfactant eliminates the interfacial yield stress. Evidence of a bulk yield stress arising from protein aggregation is presented, and correlated with results from standard characterization techniques used in the bio-pharmaceutical industry. The protein film at the air/water interface and bulk aggregates both lead to an apparent viscosity increase and their contributions are quantified using a dimensionless ratio of the interfacial and total yield stress. While steady shear viscosities at shear rates below ∼1 s−1 contain rich information about the stability of protein solutions, embodied in the measured yield stress, such low shear rate data are regrettably often not measured and reported in the literature.
1. Introduction
The rheology of therapeutic protein solutions has a significant impact on their delivery and fill/finish operations. Subcutaneous parenteral delivery of bio-therapeutics is preferred over intravenous delivery for many therapeutic indications because of added conveniences such as simple dosing and potential for self-administration. However this can be challenging for bio-therapeutics of lower potency because of the volume limitation of dosing into the subcutaneous tissue (∼1 mL). Highly concentrated antibody formulations are therefore desired, creating several challenges, including higher propensity for protein aggregation and higher viscosities.1 Additionally, bio-therapeutic formulations are subject to a variety of complex flow fields during operations that promote formation of aggregates (filtration, transportation, etc.), which is undesirable due to possible immunogenic responses and loss of biological activity.2,3 Therefore, understanding the bulk and interfacial shear rheology of protein solutions under different conditions, and the relationship between viscosity and stability remains an unfulfilled scientific challenge in the development and delivery of therapeutic proteins.Protein aggregation is a kinetic process that occurs through different mechanisms, such as the formation of partially unfolded intermediate precursors (non-native) or self-associated native proteins, which in many cases form a nucleus that further grows into a protein aggregate.4–6 The aggregates formed depend on the aggregation pathway, and even different types of aggregates can be formed by the same protein.7,8 In a recent study, we reported results on a monoclonal antibody (mAb) that had aggregated at storage conditions (5 °C) over months.9 The present study exploits thermally driven aggregation of a mAb at 40 °C, which is used as a standard accelerated stability test condition in the bio-pharmaceutical industry. The aggregation kinetics of a mAb solution has been suggested to be second order with rate constants that follow non-Arrhenius behavior, and accelerated aggregation studies could be used to predict long-term storage stability.10 The mAb formulation of this study is incubated at 40 °C for prolonged times, where molecules have higher Brownian mobility and experience faster conformational changes that promote the formation of partially unfolded intermediates. Furthermore, as temperature increases molecules diffuse faster and thus have higher probabilities for aggregation. The mAb studied here has poor conformational stability at 40 °C (see ESI†), and shows ∼20% monomer loss by size-exclusion chromatography in 2 weeks, which motivated its choice for this study.
Despite the relevance of protein aggregation in bio-therapeutics, limited research has been performed on the flow properties of aggregated protein solutions. While numerous studies have investigated the viscosity of protein solutions,11–19 many of them report the viscosity at a single shear rate or frequency, or do not comprehensively characterize the protein aggregates under the conditions studied. Here we demonstrate that measuring the viscosity for a broad range of shear rates can provide information about the stability of protein solutions. Since interfacial effects could corrupt the measurement of the true bulk viscosity,17 we present an approach to account for interfacial and bulk contributions in the rheology measurements. Moreover, we show that the interfacial viscosity of protein solutions decreases in the presence of the surfactant polysorbate 80, which is commonly added to therapeutic formulations to prevent surface-induced aggregation.
The purpose of this work is to gain a better understanding of the contribution of protein aggregation to interfacial and bulk viscosities at various shear rates. Bovine Serum Albumin (BSA) is studied for an extensive range of concentrations, from 11 mg mL−1 up to 386 mg mL−1. Additionally, BSA is a relatively inexpensive protein that can be easily prepared at high concentrations in large quantities, in order to perform measurements in interfacial rheometers that require large volumes. This study proves that while air/water (A/W) interfaces influence the rheology of protein solutions, they are not the only factor responsible for non-Newtonian rheology, as protein aggregation in the bulk solution also contributes to the yield stress. The yield stress refers to the minimum stress required for the protein solution to flow like a liquid, whereas a solid-like response is observed below the yield stress. The term aggregation is used here to refer to any kind of supra-monomeric structure formed, including reversible self-association, reversible clustering and irreversible clustering. The types of aggregates observed are discussed according to the characterization techniques used.
To summarize organization, in Section 2 a brief literature review on the rheology of protein solutions is presented. Section 3 describes materials, methods and characterization techniques. In Section 4 we discuss the rheology of BSA solutions in the absence of surfactant, followed by the rheology of a surfactant-laden mAb solution as aggregation proceeds, and correlations between the yield stress and the results from biophysical characterization. We conclude with a summary of the main results of the study on the contributions to shear yielding in protein solutions.
2. Protein solution rheology review
One of the main areas of study in protein solution rheology is the dependence of viscosity on concentration. The high shear rate viscosity of protein solutions shows a monotonic exponential increase with concentration, comparable to the rheological response of colloidal particles that follow Krieger–Dougherty type models,20,21 or models for charged colloidal particles interacting by attractive and repulsive Derjaguin–Landau–Verwey–Overbeek (DLVO) type potentials.17,22 However, these models fail at high protein9 and salt concentrations,23,24 where intermolecular interactions increase in importance, and surface patchiness, charge distribution,16,19 relative orientation, hydrophobic interactions, etc., become relevant to describe the viscosity of protein solutions.25,26Another important aspect regarding the rheology of protein solutions is their characteristic flow curves. Steady shear rheology experiments on surfactant-free protein solutions show a non-Newtonian viscosity at low shear rates that decreases by as much as 4 orders of magnitude at the highest shear rates, where the viscosity shows a plateau.13,14,27 This extreme change in the low shear rate viscosity due to shear yielding (i.e. presence of a yield stress under shear leading to higher viscosities) was attributed to a weak network of protein aggregates.27 Rheopexy (increase of viscosity with time at a constant shear rate) was observed in BSA solutions and synovial fluid, and attributed to a weak dipolar attractive interaction energy of ∼3 kT (estimated in ref. 27).27 Another study with a surfactant-free mAb formulation showed an apparent non-monotonic dependence of the low shear rate viscosity with concentration.9 It was verified that when clusters larger than 0.2 μm were filtered, monotonic dependence of viscosity with concentration was restored, but with shear rate dependent viscosities (non-Newtonian behavior).9 These results suggest that the common practice of reporting viscosity at a single shear rate or frequency19,28,29 is inadequate for describing the rheology of protein solutions.
