Formation of supported lipid bilayers on silica: relation to lipid phase transition temperature and liposome size†
Yujia
Jing
ab,
Hana
Trefna
b,
Mikael
Persson
b,
Bengt
Kasemo
a and
Sofia
Svedhem
a
aDepartment of Applied Physics, Chalmers University of Technology, SE-412 96 Göteborg, Sweden. E-mail: sofia.svedhem@chalmers.se
bDepartment of Signals and Systems, Chalmers University of Technology, SE-412 96 Göteborg, Sweden
First published on 19th November 2013
Abstract
DPPC liposomes ranging from 90 nm to 160 nm in diameter were prepared and used for studies of the formation of supported lipid membranes on silica (SiO2) at temperatures below and above the gel to liquid-crystalline phase transition temperature (Tm = 41 °C), and by applying temperature gradients through Tm. The main method was the quartz crystal microbalance with dissipation (QCM-D) technique. It was found that liposomes smaller than 100 nm spontaneously rupture on the silica surface when deposited at a temperature above Tm and at a critical surface coverage, following a well-established pathway. In contrast, DPPC liposomes larger than 160 nm do not rupture on the surface when adsorbed at 22 °C or at 50 °C. However, when liposomes of this size are first adsorbed at 22 °C and at a high enough surface coverage, after which they are subject to a constant temperature gradient up to 50 °C, they rupture and fuse to a bilayer, a process that is initiated around Tm. The results are discussed and interpreted considering a combination of effects derived from liposome–surface and liposome–liposome interactions, different softness/stiffness and shape of liposomes below and above Tm, the dynamics and thermal activation of the bilayers occurring around Tm and (for liposomes containing 33% of NaCl) osmotic pressure. These findings are valuable both for preparation of supported lipid bilayer cell membrane mimics and for designing temperature-responsive material coatings.
1. Introduction
Liposomes are used as drug carriers in many commercial products, and they are promising candidate carriers for more advanced controlled delivery and release of therapeutic or diagnostic agents.1 Liposomes are also a common starting point for the preparation of supported lipid membranes (through rupture and fusion of surface-adsorbed liposomes),2 which in turn has developed into a generic platform for a number of applications where the supported membranes are used as cell membrane mimics to investigate, e.g. membrane dynamics,3 protein–lipid interactions,4,5 surface–lipid interactions,6 and as supports for cell culture.7 In both of these two categories of applications, it is of interest to investigate the role of temperature and especially the role of lipid phase transitions. The phase transition between the solid gel and the liquid disordered (liquid–crystalline) state of the membrane is defined by the phase transition temperature (Tm),8 the point at which the physical properties of lipid membranes are drastically altered with respect to, e.g. permeability, viscosity and mechanical strength.9 The permeability of the membrane is the greatest around the phase transition temperature, as a result of the coexistence of lipids in the solid and in the liquid phases.10 Importantly, the distinct temperature-dependent changes of liposome properties can be used to release the loaded drug above a certain temperature, which is especially attractive for chemotherapy combined with hyperthermia (i.e., elevation of the tissue temperature, usually to about 42 °C).11 For example, DPPC, with a phase transition temperature of 41 °C,12 has been used to prepare liposomes which encapsulate anticancer drugs,13 or contrast enhancing agents,14 to be released between 40 and 45 °C in hyperthermia treatments. Recently, temperature-triggered release from stealth liposomes modified by PEG was studied in vitro.15 For the further development of these drug delivery systems, it is important to understand the liposome–liposome and liposome–surface interactions around the phase transition temperature. Attractive model systems for such studies are adsorbed liposomes on surfaces and processes related to such adsorption.A quartz crystal microbalance with dissipation monitoring (QCM-D) is an advanced technique for real time monitoring of small mass changes on a sensor surface together with the corresponding viscoelastic properties of the surface adlayer.16 It has the capacity of providing unique information of liposome deposition and fate on various surfaces,17–19 and can record the process of supported bilayer formation at different conditions such as pH,20 temperature,21 ion concentration in bulk solution,22,23 or the presence of domains in lipid membranes.24 Extensive studies have been performed, and a detailed mechanistic understanding has been obtained for lipid bilayer formation for a range of liposomes (commonly based on phosphatidylcholine lipids) on silica and other surfaces.2,25–30 However, the formation of supported lipid bilayers at or in the proximity of the lipid phase transition has been rarely studied (there are, e.g. only few studies with DPPC mixtures31,32) and the parameters governing the process have only been partially discussed, such as the influence of the support materials,28 the ionic content of the solution,27 or pH.28
Here we describe DPPC liposome–surface interactions over a temperature range including the phase transition temperature, both by experiments at constant temperature above and below the phase transition temperature (Tm) and by applying temperature gradients through Tm. The pathway of DPPC liposome rupture on a SiO2 surface under physiological conditions was compared to that of POPC liposomes, which rupture into a supported lipid membrane as critical surface liposome coverage is reached.33 In addition to studying the formation of lipid bilayers, liposomes were studied at different surface coverage on the sensor surface to separate features resulting from liposome crowding (dependent both on liposome–liposome interaction and liposome–surface interaction) at the surface from the behavior of intact, single liposomes (governed by liposome–surface interaction).18,34 Furthermore, we hypothesized that the release of liposome content could be detected by monitoring the viscoelastic properties of the layer of adsorbed liposomes. In the present study, this is exemplified for DPPC liposomes containing a concentrated (33%) NaCl solution undergoing a temperature gradient through Tm in a lower ionic strength ambient buffer.
2. Materials and methods
2.1. Materials
Unless otherwise stated, chemicals were purchased from commercial suppliers and used without further purification. The 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine (DPPC) lipid was purchased from Avanti Polar Lipids Inc, USA. Water was purified using a Milli-Q Water Purification System (Millipore Co.) to a minimum resistivity of 18.2 MΩ cm. Phosphate buffered saline (PBS) (containing 137 mM sodium chloride, 10 mM sodium phosphate, 2.7 mM potassium chloride, 2.0 mM potassium phosphate, pH 7.4) was prepared from tablets (Sigma), filtered through 0.2 μm filters, and degassed. When preparing NaCl containing liposomes, 0.56 M sodium chloride was added to the PBS. 10 mM sodium dodecyl sulfate (SDS) solution was prepared in water.2.2. Liposome preparation
DPPC liposomes were prepared using the following procedure: 10 mg of the DPPC lipid was dissolved in chloroform in a round bottomed flask. The solvent was evaporated under a gentle nitrogen flow in a fume hood, after which the flask was connected to a vacuum pump to remove residual chloroform. The subsequent rehydration and extrusion procedures were carried out at 50 °C, i.e., above Tm for DPPC (41 °C). The lipid film was rehydrated with 1 mL of preheated PBS buffer and the suspension was kept at 50 °C in an oven for at least 2 hours with occasional vortexing until fully dissolved. The solution was then extruded through polycarbonate membranes with different pore sizes (30 nm for preparing liposome with the size of 90 nm, 50 nm for preparing liposome with the size of 110 nm, 100 nm for preparing liposome with the size of 130 nm and 160 nm, Whatman, UK) in a preheated mini-extruder (Avanti Polar Lipids) maintained above the Tm on a heating plate. The liposomes were obtained by extruding the suspension 21 times. Samples in which concentrated NaCl solution was encapsulated inside the liposomes were desalted by a mini PD-10 gel column (GE Healthcare, UK) using PBS as an eluent. The prepared liposome solution was stored at 4 °C.2.3. Determination of liposome size by nanoparticle tracking analysis (NTA)
The prepared liposomes were characterized by nanoparticle tracking analysis (NTA) (LM10, Nanosight, UK) with respect to size and size distribution. Measurements were performed at room temperature, and the liposome solution was diluted in PBS buffer to a total lipid concentration of 0.0005 mg mL−1. The size distribution was determined as the difference between minimum and maximum diameters (see Fig. S1†).2.4. Quartz crystal microbalance with dissipation (QCM-D) measurements
Liposome adsorption and the supported lipid bilayer formation processes were monitored by QCM-D and repeated several times. Just prior to the measurements, the sensor crystals (silica-coated QCM-D sensors with a fundamental frequency of 5 MHz obtained from Q-Sense AB) were rinsed with water, a 10 mM SDS solution and further cleaned in a UV –ozone cleaner. QCM-D measurements were performed at several harmonics (3, 5, 7, 9, 11 and 13) using a QCM-D E4 system (Q-Sense AB, Sweden). The frequency and dissipation changes were plotted and analyzed using Q-tools software (Q-Sense AB, Sweden). The presented frequency shifts obtained at different overtones were normalized by division by the overtone number.A typical experiment was performed as follows: liposomes with a selected size distribution were adsorbed onto a silica coated QCM-D sensor at room temperature. The temperature was initially kept constant at 22 °C to stabilize the liposomes, and later it was increased to 50 °C (above Tm) at a rate of 0.46 °C min−1 and maintained at 50 °C for 60 min, after which it was decreased to 22 °C using the opposite temperature gradient. Since the QCM-D response, especially the frequency shift, is sensitive to temperature-induced changes in the sensor properties, it is important to subtract the inherent frequency contribution from the QCM-D sensor to the recorded signals, and for this purpose a reference measurement was in all cases performed using the same blank sensor exposed to the same conditions except for the lipids. An example is given in Fig. S2.†
The obtained reference subtracted (to compensate for inherent temperature effects, see above) QCM-D signals (overtones n = 3, 5, 7, 9, 11, 13) were fitted to a Voigt-based viscoelastic model implemented in QTools (Q-sense AB, Sweden). In this model, the viscoelastic solid on the sensor surface is represented by a homogeneous mass (described by its thickness and density) and a complex shear modulus (G = μ + iωη = μ + i2πfη, where μ is the shear modulus and η is the shear viscosity). Due to the frequency dependence of a viscoelastic layer, in QCM-D experiments, both frequency and dissipation responses of the adsorbed layer will differ depending on the overtone that is used to probe the layer properties. Signals obtained at different overtones are included in the modelling to determine effective values of film thickness (under the assumption that density is known), shear modulus, and viscosity.35 One of the output parameters, χ2, is a measure of the goodness of the fitting with respect to a new set of frequency and dissipation curves based on the modeled parameters (i.e., the total error of the modeled curves comparing with the original signals), which is minimized by optimization of range (from wide to narrow) of parameters used as starting values for the iterative fitting procedure.