Additional studies proposed that the yield stress observed in bulk torsional rheometry measurements is only an interfacial artifact, due to the viscoelastic film that proteins form at the A/W interface.17,30 Proteins at interfaces are viscoelastic,31–39 and addition of non-ionic surfactants changes the interfacial properties, because surfactant molecules with higher surface activity than proteins preferentially adsorb at the interface and reach surface coverage.30,40–42 A/W interfaces not only affect the viscosity of protein solutions, but also promote protein aggregation when the interface is disturbed.43–46 Most studies of interface-driven aggregation have failed to characterize the interfacial viscoelasticity, without which an understanding of interface-driven protein instability is incomplete.43–45
3. Methodology
3.1. Protein solutions
Bovine Serum Albumin (BSA fraction V, Sigma-Aldrich, A-3059) was used as received and dissolved in Phosphate Buffered Saline solution (PBS, Sigma-Aldrich P-3813, pH = 7.4, 138 mM NaCl) at concentrations from 11 mg mL−1 up to 386 mg mL−1. Concentration was measured by gravimetric A-280 (absorbance at 280 nm) using an UV spectrophotometer with an extinction coefficient for BSA of 0.667 cm2 mg−1 for a 0.1% solution.47 Each sample was quiescently dissolved (no stirring) for about 1 day while stored at 4 °C. For BSA solutions containing surfactant, polysorbate 80 (PS80, (sorbitan)[–(C2H4O)x(OH)][–(C2H4O)y(OH)][–(C2H4O)z(OH)][–(C2H4O)w]–OC(O)–(CH2)7–(CH)2–(CH2)7–CH3 where w + x + y + z = 20 or C64H124O26, Sigma-Aldrich, P1754, critical micelle concentration (CMC) of 0.012 mM)48 was added at concentrations of 1 × 10−3 weight% (∼0.7 CMC) by successive dilutions.The mAb used in this study is a fully humanized IgG1,49 supplied as a lyophilized formulation. Reconstitution with 2.17 mL of water for injection yields a protein concentration of 53 mg mL−1 in 20 mM histidine buffer (pH 6.0), sugar and a low level of PS80. mAb samples were incubated at 40 °C for up to 44 days. Centrifugal filtration (Amicon® Ultra-15, 50k MWCO filters, EMD Millipore, Catalogue Number UFC905024) at a speed of 4000 rcf was used to concentrate the mAb to 114 ± 2 mg mL−1. Antibody concentration was determined by A-280 using an extinction coefficient of 1.45 cm2 mg−1 for a 0.1% solution. For filtration studies, samples were filtered through a 0.2 μm Sterile Acrodisc® filter (Gelman Sciences, Product number 4192). The triple mutation mAb of this work was previously observed to have lower stability than the corresponding wild-type protein, with three defined regions for domain unfolding: the CH2 domain unfolds at 49 °C, Fab unfolds at 72 °C, and CH3 at 83 °C.49,50 Due to the low stability at 40 °C, the least conformationally stable domain (CH2, where mutations where introduced) is the most aggregation prone domain,49 although this might not be always the case.7
3.2. Rheology experiments
Steady shear experiments were conducted using conventional rotational rheometers with concentric cylinders, a microfluidic rheometer and an interfacial shear rheometer.Contraves LS-30 is a strain controlled rheometer consisting of a cup and a stationary bob (cup and bob rheometer, CB) that rotates at different speeds depending on the specified shear rate. This rheometer is capable of applying shear rates between 0.02 and 100 s−1. It can measure any stress in the range 0.2–3000 mPa, by application of a compensating torque with a drag cup motor working against the torque from the sample and a fine torsional wire.27 Temperature is controlled at 25 °C using a water bath. Two bobs with lengths 8.0 mm and 15.2 mm were used, both with bob and cup radii of 5.5 mm and 6.0 mm respectively. A sample volume of 2 mL or 3 mL (depending on the bob used) was loaded in the rheometer cup while avoiding visible air bubbles. Since the non-Newtonian viscosity in protein solutions depends on time and shear history (as shown below in Section 4.1), samples were pre-sheared for 5 minutes at 60 s−1, followed by the actual measurement starting from low to high shear rates and a final step from high to low shear rates, and this final step is reported. Samples were sheared until reaching an apparent steady value. The start-up of steady shear (application of a constant shear rate while monitoring the build-up of stress) was utilized to ensure that the data reported are steady state viscosities. The average age of the interface after pre-shearing was between 5 and 10 minutes. Similar protocols were used for steady shear experiments in other rheometers.
The double gap stress controlled rheometer (DG26.7, Anton-Paar MCR-301) has an outer cylinder with internal and external radii of 11.910 mm and 13.801 mm, inner cylinder with an internal and external radii of 12.329 mm and 13.329 mm, and an effective bob length of 40 mm. Shear rates were applied covering the range from 0.01 s−1 to 1000 s−1. The instrument can measure viscosities for which the corresponding torque exceeds 0.1 μN m, and thus it resolves a shear stress as small as 1 mPa.
A microfluidic (A/W interface-free) Viscometer/Rheometer-on-Chip device (m-VROC, Rheosense) was used to measure the true high shear rate bulk viscosity. The m-VROC consists of a rectangular slit with an array of three pressure sensors that can sense a maximum pressure drop of 200 kPa. A 50 micron depth chip type B05 was used to measure shear rates from 500 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s−1. The sensors measure the pressure drop as the solution flows from a syringe down the slit. Additional details on this instrument can be found in ref. 9 and 17.
000 s−1. The sensors measure the pressure drop as the solution flows from a syringe down the slit. Additional details on this instrument can be found in ref. 9 and 17.
Interfacial viscosities were measured using a double-wall ring (DWR) in a stress controlled rheometer (AR-G2, TA Instruments), as described by Vandebril et al.51 Corrections due to non-linearity of the surface flow field were not required, as confirmed from the Matlab script from ref. 51, kindly provided by Jan Vermant. The smallest torque that this rheometer can apply is 0.05 μN m, corresponding to an interfacial shear stress of 3 μPa m.