2.5. Dielectric probe measurement
The release of encapsulated NaCl from DPPC liposome was detected by measuring conductivity changes with a 85070E Dielectric probe kit (Agilent Technologies, USA). The data were recorded in the frequency range of 500 MHz to 3 GHz and in addition to the determination of the solution permittivity (dielectric constant) obtained from the recorded data, the dielectric losses could be converted into conductivity. The probe tip was immersed in 1 mL of solution in a 4 mL flask, and the measurement temperature was adjusted using a water bath on a heating plate. The temperature of the solution was increased from 22 °C to 51 °C for one hour, and conductivity measurements were performed during this time at a frequency of 500 MHz.3. Results
DPPC liposomes with different average sizes in the range from 90 nm to 160 nm in diameter were prepared by extrusion. The measured hydrodynamic sizes and the corresponding size distributions are given in Table 1. In the following, liposomes are referred to as 90 nm, 110 nm, 130 nm, and 160 nm liposomes. Larger liposomes were obtained, along with broader size distributions, for increasing pore sizes of the membrane filters used during the liposome extrusion procedure.| Name | Membrane pore size used for extrusion | Average liposome hydrodynamic diametera | Liposome diameter distribution |
|---|---|---|---|
| a Unless otherwise stated liposomes contained buffer. b These liposomes were extruded in a 33% NaCl solution, followed by ion exchange; see the Materials and methods section for details. | |||
| 90 nm liposomes | 30 nm | 91 ± 2 nm | Min: 31 ± 2 nm |
| Max: 210 ± 10 nm | |||
| 110 nm liposomes | 50 nm | 114 ± 1 nm | Min: 46 ± 4 nm |
| Max: 255 ± 5 nm | |||
| 130 nm liposomes | 80 nm | 130 ± 1 nm | Min: 46 ± 6 nm |
| Max: 280 ± 2 nm | |||
| 160 nm liposomes | 100 nm | 156 ± 1 nm | Min: 45 ± 3 nm |
| Max: 330 ± 20 nm | |||
| 160 nm liposomes (filled with 33% NaCl solution)b | 100 nm | 158 ± 1 nm | Min: 45 ± 2 nm |
| Max: 336 ± 30 nm | |||
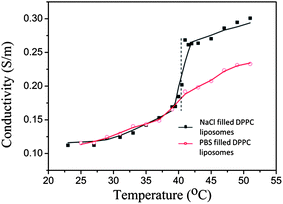
Two variants of the largest liposomes were used: one where the liposomes contained the same buffer as in the solution and one where they contained a 33% NaCl solution (both types have the same size after extrusion, Table 1). The NaCl solution is used to create osmotic pressure across the membranes and as a model medium to monitor release from the liposomes. The diffusion of NaCl across the liposome membrane when reaching Tm gives rise to an increase in conductivity of the liposome solution (Fig. 1) and also a lowering of the density of the liposome content (see below). As seen in Fig. 1, NaCl release occurs over a temperature regime of approximately Tm = 41 °C ± 2 °C.
The behavior of the DPPC liposomes, as functions of size, coverage, temperature and content, when adsorbed on a silica surface was monitored by QCM-D.
Dependence on liposome size for the formation of DPPC supported lipid membranes at a constant temperature above the phase transition temperature T m: we first present QCM-D results for liposomes of varying liposome sizes adsorbed on silica at a constant temperature of 50 °C, see Fig. 2. All liposomes initially attach to the surface as intact liposomes, which is signaled by the significant increase in dissipation, D.
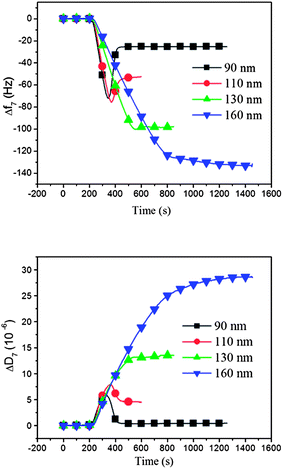 | ||
| Fig. 2 Adsorption kinetics of DPPC liposomes of different sizes (ranging from 90 nm to 160 nm) on silica surfaces at 50 °C (i.e. > Tm), displayed as (a) a QCM-D frequency shift (Δf) and (b) a QCM-D dissipation shift (ΔD) versus time. The smallest liposomes give rise to results, which are typical for the formation of a supported lipid membrane, whereas the results obtained with the largest liposomes are characteristic for adsorption of intact liposomes.18 | ||
The smallest liposomes, with an average diameter of 90 nm (black curves in Fig. 2), attach as intact liposomes until a certain critical surface coverage is reached, where the “crowded” liposome layer transforms to a supported lipid bilayer, following a well-established process characterized by typical QCM-D frequency and dissipation responses.18 That is, initially, a frequency decrease (mass increase) and a dissipation increase are observed during the adsorption of intact liposomes, reaching a minimum in frequency (maximum in mass) and maximum in dissipation, followed by a subsequent rise in frequency and a decrease in dissipation when the critical coverage of DPPC liposome has been reached, where the liposome transformation to a supported membrane begins via liposome rupture. For the 90 nm liposomes, the final frequency and dissipation values stabilize at around −25 Hz and 0 × 10−6, respectively, which are the typical values for an intact supported lipid bilayer formed on a QCM-D sensor surface, with no or a minor fraction of intact liposomes left in the final bilayer.
Liposomes with larger diameters (110–130 nm) initially adsorbed on the sensor surfaces in a similar way as the 90 nm liposomes, but when inspecting Fig. 2, and comparing the frequency shift and dissipation curves, it is obvious that they do not rupture as efficiently as the smaller liposomes, as judged from the asymptotic D and f signals (they are both very sensitive to the remaining small fractions of intact liposomes, especially the D change, which should be nearly zero for a perfect bilayer). If complete transformation to lipid bilayers had occurred, the asymptotic frequency and dissipation values would have all been the same at −25 Hz and 0 × 10−6, respectively. The asymptotic values increase for both frequency and dissipation with increasing liposome size. When intact liposomes are left on the surface after saturation, the final frequency shift is larger than for a pure bilayer, because the intact liposomes contain liquid that is detected as bilayer mass, in addition to the liposome mass. At the same time the dissipation is higher because intact liposomes have much more dissipative structures compared to the bilayer. As judged both from the frequency and dissipation responses in Fig. 2, the fraction of intact adsorbed liposomes thus increases for increasing liposome size, and mixed layers are formed, composed of the supported lipid bilayer and supported liposomes. For example, comparing the results for 90 and 110 nm liposomes, we note that the final frequency shift is almost twice as large for the 110 nm liposomes and the dissipation has risen from essentially 0 to nearly 5 × 10−6.
The largest liposomes (160 nm) exhibit a monotonically increasing frequency shift, with no minimum, indicating very little or no liposome rupture, and thus essentially the formation of a layer of intact liposomes. This conclusion is consistent with the monotonic and large rise of D (no maximum), signaling that intact liposomes are adsorbed even at the highest coverage.
Taken together, these results are essentially in accordance with previous studies where liposomes of increasing size were observed to rupture and to form supported membranes with increasing difficulty.26 The sensitivity to liposome size is, however, different for different lipid types and other conditions, like temperature and the type of the surface. The mechanistic explanation for the size dependence of the transformation from intact liposomes to a bilayer is that the process (at constant temperature) is governed by a competition between three main factors: the cohesive energy of the liposomes, the liposome–surface interaction and the liposome–liposome interaction.36,37 When liposomes are adsorbed due to attractive surface interaction, they deform, as has been shown by AFM.33,37 As liposome size is increased, it is mainly the cohesion that is influenced, more specifically the forces exerted onto the adsorbed liposomes at the point of the lowest radius of curvature, where the adsorbed and flattened liposomes leave the contact with the surface.
Liposome deposition at low temperature followed by increase of the temperature through the phase transition temperature T
m: it is interesting to note from the results described above that liposomes larger than 90 nm in diameter did not form complete lipid bilayers even at 50 °C, or not at all (160 nm liposomes), despite the fact that this temperature is well above the phase transition temperature of the DPPC lipid (Tm = 41 °C). However, when the experiment was conducted differently, by first depositing 160 nm liposomes at a lower temperature (22 °C), after which the temperature was raised to 50 °C at a rate of 0.46 °C min−1, the adsorbed liposomes ruptured around Tm, as judged from the final f and D shifts in Fig. 3, where the QCM-D data are plotted as a function of temperature. The first part in Fig. 3 shows the same intact liposome adsorption behavior (at 22 °C) as in Fig. 2 (at 50 °C) for 160 nm liposomes. When the temperature is increased (starting at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 s) and reaches the region of Tm, dramatic changes in both f and D occur, and the final f and D values when 46 °C is reached correspond to a lipid membrane on the surface. One key to understand this behavior, when comparing with the experiment performed at a constant temperature of 50 °C from zero to full coverage, is that the liposome coverage established at 22 °C before the temperature gradient was initiated, was actually larger than the final coverage attainable at 50 °C. The second key to understand this behavior is the dynamics occurring in the liposome bilayer around Tm – this will be discussed in more detail below.
500 s) and reaches the region of Tm, dramatic changes in both f and D occur, and the final f and D values when 46 °C is reached correspond to a lipid membrane on the surface. One key to understand this behavior, when comparing with the experiment performed at a constant temperature of 50 °C from zero to full coverage, is that the liposome coverage established at 22 °C before the temperature gradient was initiated, was actually larger than the final coverage attainable at 50 °C. The second key to understand this behavior is the dynamics occurring in the liposome bilayer around Tm – this will be discussed in more detail below.
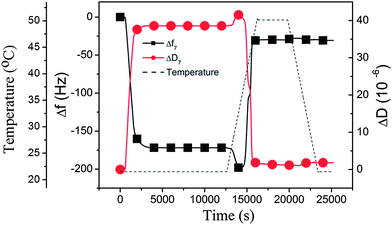 | ||
| Fig. 3 QCM-D results (Δf and ΔD at the 7th overtone) obtained when adsorbing 160 nm DPPC liposomes onto silica at 22 °C, followed by a temperature gradient from 22 °C to 50 °C, leading to a transformation of the liposome layer into a supported lipid membrane around Tm. The temperature is indicated by the dashed line. The presented data have been corrected with respect to the inherent temperature dependence of the QCM-D signals (see the ESI, Fig. S2†). | ||
Inspecting more closely Fig. 3, we see that, as the temperature was increased, the corresponding changes in the properties of the adsorbed DPPC liposomes give rise to several interesting features in the QCM-D signals. The net effects on Δf and ΔD when increasing the temperature from 22 °C to 50 °C is a large reduction in Δf, to a value corresponding to a bilayer on the surface, and a large reduction in ΔD also to a value consistent with bilayer formation. However, during this process and starting at about 39 °C (at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s) and continuing up to 44 °C, there is a complex behavior; first there is a small downward shift in Δf (“apparent” increasing mass load) accompanied by a slight increase in dissipation, followed by a steep upward shift in frequency and an accompanying decrease in dissipation. The latter is no doubt signaling the rupture–fusion process (leading to the formation of a supported membrane, discussed in more detail below), while the origin of the downward shift of Δf is more uncertain. Most likely, at first there is a “pre-melting” type of phenomenon, since it occurs very close to the phase transition temperature. Possible origins are swelling (would be consistent with both increasing mass load and increasing dissipation) and/or a change of the liposome membrane properties. The measured QCM-D signals at several overtones were fitted to a one-layer-viscoelastic model from which the adsorbed liposome mass (the red curve in Fig. 4) and the corresponding viscoelastic parameters were deduced (see the ESI, Table S1†). Based on these results, it was concluded that the origin of the peaks in the QCM-D responses as the temperature gradient initiated was rather related to changes in the liposome membrane properties (indicated by an increased viscosity value) as opposed to swelling (the adsorbed mass stayed constant). After the system had stabilized at 50 °C, the characteristic QCM-D responses for a supported lipid membrane were obtained; Δf was −27 Hz and ΔD was near 0, referenced to the values before exposure to liposomes. These values did not change significantly as the temperature was decreased back to 22 °C (see Fig. 3 to the right), i.e., the supported membrane was stable from mass and dissipation points of view.