3.3. Biophysical characterization of protein solutions
Monomer purity was determined with UV-detection based high performance size exclusion chromatography (HP-SEC, Agilent 1100 series with a Tosoh G3000SWXL Column, 25 nm average pore size) after sample dilution to 10 mg mL−1 to prevent column overloading. Autocorrelation functions and diffusion coefficients of bubble-free samples were determined at 25 °C using dynamic light scattering (DLS) on a DynaPro Plate Reader (Wyatt Technology, wavelength λ = 830 nm, scattering angle 2θ = 158°, wavevector q = 0.020 nm−1) after centrifuging at 2000 rpm for 2 minutes. Subvisible (microscopic) particles in the range from 2 μm to 300 μm ECD (Equivalent Circular Diameter) were counted and imaged without dilution using a Micro-Flow Imaging device (MFI, DPA 4100, Brightwell Technologies) at a flow rate of 0.22 mL min−1 in a 40 mm wide × 1.3 mm long × 0.4 mm flow cell. For BSA solutions, the dynamic surface tension was measured using a K100 Wilhelmy plate tensiometer (Krüss GmbH) for the same concentrations and time scales studied in the rheology experiments. The surface of the instrument was leveled before measurements and a small beaker with ∼15 mL of sample was used. Surface tension of water was verified to be 72.8 ± 0.5 mN m−1 at 25 °C before performing any measurements.4. Results and discussion
4.1. Rheology of BSA solutions
BSA is a globular protein with isoelectric point pI = 5.1 and 67 kDa molecular weight. When BSA is buffered in PBS at pH 7.4, the protein carries a net negative charge,52 but the 138 mM NaCl screens the charges, thus decreasing the Debye length (κ−1 = 0.8 nm). The viscosity of surfactant-free BSA in PBS measured in different bulk rheometers at a variety of protein concentrations is presented in Fig. 1. The high shear rate viscosity increases with concentration consistently for all rheometers. However, at low shear rates the viscosity is much higher than expected for a Newtonian liquid and apparently independent of protein concentration. Unfortunately, the m-VROC cannot measure the bulk contribution to the viscosity at the lower shear rates, when shear yielding occurs in the conventional rheometers (below 10 s−1). Adding a small amount of non-ionic surfactant makes the response Newtonian and eliminates the apparent and surface yield stress (see data in ESI†). A zero shear bulk viscosity was not observed in samples with a yield stress, and should clearly not be extrapolated from the high shear rate viscosity data.53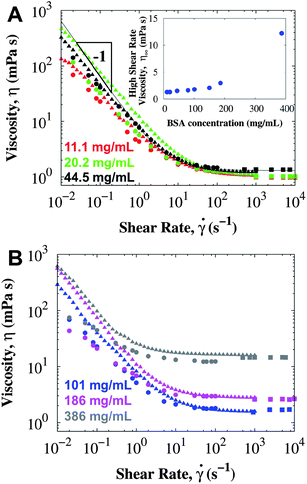 | ||
| Fig. 1 The viscosity of surfactant-free BSA in PBS (pH 7.4) at 25 °C using conventional rotational rheometers: CB (circles), DG (triangles), and an A/W interface-free rheometer m-VROC (squares). Each color indicates a different concentration: (A) 11.1 ± 0.2 mg mL−1 (red), 20.2 ± 0.1 mg mL−1 (green), 44.5 ± 0.3 mg mL−1 (black), (B) 101 ± 1 mg mL−1 (blue), 186 ± 2 mg mL−1 (pink) and 386 ± 3 mg mL−1 (gray). Inset in (A) corresponds to the high shear rate viscosity measured in the CB rheometer. Line corresponds to a Bingham model fit (eqn (1)) with a yield stress σY = 6.4 mPa. The short bob of the CB was used for these measurements. | ||
Bingham54 proposed that the viscosity η(![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) ) (ratio of measured steady-state stress σ and applied shear rate
) (ratio of measured steady-state stress σ and applied shear rate ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) ) can be described as:
) can be described as:
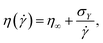 | (1) |
Since the increase in low shear rate viscosity can be influenced by the viscoelastic protein film at the A/W interface,17 the presence of interfacial effects in the measured yield stress (and therefore in the viscosity) needs to be considered. Contributions to the total torque M from the bulk MB and the interface MS were calculated using a torque balance:
| M = MB + MS. | (2) |
Solving the torque balance depends exclusively on geometrical parameters. A general expression can be obtained (see details in ESI†), where the total measured stress σ depends on the stress coming from the bulk σB and the interface σS:
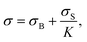 | (3) |
Consistent results are observed for the high shear rate viscosities using the rheometers of this study. However, for geometries that have a higher interfacial contribution, such as cone and plate, the high shear rate viscosity might not be representative of the true bulk viscosity. In cone and plate, the K parameter could be an order of magnitude smaller than in concentric cylinders, and interfacial effects will be important even at 1000 s−1. To determine the contribution of interfacial effects to the total measured viscosity, the parameter σS/(Kσ) is proposed, which corresponds to the fraction of the measured stress σ that comes from the A/W interface. If this ratio is of order unity, interfacial effects dominate, whereas the measured viscosity represents the true bulk solution viscosity as this parameter approaches zero. Further analysis of these estimates is presented in Section 4.4, where for the surfactant-free BSA system the yield stress comes mainly from the interface.
A dimensionless parameter known as the Bousinessq number (Bo)55,56 describes the surface drag relative to the bulk or sub-phase drag. It can be equivalently defined as the ratio of surface viscosity ηS to bulk viscosity ηB divided by a characteristic length l; l corresponds to the ratio of the area of the probe in contact with the sub-phase and the perimeter of the probe in contact with the interface:51
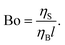 | (4) |
If 0 < Bo ≪ 1, bulk effects are important, and if Bo ≫ 1 surface effects dominate; for Bo close to unity, both surface and bulk can have important effects on the measurement. For our purposes, the Bousinessq number is not so helpful because the bulk viscosity cannot be cleanly measured when it has contributions from the A/W interface. Eqn (3) and the parameter σS/(Kσ) are more useful in practice, unless the bulk viscosity can be measured with no surface effects (e.g. rheometer with no A/W interface). In addition, the Bousinessq number could be shear rate dependent if either the surface or bulk viscosity is non-Newtonian.
The viscosity at the A/W interface was measured using the DWR for the same range of concentrations studied with the bulk rheometers, and results agree with the presence of an interface with viscoelastic properties (results not shown). Applying the Bingham model (eqn (1)), an interfacial yield stress of 7 × 10−4 Pa m is obtained, which is slightly higher than the yield stress from oscillatory shear measurements (2.3 × 10−4 Pa m).17 The surface yield stress is independent of BSA concentration, although smaller interfacial viscosities could be observed for the lower concentrations, as discussed next.