000 s) and continuing up to 44 °C, there is a complex behavior; first there is a small downward shift in Δf (“apparent” increasing mass load) accompanied by a slight increase in dissipation, followed by a steep upward shift in frequency and an accompanying decrease in dissipation. The latter is no doubt signaling the rupture–fusion process (leading to the formation of a supported membrane, discussed in more detail below), while the origin of the downward shift of Δf is more uncertain. Most likely, at first there is a “pre-melting” type of phenomenon, since it occurs very close to the phase transition temperature. Possible origins are swelling (would be consistent with both increasing mass load and increasing dissipation) and/or a change of the liposome membrane properties. The measured QCM-D signals at several overtones were fitted to a one-layer-viscoelastic model from which the adsorbed liposome mass (the red curve in Fig. 4) and the corresponding viscoelastic parameters were deduced (see the ESI, Table S1†). Based on these results, it was concluded that the origin of the peaks in the QCM-D responses as the temperature gradient initiated was rather related to changes in the liposome membrane properties (indicated by an increased viscosity value) as opposed to swelling (the adsorbed mass stayed constant). After the system had stabilized at 50 °C, the characteristic QCM-D responses for a supported lipid membrane were obtained; Δf was −27 Hz and ΔD was near 0, referenced to the values before exposure to liposomes. These values did not change significantly as the temperature was decreased back to 22 °C (see Fig. 3 to the right), i.e., the supported membrane was stable from mass and dissipation points of view.
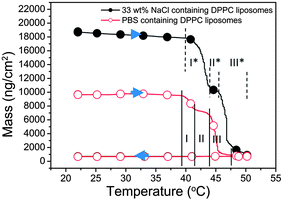
Liposomes with different contents – buffer versus 33% NaCl: in additional experiments performed using the same temperature gradient as above, the temperature dependence of the properties of DPPC liposomes with different contents was compared; i.e., the behavior of liposomes prepared in PBS (thus containing PBS, the same experiment as above) was compared to that of liposomes containing a 33% NaCl solution. The results are shown in Fig. 4, where the modeled adsorbed mass (using Voigt-based viscoelastic modeling as described in the Materials and methods section) is now plotted as a function of temperature. A first striking observation is that the 33% NaCl-loaded liposomes yield much larger QCM-D responses (the black curve in Fig. 4) compared to the liposomes containing PBS (the red curve in Fig. 4). This is explained by the higher density (around 1.25 g cm−3) of the liposomes containing 33% NaCl (modeled values are given in Table S1†). By comparing the mass changes of the two types of liposomes, the liposome membrane property changes leading to ion permeability increase is judged to occur between 39 °C and 50 °C. This is in the same temperature range where release of NaCl was found to occur in the bulk phase by the conductivity measurements (see Fig. 1). The details of the QCM-D responses (several regimes I, II and III, were identified from the modeled adsorbed mass vs. temperature plots) will be discussed below.
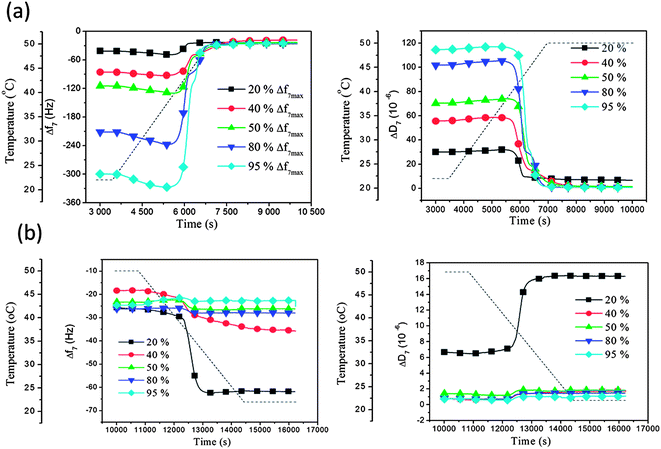
160 nm liposomes adsorbed at different surface coverage and subject to increasing temperature through the phase transition temperature T m: the liposome rupture and formation of a supported membrane process were further studied for 160 nm liposomes deposited at 22 °C and then subject to a temperature gradient through Tm and up to 50 °C, as in Fig. 3 and 4, but now for different initial liposome coverage. We remind that for this liposome size no sign of rupture was observed during deposition to saturation coverage at either of the constant temperatures 22 °C or 50 °C. Different initial coverages for these experiments were prepared by interrupting the liposome adsorption/exposure process at different times at 22 °C. Specifically, the exposure was interrupted when the frequency shifts had reached 20%, 40%, 50%, 80% or 95% of the maximum attainable Δf value (7th overtone). (In the following we refer to these values as coverage, although strictly speaking there is not perfect linearity between coverage and frequency shift.38 The latter is of minor importance here, however.)