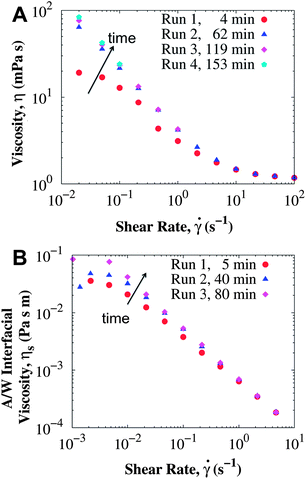
Besides the geometry of the rheometry fixtures, the age of the interface (time between loading the protein solution into the rheometer and the start of the measurement) also influences the apparent viscosity. Fig. 2 depicts the total and surface viscosity of BSA at 11.1 mg mL−1 as the age of the interface increases. For measurements performed only a few minutes after the interface is formed, the viscosity is lower than expected for the Bingham fluid. Repeating the same measurement procedure leads to higher viscosities at low shear rates. The increase in viscosity is not merely due to water evaporation, as the high shear rate viscosity changed less than 0.1 mPa s, even after about 3 hours for the concentration of 186 mg mL−1. Changes in the low shear rate viscosities are more pronounced for the lower protein concentrations: for BSA solutions at 1 mg mL−1 the viscosity at 0.02 s−1 changed from 55 to 154 mPa s ∼3 hours after the interface was formed. For the concentration of 186 mg mL−1, the viscosities did not change for any of the shear rates studied during a similar time interval (results not shown). These results account for the apparent rheopexy observed in ref. 27.
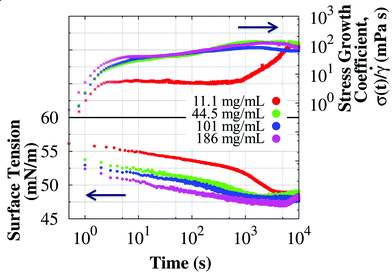
A Wilhelmy plate tensiometer was used to determine the relevant time scales at which the interfacial properties changed and reached steady state. Surface tension data of surfactant-free BSA solutions have been extensively published in the literature,57–62 but most studies report surface tension data at low concentrations (<1 mg mL−1) and measured for less than an hour. Fig. 3 shows the dynamic surface tension and the stress growth coefficient (with units of viscosity) from the start-up of steady shear measured in the short bob of the CB at 0.05 s−1 in surfactant-free BSA solutions. Interfacial changes occur at similar time scales as the apparent viscosity, reaching a concentration independent steady value after about 1 hour. Therefore, protein adsorption at the interface influences the apparent viscosity and rheopexy of surfactant-free BSA solutions. The steady value of the surface tension does not change for the BSA concentrations studied,57 but the kinetic process does: solutions with higher protein concentrations reach the steady value faster, and therefore show less rheopexy.
4.2. Rheology of mAb solutions
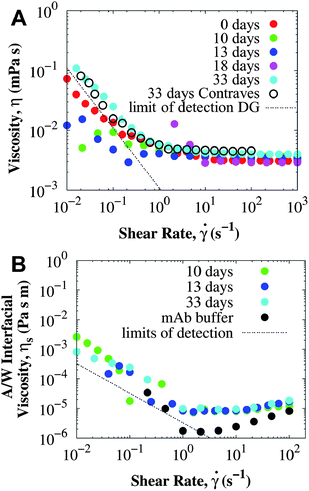
A mAb49 with molecular weight 145 kDa and pI 8.6 was also studied. The mAb is positively charged at pH 6.0, it contains a very small concentration of surfactant in its formulation, and has poor conformational stability (see ESI† for DSC data). Fig. 4 presents steady shear rheology studies of the mAb solutions after incubation at 40 °C. Since the solution contained surfactant, no yield stress is observed in freshly reconstituted samples. However, as the incubation time at 40 °C increases, a yield stress develops and higher viscosities are observed than those expected for a Newtonian fluid. After 10 days, low shear rate rheology was sensitive to the higher order structures formed, which increased the yield stress up to about 10 mPa after 41 days of incubation at 40 °C. The interfacial viscosity was negligible in the presence of surfactant (see ESI†), and consequently the measured viscosity is a good estimate of the true bulk viscosity with minimal interfacial effects. The yield stress was removed (i.e. Newtonian behavior was restored) after filtering through a 0.2 μm sterile filter, and did not recover within 5 hours. This result suggests that subvisible particles are implicated in the low shear viscosity increase, which is due to protein aggregation in the bulk.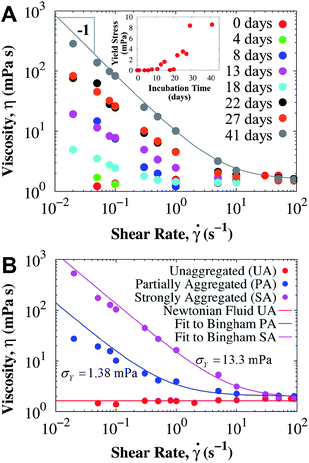 | ||
| Fig. 4 Viscosity of surfactant-laden mAb solutions measured in the CB (short bob) at 25 °C, clearly showing that sensitive conventional rheometry at low shear rates is very useful for monitoring the aggregation state of the mAb. (A) Rheology of mAb solution at 52.7 ± 0.9 mg mL−1 incubated at 40 °C for different periods of time. Line corresponds to the Bingham model fit with σY = 8.6 mPa. The inset shows the evolution of the yield stress as the 40 °C incubation time increases. (B) Rheology of mAb solution at 58.1 ± 0.5 mg mL−1 incubated at different temperatures: unaggregated sample with 99.1% monomer (UA, freshly reconstituted and only stored at 4 °C for 2 days before the measurement), partially aggregated sample with 93.9% monomer (PA, incubated at 25 °C for about 7 months), and a strongly aggregated sample with 79.4% monomer (SA, incubated an additional 44 days at 40 °C in comparison with PA). Lines are fits to the Bingham model (eqn (1)). In both examples, the low shear rate viscosity (and therefore the yield stress σY) increases as more aggregates are formed. | ||
Shear yielding was also observed for the concentrated sample after prolonged ex situ incubation at 40 °C. Fig. 5A shows the rheology of surfactant-laden mAb solution at 114 mg mL−1 using the DG geometry. After incubating for 33 days, a yield stress of O(2 mPa) was obtained from fitting the experimental data to the Bingham model (eqn (1)). An additional measurement using the long bob of the CB showed that the experimental data for 33 days were fully consistent in both the DG and the CB at all shear rates, confirming that the true bulk viscosity is being measured. In other words, if interfacial effects were responsible for shear yielding, higher viscosities would be observed for the DG than for the CB. A yield stress was detected only after 33 days, because the low shear rate viscosity data for other incubation times in the DG are below the minimum torque resolved by the MCR-301 rheometer. While the change in yield stress as aggregation proceeds is consistently observed, the time evolution between the stable and the non-equilibrium aggregated state at which the yield stress is observed varies.
Interfacial rheology was measured on samples from the same batch at 114 mg mL−1 and is presented in Fig. 5B. The A/W interfacial viscosity of surfactant-laden mAb is independent of the incubation time and comparable to the interfacial viscosity of the buffer. Since, the interfacial viscosity is determined primarily by the surfactant adsorbed at the interface, both BSA and mAb solutions with a small amount of PS80 have the same A/W interfacial viscosity independent of the protein (see details in ESI†). The surfactant preferentially adsorbs at the A/W interface and eliminates the interfacial yield stress, and aggregates in the bulk do not change the interfacial viscosity. For the mAb sample incubated at 40 °C for 33 days, the surface yield stress was not greater than 2 × 10−6 Pa m, resulting in less than 3% of surface contribution to the bulk yield stress.
4.3. Characterization of aggregates and contributions to the yield stress
It has been shown that the yield stress in protein solutions can be attributed to either the formation of a viscoelastic protein film at the A/W interface or the presence of protein aggregates in the bulk solution. For protein solutions with non-ionic surfactant, the low shear rate viscosity is dependent on the presence of aggregates. However the nature and structure of those aggregates responsible for a bulk yield stress still need to be elucidated. Rheology by itself cannot provide this information and other techniques are required (rheology was aided here by SEC, DLS, and MFI).SEC is a standard high-throughput technique used in the bio-pharmaceutical industry to determine the stability of a protein solution based on the presence of oligomeric aggregates.3 For BSA solutions, the percentage of monomer remains constant at 84% monomer for all protein concentrations studied, with the remaining 16% corresponding to aggregates. After addition of surfactant, the yield stress is eliminated but the percentages of monomer and oligomers remain constant, implying that the surfactant does not affect the aggregate content. For the surfactant-laden mAb solution, a yield stress is associated with a decrease in the monomer content, as more oligomeric aggregates are formed with increasing incubation time at 40 °C. However, a decrease in the percentage of monomer from SEC does not always correlate with a higher yield stress, in particular during the first stages of incubation where a yield stress is not detected.
In contrast, the time-resolved DLS autocorrelation function appears to be correlated with the evolution of the bulk yield stress: the autocorrelation function shows slower relaxations with additional exponential decays from aggregates that diffuse about two orders of magnitude slower than protein monomers. Fig. 6 presents a comparison between the autocorrelation function of surfactant-free BSA solutions and surfactant-laden mAb solutions, which have a yield stress from the A/W interface and from protein aggregation respectively. For BSA (Fig. 6A), the autocorrelation function does not change significantly with concentration: the fast mode diffusion coefficient D1 (τi−1 = Diq2, where τi corresponds to the i-th mode relaxation time) only increases from 6.0 × 10−11 to 7.2 × 10−11 m2 s−1 for 11.1 and 386 mg mL−1 respectively, although at the very high concentrations an additional exponential decay is observed due to crowding. These diffusion coefficients are in agreement with previously reported results for BSA at similar conditions.63 Contrary to BSA, the autocorrelation functions of incubated mAb solutions reveal slower relaxation modes (i.e. longer relaxation times) due to protein aggregation (Fig. 6B). The fast diffusion coefficient decreases as aggregation proceeds. When a yield stress is observed, additional relaxation modes coming from larger aggregates are required to describe the autocorrelation function. Similar responses occur in samples at other concentrations, suggesting that both DLS and rheology are sensitive to the type of protein aggregates that contribute to a yield stress.
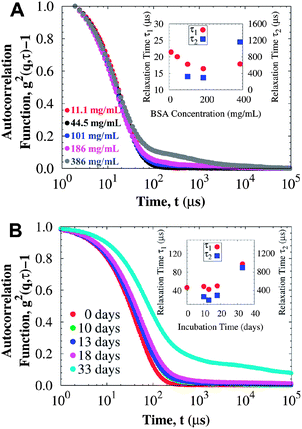 | ||
| Fig. 6 DLS data at 25 °C for (A) BSA solutions at different protein concentrations (rheology data presented in Fig. 1), and (B) mAb solutions at 114 mg mL−1 incubated at 40 °C for different periods of time (rheology data presented in Fig. 5, where a yield stress is detected after 33 days of incubation). Insets correspond to the relaxation times for the faster decay (τ1) and the slower second exponential decay (τ2). For surfactant-free BSA solutions, most of the yield stress comes from the A/W interface and the autocorrelation function can be mainly described by a fast relaxation mode corresponding to protein monomers, unless crowding becomes important. For the surfactant-laden mAb solutions, where the yield stress comes from protein aggregation in the bulk, relaxation modes are slower as the yield stress increases, and even a third relaxation mode is observed for the sample incubated at 40 °C for 33 days (of order 105 μs, not shown). | ||
Subvisible particles were observed using MFI in both BSA and the mAb solutions, but the concentration of particles was an order of magnitude lower than in samples incubated at 40 °C. In BSA solutions, subvisible particles with concentrations from 5000 to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 particles per mL were observed, independent of protein concentration. Conversely, mAb solutions show an increase in the concentration of subvisible particles upon incubation at 40 °C with minimal changes in the size distribution (i.e. most particles are in the order of 2 to 15 μm independent of incubation time). Fig. 7 presents the size distribution of mAb samples at 114 mg mL−1 with rheology data available (Fig. 5). The size of the particles decays exponentially:
000 particles per mL were observed, independent of protein concentration. Conversely, mAb solutions show an increase in the concentration of subvisible particles upon incubation at 40 °C with minimal changes in the size distribution (i.e. most particles are in the order of 2 to 15 μm independent of incubation time). Fig. 7 presents the size distribution of mAb samples at 114 mg mL−1 with rheology data available (Fig. 5). The size of the particles decays exponentially:
C = C0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−r/d), exp(−r/d), | (5) |
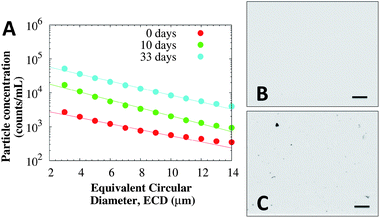 | ||
| Fig. 7 (A) MFI cumulative size distribution of subvisible particles present in mAb samples at 114 mg mL−1 and 25 °C, after incubation at 40 °C. Representative micrographs of the mAb samples incubated at 40 °C for (B) 0 days (showing no particles) and (C) 33 days (showing many small particles). Rheology data of these samples is presented in Fig. 5, and DLS autocorrelation functions in Fig. 6B. Lines correspond to fitting eqn (5). Scale bars in both micrographs correspond to 200 μm. | ||
Powerful and high-throughput techniques do not comprehensively characterize all types of aggregates in protein solutions and several complementary techniques are required.64,65 Protein aggregation leads to an increase in the low shear rate viscosity, accompanied by an increase in monomer loss (by SEC), the formation of aggregates that diffuse much slower than the monomer (by DLS), and subvisible particles (by MFI). Additional studies are being conducted using small-angle neutron scattering to identify the inter-molecular interactions and morphology of the aggregates responsible for shear yielding, which decrease the ability of a protein solution to flow.