After completion of the adsorption to a pre-selected liposome coverage on the surface, the temperature was raised (at a rate of 0.46 °C min−1), while Δf and ΔD were recorded. From Fig. 5 it is clear that the Δf and ΔD signals for the lowest coverage, 20%, deviate from the common behavior for all the larger coverages, from 40% and above. The latter exhibited similar profiles as we saw already in Fig. 3 and 4. Even with a much lower amount of liposomes accumulated on the QCM-D sensor surface, compared to Fig. 3, they still started changing (see above) at around 39 °C and, except for the lowest coverage of 20%, they all turned into a planar supported lipid membrane structure above 41 °C. When the temperature after the increase from 22 °C to 55 °C was again lowered, from 55 °C to 22 °C, using a similar but reversed temperature profile, Δf and ΔD of the samples with 40%, 50%, 80% and 95% coverage showed minor variations, as was also the case in Fig. 4, indicating that the formed supported lipid bilayer on the surface was residing there without any mass (ex)change or morphological rearrangements. In contrast, the Δf and ΔD signals from the sample with 20% coverage showed a partly reversible behavior and returned to levels between the original levels after adsorption at 22 °C, and those corresponding to a supported membrane. Thus, the liposomes at 20% coverage, had not undergone complete liposome-supported membrane transformation, but partly stayed as intact liposomes on the surface during the whole temperature cycle. This behavior is attributed to the small coverage, preventing or reducing liposome–liposome interactions, an interaction that has been shown earlier often, but not always (it depends on lipid type39), to be necessary for liposome-supported membrane transformation.30 In other words, when the coverage is sufficiently small, the liposomes on the surface behave as individual objects, subject only to the liposome–surface interaction and to the influence of the temperature on the liposomes. Obviously, the latter two are not enough to induce the structural transformation leading to a supported membrane (in the present case), but the additional liposome–liposome interaction is needed for this to occur.
4. Discussion
Briefly, from the adsorption results with DPPC liposomes of different sizes at 50 °C, i.e., well above Tm, we found that 90 nm liposomes differ from larger liposomes. The former behave in a similar way as reported earlier for, e.g. POPC liposomes at 20 °C on silica – initial adsorption of intact liposomes is followed by their rupture and fusion to a supported membrane at a critical coverage, leading to a membrane with few defects and completely covering the support. In contrast, the three larger liposome types showed partial supported membrane formation (110 and 130 nm) or essentially only intact liposome adsorption (160 nm) at 50 °C. While the 90 nm result was expected, the results for the larger liposomes was somewhat surprising, especially in view of the fact that 50 °C is well above Tm (41 °C).Based on the current picture of liposome to bilayer conversion on silica surfaces,2,23,25 rupture is due to combined liposome–surface and liposome–liposome interactions. Due to the coverage-dependent mechanisms observed above, we conclude that under present conditions the DPPC liposome–surface interaction is too weak for the liposomes to rupture at any coverage. Thus, the key component to explain the behavior of the larger DPPC liposomes is coverage-dependent liposome–liposome interactions. For the 90 nm liposomes, the larger internal stress of these deformed liposomes (deformed due to surface interaction), dominant at the point of minimum curvature, makes them rupture at a critical coverage, where also liposome–liposome interaction is a contributing factor (see Discussion in our previous studies22). Another way of expressing this is that the activation energy for rupture at the point of minimum curvature is smaller for the smaller liposomes with more constrained membranes. The fact that the intermediate size liposomes showed partial rupture (Fig. 5) tells that these liposomes are just on the verge of rupturing; some rupture and some do not, most likely due to local variations in close packing (“crowding”), causing local variations in the strength of the liposome–liposome interactions.
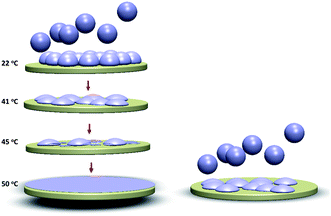
Contribution to the understanding of adsorbed liposome interactions: a plausible model for the bilayer formation process for 160 nm liposomes under different conditions employed in the experiments is schematically illustrated in Fig. 6. The conventional understanding of bilayer membrane formation from adsorbed intact liposomes, until a critical surface coverage is reached, is based on a combination of liposome–surface and liposome–liposome interaction competing with liposome cohesive forces, under certain conditions causing the liposomes to rupture.40 The lipid fragments fuse until a final continuous bilayer is formed (in some special cases some fragments may be lost to the bulk). All these interactions are of course also subject to influence by temperature. In the present study, it was shown that large (160 nm) DPPC liposomes could be adsorbed to crowd together without rupturing, even as the critical coverage is reached and the temperature is above Tm (this behavior was contrasted by small liposomes under the same conditions – the latter formed a bilayer). If, however, the adsorption of the 160 nm liposomes was performed at 22 °C and followed by increasing the temperature, we suggest that intact liposomes deform into more flat structures, leading to increased liposome–liposome interactions and liposomes with smaller edge curvatures when reaching 41 °C, eventually forming complete lipid bilayers upon longer incubation at 50 °C.
An interesting feature in the QCM-D data in Fig. 4, where we plotted mass vs. temperature for the temperature gradients, is the rather complex structure in the mass curves around Tm, most likely reflecting rich dynamics in the lipid structures and mobility around the phase transition. We have somewhat arbitrarily divided this into three temperature regimes (temperature ranges within parentheses are given for the PBS filled DPPC liposomes); I (39–41.5 °C), II (41.5–44 °C), and III (44–47.5 °C). In the first regime the mass decreases as a consequence of liposomes deformation (likely due to pre-melting). In regime II, there is a plateau. In regime III, the adsorbed mass decreases again, indicating further rupture/loss of intact liposomes/bilayer formation. This process continues and is completed upon further heating to 50 °C. The fact that there is a plateau in the rupture–bilayer formation process could possibly be due to difference in the dynamics for the fraction of the liposome bilayer that is in contact with the surface and the fraction facing the bulk. For example, if these fractions had slightly different Tm it could explain the plateau, but this is pure speculation.
For the NaCl-filled liposomes, we see a similar structure around Tm as for PBS filled ones but the T regimes I*, II*, III* are slightly different. The prolongation and the delay of regime I* (40–43 °C) and regime III* (46–50 °C) in this case might indicate that the osmotic pressure hinders the deformation of the liposomes, thus the initial deformation and the later fusion and rupturing of the liposomes are delayed.
From Fig. S3,† the assumption can be made that a majority of intact DPPC liposomes were surrounded by some small pieces of the supported lipid bilayer after the first temperature cycling, and gradually ruptured during the following cycles. By this, we can speculate that the bilayer patches are catalyzing rupture of adjacent liposomes as suggested theoretically.41
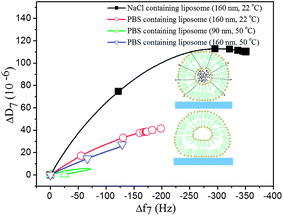
Influences of osmotic pressure on the liposome layer viscoelastic properties: the difference between a constant temperature and a temperature gradient experiment through Tm is further illustrated in Fig. 7, where we have plotted the adsorption parts of the two experiments (data in Fig. 2 and 4 for 160 nm liposomes) in so-called D–f plots,26 where the measured dissipation change is plotted versus the corresponding frequency change. The red curve is for adsorption at 22 °C and the blue curve for adsorption at 50 °C of 160 nm liposomes. The former curve reaches a significantly higher frequency shift at saturation signaling a much higher attainable coverage, supported also by the higher dissipation shift. The origin for the higher attainable coverage at 22 °C compared to 50 °C is most likely that the vesicles are stiffer and more spherical at 22 °C and softer and more flattened (due to the surface interaction) at 50 °C, above Tm. When the liposome system adsorbed at 22 °C is heated through Tm, it is much more close-packed than the saturated 50 °C system, and therefore the liposome–liposome interaction is stronger, leading to rupture when the system is heated through Tm (not shown in Fig. 7 but shown in Fig. 4, above). However, importantly, as pointed out above, the temperature gradient experiment produces a bilayer even at smaller coverage than the saturation coverage at 50 °C, highlighting the importance of the dynamics around Tm in the temperature gradient experiments.
We now return to the D–f plot of Fig. 7 and the NaCl filled 160 nm liposomes. The higher slope of the D–f-curve for the latter (black curve) below the phase transition temperature can be explained by the osmotic stress exerted on the liposomes, resulting in a less soft layer of liposomes on the sensor surface. The osmotic gradient across the lipid membrane causes a more expanded spherical shape of the liposome (see the inset figures in Fig. 7 for a schematical illustration), associated with higher shifts in ΔD and Δf upon adsorption, compared to experiments without an osmotic gradient as shown in Fig. 7. The higher density of the NaCl-containing liposomes as such might also affect the D–f plot. We note that the fitted effective viscoelastic parameters in Table S1† are consistent with a liposome layer below the phase transition temperature, and a softer layer for the liposomes under less osmotic pressure.
These results suggest that the QCM-D technique provides a new platform for monitoring conditions for content release from liposomes, where the effective mass loss of the encapsulated compounds can be determined. By using a different sensor coating, e.g. titania, it will most likely be possible to keep the DPPC liposomes intact without rupturing during temperature cycles.34 Previously, studies of liposome permeability have been performed by surface plasmon resonance (SPR), measuring passive diffusion of uncharged sugar molecules or membrane protein mediated transport across the lipid membrane of surface immobilized liposomes.42 Using surface-adsorbed liposomes, liposome sample consumption can be greatly reduced compared to conventional drug release measurements based on spectroscopic techniques.
5. Conclusion
By studying the viscoelastic properties of surface-adsorbed DPPC liposomes and the relation to liposome rupture, structural changes, permeability and formation of supported membranes as a function of temperature above, below and through the phase transition temperature, we conclude that there is significant dependence on liposome size and surface coverage on the formation of supported membranes at temperatures at and above Tm. Smaller DPPC liposomes (∼90 nm in diameter) readily rupture at temperatures above the lipid phase transition temperature, whereas larger liposomes (∼160 nm in diameter) stay intact. However, DPPC liposomes of 160 nm diameter that were adsorbed onto silica at room temperature, and then heated, eventually ruptured and formed a supported lipid membrane, provided that the initial coverage was not too low. This process could be followed as first deformation (flattening) of the liposomes at increasing temperature, followed by melting and rupture through distinct temperature regimes. Furthermore, it was demonstrated that changes in viscoelastic properties can be used to detect liposome permeability, when a high ionic content equilibrated with the ambient buffer as the temperature reached Tm.Acknowledgements
We gratefully acknowledge financial support for this project from the Swedish Research Council Formas (Nanosphere) and the Chinese Scholarship Council (CSC).References
- V. P. Torchilin, Nat. Rev. Drug Discovery, 2005, 4, 145–160 CrossRef CAS PubMed.
- R. P. Richter, R. Bérat and A. R. Brisson, Langmuir, 2006, 22, 3497–3505 CrossRef CAS PubMed.
- N. Oppenheimer and H. Diamant, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat., 2010, 82, 041912 CrossRef.
- M. de Planque and J. A. Killian, Mol. Membr. Biol., 2003, 20, 271–284 CrossRef CAS PubMed.
- H. Brockman, Curr. Opin. Struct. Biol., 1999, 9, 438–443 CrossRef CAS.
- M. Tanaka and E. Sackmann, Nature, 2005, 437, 656–663 CrossRef CAS PubMed.
- D. Thid, K. Holm, P. S. Eriksson, J. Ekeroth, B. Kasemo and J. Gold, J. Biomed. Mater. Res., Part A, 2008, 84A, 940–953 CrossRef CAS PubMed.