4.4. Origins of the yield stress in protein solutions
Depending on the conditions of the system, viscoelastic protein films at A/W interfaces and protein aggregation in bulk solution can contribute to shear yielding in protein solutions. The main results of this study are summarized in Fig. 8, where the fractions of yield stress and high shear rate viscosity that come from the A/W interface are compared for surfactant-free BSA solution and surfactant-laden mAb solution. For surfactant-free BSA solutions, the yield stress ratio is independent of concentration, since neither the interfacial nor the apparent yield stress depends on bulk protein concentration. For the surfactant-laden mAb, the yield stress ratio is sensitive to bulk aggregates and σS/(Kσ) tends to 0 as the incubation time at 40 °C increases, because while the interfacial yield stress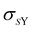 saturates rapidly, the bulk yield stress
saturates rapidly, the bulk yield stress 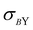 continues to increase. High shear rate viscosities for both systems are dominated by the bulk viscosity of the solution. At high shear rates for which σ ≫ σY, protein solutions behave like simple Newtonian liquids and bulk solution contributions generally dominate over interfacial contributions.
continues to increase. High shear rate viscosities for both systems are dominated by the bulk viscosity of the solution. At high shear rates for which σ ≫ σY, protein solutions behave like simple Newtonian liquids and bulk solution contributions generally dominate over interfacial contributions.
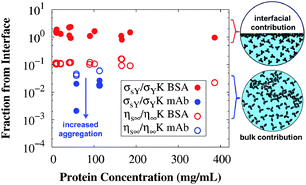 | ||
| Fig. 8 Fractions of the measured yield stress (filled symbols) and high shear rate viscosity (open symbols) that originate from the A/W interface, expressed as dimensionless ratios of Bingham parameters (eqn (1)) for interfacial and conventional bulk rheology measurements at 25 °C. Values for surfactant-free BSA solutions are in red and for the surfactant-laden mAb solutions in blue. The yield stress in surfactant-free BSA solutions is dominated by the A/W interfacial contribution, while the yield stress in surfactant-laden mAb solutions and both of the high shear rate viscosities have minimal contributions from the A/W interface. Cartoon representations use the structures of serum albumin66 and an IgG167 for the interfacial and bulk contribution respectively. | ||
A simple torque balance and the Bingham model applied to bulk and interfacial measurements can be used to quantify the interfacial contributions to the total yield stress. This quantification method is one of the key contributions of this work, along with the observation that low shear rheometry contains information about the stability of protein solutions. The results reported in ref. 9 on a quiescently aggregating surfactant-free antibody solution have been confirmed here with another antibody containing surfactant, wherein aggregation has been driven thermally. It is also important to note that many factors affect the trajectory of the time-dependent rheology measurements, and therefore results should not be expected to be quantitatively but only qualitatively reproducible27 as irreversible aggregation is a non-equilibrium process that contributes to yielding.9 Factors that can affect the reproducibility68 include small variations in concentration, presence of protein aggregates, presence of impurities, etc. Consequently, attention should be given to the order of magnitude of σS/(Kσ) instead of its actual value.
In surfactant-free BSA solutions, interfacial effects are the predominant cause of the yield stress. In the surfactant-laden mAb solutions with aggregates, interfacial properties are dominated by the surfactant and bulk aggregation is responsible for the higher viscosities at the low shear rates. The essential physics here lies in the competition between the amphiphilic species, protein and surfactant. The species that preferentially adsorbs to the interface also seems to dictate the interfacial rheology.
5. Conclusions
Both protein adsorption and aggregation contribute to shear yielding and a low shear viscosity increase in protein solutions. The yield stress in surfactant-free BSA solutions is mostly due to a viscoelastic protein film at the air/water interface. However, protein adsorption at interfaces is not the only contributing factor, and aggregates in the bulk can also influence the yield stress, as observed for the surfactant-laden mAb solution. These results have important consequences for the flow properties of protein solutions during filling and delivery of bio-pharmaceutical formulations, as the formation of aggregates can occur and presence of interfaces is ubiquitous in these operations. Interpretation of viscosity measurements must be done with careful consideration of the experimental conditions and the geometry used. Both protein adsorption and aggregation are time-dependent processes that can cause the viscosity to increase with time (i.e. apparent rheopexy).Shear yielding in protein solutions can be described using the Bingham model. Along with a torque balance, the yield stress and high shear rate viscosities from the Bingham model quantify contributions from the air/water interface and bulk to protein solution rheology measurements. Surfactant-free BSA solutions have an apparent yield stress from interfacial effects, whereas the surfactant-laden mAb has a yield stress in the bulk solution due to protein aggregation.
After accounting for interfacial effects on the rheology measurements, the low shear viscosity can provide important information about the stability of protein solutions. Increases in the bulk yield stress are accompanied by a loss of monomer and an increase in the concentration of subvisible particles. DLS is particularly sensitive to protein aggregates that contribute to the yield stress. These aggregates produce slow relaxation modes in the DLS autocorrelation function and decrease the diffusivity of the particles in the system, making the solution more difficult to flow under an imposed shear. Upcoming studies will focus on addressing characteristics of these aggregates using small-angle neutron scattering.
Acknowledgements
The authors are very grateful to MedImmune for the financial support to this research, supplying the mAb formulation and allowing the use of their facilities and equipment. MMC thanks the Formulation Team at MedImmune and in particular Reza Esfandiary, Natalie DeJesus, Jared Bee, Paul Santacroce, Quanmin Chen, Prasad Sarangapani and Nayoung Kim for their assistance in the use of the characterization instruments used in this work and discussions. Tom Leach (MedImmune) kindly shared the DSC data of the mAb of this study (data taken by Martin Gonzalez), and Steven Bishop kindly provided support and helpful comments. For performing BSA rheology measurements in the CB rheometer, the authors thank Joel Millan (University of Puerto Rico) supported by the NSF-REU program in Soft Materials at Penn State, and Christopher Kee (Penn State) supported by the Undergraduate Research Fellow Program at Penn State.References
- S. J. Shire, Z. Shahrokh and J. Liu, J. Pharm. Sci., 2004, 93, 1390–1402 CrossRef CAS PubMed.
- N. Rathore and R. S. Rajan, Biotechnol. Prog., 2008, 24, 504–514 CrossRef CAS PubMed.
- C. J. Roberts and W. Wang, Aggregation of Therapeutic Proteins, John Wiley & Sons, Hoboken, New Jersey, 2010 Search PubMed.
- E. Y. Chi, S. Krishnan, T. W. Randolph and J. F. Carpenter, Pharm. Res., 2003, 20, 1325–1336 CrossRef CAS.
- W. Wang, Int. J. Pharm., 2005, 289, 1–30 CrossRef CAS PubMed.
- W. Wang, S. Nema and D. Teagarden, Int. J. Pharm., 2010, 390, 89–99 CrossRef CAS PubMed.
- R. K. Brummitt, D. P. Nesta, L. Chang, A. M. Kroetsch and C. J. Roberts, J. Pharm. Sci., 2011, 100, 2104–2119 CrossRef CAS PubMed.
- E. Sahin, A. O. Grillo, M. D. Perkins and C. J. Roberts, J. Pharm. Sci., 2010, 99, 4830–4848 CrossRef CAS PubMed.
- J. A. Pathak, R. R. Sologuren and R. Narwal, Biophys. J., 2013, 104, 913–923 CrossRef CAS PubMed.
- V. Kayser, N. Chennamsetty, V. Voynov, B. Helk, K. Forrer and B. L. Trout, J. Pharm. Sci., 2011, 100, 2526–2542 CrossRef CAS PubMed.
- V. Tirtaatmadja, D. V. Boger and J. R. E. Fraser, Rheol. Acta, 1984, 23, 311–321 CrossRef CAS.
- H. Inoue and T. Matsumoto, Colloids Surf., A, 1996, 109, 89–96 CrossRef CAS.
- S. Ikeda and K. Nishinari, Biomacromolecules, 2000, 1, 757–763 CrossRef CAS.
- G. J. Brownsey, T. R. Noel, R. Parker and S. G. Ring, Biophys. J., 2003, 85, 3943–3950 CrossRef CAS.
- S. M. Loveday, L. K. Creamer, H. Singh and M. A. Rao, J. Food Sci., 2007, 72, R101–R107 CrossRef CAS PubMed.
- S. Yadav, S. J. Shire and D. S. Kalonia, J. Pharm. Sci., 2010, 99, 4812–4829 CrossRef CAS PubMed.
- V. Sharma, A. Jaishankar, Y.-C. Wang and G. H. McKinley, Soft Matter, 2011, 7, 5150–5160 RSC.
- S. Yadav, S. Shire and D. Kalonia, Pharm. Res., 2011, 28, 1973–1983 CrossRef CAS PubMed.
- S. Yadav, T. M. Laue, D. S. Kalonia, S. N. Singh and S. J. Shire, Mol. Pharm., 2012, 9, 791–802 CrossRef CAS PubMed.
- I. M. Krieger and T. J. Dougherty, Trans. Soc. Rheol., 1959, 3, 137–152 CrossRef CAS.
- R. Larson, The Structure and Rheology of Complex Fluids, Oxford University Press, Oxford, 1999 Search PubMed.
- R. Nossal, C. J. Glinka and S. H. Chen, Biopolymers, 1986, 25, 1157–1175 CrossRef CAS PubMed.
- M. Boström, D. R. M. Williams and B. W. Ninham, Phys. Rev. Lett., 2001, 87, 168103 CrossRef.
- J. J. Grigsby, H. W. Blanch and J. M. Prausnitz, Biophys. Chem., 2001, 91, 231–243 CrossRef CAS.
- O. D. Velev, E. W. Kaler and A. M. Lenhoff, Biophys. J., 1998, 75, 2682–2697 CrossRef CAS.
- C. J. Coen, H. W. Blanch and J. M. Prausnitz, AIChE J., 1995, 41, 996–1004 CrossRef CAS.
- K. M. N. Oates, W. E. Krause, R. L. Jones and R. H. Colby, J. R. Soc., Interface, 2006, 3, 167–174 CrossRef CAS PubMed.
- J. Liu, M. D. H. Nguyen, J. D. Andya and S. J. Shire, J. Pharm. Sci., 2005, 94, 1928–1940 CrossRef CAS PubMed.
- B. D. Connolly, C. Petry, S. Yadav, B. Demeule, N. Ciaccio, J. M. R. Moore, S. J. Shire and Y. R. Gokarn, Biophys. J., 2012, 103, 69–78 CrossRef CAS PubMed.
- A. Jaishankar, V. Sharma and G. H. McKinley, Soft Matter, 2011, 7, 7623–7634 RSC.
- E. M. Freer, K. S. Yim, G. G. Fuller and C. J. Radke, J. Phys. Chem. B, 2004, 108, 3835–3844 CrossRef CAS.
- J. Krägel and S. R. Derkatch, Curr. Opin. Colloid Interface Sci., 2010, 15, 246–255 CrossRef PubMed.
- D. E. Graham and M. C. Phillips, J. Colloid Interface Sci., 1980, 76, 240–250 CrossRef CAS.
- B. S. Murray and E. Dickinson, Food Sci. Technol. Int., 1996, 2, 131–145 CrossRef CAS PubMed.
- A. H. Martin, M. A. Bos and T. van Vliet, Food Hydrocolloids, 2002, 16, 63–71 CrossRef CAS.
- J. Krägel, S. R. Derkatch and R. Miller, Adv. Colloid Interface Sci., 2008, 144, 38–53 CrossRef PubMed.
- K. Sankaranarayanan, A. Dhathathreyan, J. Krägel and R. Miller, J. Phys. Chem. B, 2012, 116, 895–902 CrossRef CAS PubMed.
- D. J. Burgess, J. K. Yoon and N. O. Sahin, PDA J. Pharm. Sci. Technol., 1992, 46, 150–155 CAS.
- E. M. Freer, K. S. Yim, G. G. Fuller and C. J. Radke, Langmuir, 2004, 20, 10159–10167 CrossRef CAS PubMed.
- R. Miller, V. B. Fainerman, A. V. Makievski, J. Krägel, D. O. Grigoriev, V. N. Kazakov and O. V. Sinyachenko, Adv. Colloid Interface Sci., 2000, 86, 39–82 CrossRef CAS.
- D. O. Grigoriev, S. Derkatch, J. Krägel and R. Miller, Food Hydrocolloids, 2007, 21, 823–830 CrossRef CAS PubMed.
- C. Kotsmar, D. O. Grigoriev, F. Xu, E. V. Aksenenko, V. B. Fainerman, M. E. Leser and R. Miller, Langmuir, 2008, 24, 13977–13984 CrossRef CAS PubMed.
- S. Rudiuk, L. Cohen-Tannoudji, S. Huille and C. Tribet, Soft Matter, 2012, 8, 2651–2661 RSC.
- J. S. Bee, J. L. Stevenson, B. Mehta, J. Svitel, J. Pollastrini, R. Platz, E. Freund, J. F. Carpenter and T. W. Randolph, Biotechnol. Bioeng., 2009, 103, 936–943 CrossRef CAS PubMed.
- J. S. Bee, D. K. Schwartz, S. Trabelsi, E. Freund, J. L. Stevenson, J. F. Carpenter and T. W. Randolph, Soft Matter, 2012, 8, 10329–10335 RSC.
- L. Liu, W. Qi, D. K. Schwartz, T. W. Randolph and J. F. Carpenter, J. Pharm. Sci., 2013, 102, 2460–2470 CrossRef CAS PubMed.
- Pierce-Biotechnology, Technical Resource, Extinction Coefficients, http://www.piercenet.com.
- http://www.gbiosciences.com/PGDTween80-desc.aspx#, Tween® 80.
- V. Oganesyan, M. M. Damschroder, W. Leach, H. Wu and W. F. Dall'Acqua, Mol. Immunol., 2008, 45, 1872–1882 CrossRef CAS PubMed.
- W. Leach, The Impact of Excipient Selection on Aggregation: A Case Study of an IgG1 with a Mutated Fc, Protein Stability Conference, Breckenridge, Colorado, 2008 Search PubMed.
- S. Vandebril, A. Franck, G. G. Fuller, P. Moldenaers and J. Vermant, Rheol. Acta, 2010, 49, 131–144 CrossRef CAS PubMed.
- V. L. Vilker, C. K. Colton and K. A. Smith, J. Colloid Interface Sci., 1981, 79, 548–566 CrossRef CAS.
- F. He, G. W. Becker, J. R. Litowski, L. O. Narhi, D. N. Brems and V. I. Razinkov, Anal. Biochem., 2010, 399, 141–143 CrossRef CAS PubMed.
- E. C. Bingham, An Investigation of the Laws of Plastic Flow, U.S. Government Printing Office, 1916 Search PubMed.
- D. A. Edwards, H. Brenner and D. T. Wasan, Interfacial Transport Processes and Rheology, Butterworth-Heinemann, Boston, 1991 Search PubMed.
- M. J. Boussinesq, Ann. Chim. Phys., 1913, 29, 349–357 Search PubMed.
- D. E. Graham and M. C. Phillips, J. Colloid Interface Sci., 1979, 70, 415–426 CrossRef CAS.
- G. Serrien, G. Geeraerts, L. Ghosh and P. Joos, Colloids Surf., 1992, 68, 219–233 CrossRef CAS.
- M. R. Rodriguez Niño and J. M. Rodriguez Patiño, J. Am. Oil Chem. Soc., 1998, 75, 1241–1248 CrossRef.
- L. G. Cascão Pereira, O. Théodoly, H. W. Blanch and C. J. Radke, Langmuir, 2003, 19, 2349–2356 CrossRef.
- S. J. McClellan and E. I. Franses, Colloids Surf., B, 2003, 28, 63–75 CrossRef CAS.
- B. A. Noskov, A. A. Mikhailovskaya, S. Y. Lin, G. Loglio and R. Miller, Langmuir, 2010, 26, 17225–17231 CrossRef CAS PubMed.
- N. Meechai, A. M. Jamieson and J. Blackwell, J. Colloid Interface Sci., 1999, 218, 167–175 CrossRef CAS PubMed.
- S. K. Singh, N. Afonina, M. Awwad, K. Bechtold-Peters, J. T. Blue, D. Chou, M. Cromwell, H.-J. Krause, H.-C. Mahler, B. K. Meyer, L. Narhi, D. P. Nesta and T. Spitznagel, J. Pharm. Sci., 2010, 99, 3302–3321 CrossRef CAS PubMed.
- J. F. Carpenter, T. W. Randolph, W. Jiskoot, D. J. A. Crommelin, C. R. Middaugh, G. Winter, Y.-X. Fan, S. Kirshner, D. Verthelyi, S. Kozlowski, K. A. Clouse, P. G. Swann, A. Rosenberg and B. Cherney, J. Pharm. Sci., 2009, 98, 1201–1205 CrossRef CAS PubMed.
- S. Sugio, A. Kashima, S. Mochizuki, M. Noda and K. Kobayashi, Protein Eng., 1999, 12, 439–446 CrossRef CAS PubMed.
- E. O. Saphire, P. W. H. I. Parren, R. Pantophlet, M. B. Zwick, G. M. Morris, P. M. Rudd, R. A. Dwek, R. L. Stanfield, D. R. Burton and I. A. Wilson, Science, 2001, 293, 1155–1159 CrossRef CAS PubMed.
- R. M. Murphy and C. J. Roberts, Biotechnol. Prog., 2013, 29, 1109–1115 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3sm51994e |
| This journal is © The Royal Society of Chemistry 2014 |