- G. Cevc, Phospholipids handbook, CRC Press, 1993 Search PubMed.
- L. Thilo, H. Traeuble and P. Overath, Biochemistry, 1977, 16, 1283–1290 CrossRef CAS.
- V. Luzzati and A. Tardieu, Annu. Rev. Phys. Chem., 1974, 25, 79–94 CrossRef CAS.
- A. M. Ponce, Z. Vujaskovic, F. Yuan, D. Needham and M. W. Dewhirst, Int. J. Hyperthermia, 2006, 22, 205–213 CrossRef CAS PubMed.
- M. Vandenbranden, C. Stil, R. Brasseur and J. M. Ruysschaert, Cell. Mol. Life Sci., 1984, 40, 715–717 CrossRef CAS.
- G. Koning, A. Eggermont, L. Lindner and T. ten Hagen, Pharm. Res., 2010, 27, 1750–1754 CrossRef CAS PubMed.
- D. Volodkin, Y. Arntz, P. Schaaf, H. Moehwald, J.-C. Voegel and V. Ball, Soft Matter, 2008, 4, 122–130 RSC.
- L. Li, T. L. M. ten Hagen, D. Schipper, T. M. Wijnberg, G. C. van Rhoon, A. M. M. Eggermont, L. H. Lindner and G. A. Koning, J. Controlled Release, 2010, 143, 274–279 CrossRef CAS PubMed.
- A. Kunze, P. Sjövall, B. Kasemo and S. Svedhem, J. Am. Chem. Soc., 2009, 131, 2450–2451 CrossRef CAS PubMed.
- M. Beneš, D. Billy, A. Benda, H. Speijer, M. Hof and W. T. Hermens, Langmuir, 2004, 20, 10129–10137 CrossRef PubMed.
- C. A. Keller and B. Kasemo, Biophys. J., 1998, 75, 1397–1402 CrossRef CAS.
- M. Sundh, S. Svedhem and D. S. Sutherland, J. Phys. Chem. B, 2011, 115, 7838–7848 CrossRef CAS PubMed.
- N. J. Cho, J. A. Jackman, M. Liu and C. W. Frank, Langmuir, 2011, 27, 3739–3748 CrossRef CAS PubMed.
- T. Zhu, F. Xu, B. Yuan, C. Ren, Z. Jiang and Y. Ma, Colloids Surf., B, 2012, 89, 228–233 CrossRef CAS PubMed.
- B. Seantier and B. Kasemo, Langmuir, 2009, 25, 5767–5772 CrossRef CAS PubMed.
- S. Boudard, B. Seantier, C. Breffa, G. Decher and O. Félix, Thin Solid Films, 2006, 495, 246–251 CrossRef CAS PubMed.
- M. Sundh, S. Svedhem and D. S. Sutherland, Phys. Chem. Chem. Phys., 2010, 12, 453–460 RSC.
- E. Reimhult, M. Zäch, F. Höök and B. Kasemo, Langmuir, 2006, 22, 3313–3319 CrossRef CAS PubMed.
- E. Reimhult, F. Höök and B. Kasemo, J. Chem. Phys., 2002, 117, 7401–7404 CrossRef CAS.
- R. Tero, M. Takizawa, Y. J. Li, M. Yamazaki and T. Urisu, Langmuir, 2004, 20, 7526–7531 CrossRef CAS PubMed.
- E. Reimhult, F. Höök and B. Kasemo, Langmuir, 2002, 19, 1681–1691 CrossRef.
- E. Reimhult, F. Höök and B. Kasemo, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat., 2002, 66, 051905 CrossRef CAS.
- E. Reimhult, B. Kasemo and F. Höök, Int. J. Mol. Sci., 2009, 10, 1683–1696 CrossRef CAS PubMed.
- B. Seantier, C. Breffa, O. Félix and G. Decher, Nano Lett., 2004, 4, 5–10 CrossRef CAS.
- Z. Wang and S. Yang, Langmuir, 2008, 24, 11616–11624 CrossRef CAS PubMed.
- R. Richter, A. Mukhopadhyay and A. Brisson, Biophys. J., 2003, 85, 3035–3047 CrossRef CAS.
- G. Ohlsson, A. Tigerström, F. Höök and B. Kasemo, Soft Matter, 2011, 7, 10749–10755 RSC.
- M. V. Voinova, M. Rodahl, M. Jonson and B. Kasemo, Phys. Scr., 1999, 59, 391 CrossRef CAS.
- K. Dimitrievski, E. Reimhult, B. Kasemo and V. P. Zhdanov, Colloids Surf., B, 2004, 39, 77–86 CrossRef CAS PubMed.
- K. Dimitrievski, M. Zäch, V. P. Zhdanov and B. Kasemo, Colloids Surf., B, 2006, 47, 115–125 CrossRef CAS PubMed.
- I. Reviakine, F. F. Rossetti, A. N. Morozov and M. Textor, J. Chem. Phys., 2005, 122, 204711–204718 CrossRef PubMed.
- K. Dimitrievski and B. Kasemo, Langmuir, 2008, 24, 4077–4091 CrossRef CAS PubMed.
- V. P. Zhdanov, K. Dimitrievski and B. Kasemo, Langmuir, 2006, 22, 3477–3480 CrossRef CAS PubMed.
- O. Allerbo, A. Lundström and K. Dimitrievski, Colloids Surf., B, 2011, 82, 632–636 CrossRef CAS PubMed.
- M. Brändén, S. R. Tabaei, G. Fischer, R. Neutze and F. Höök, Biophys. J., 2010, 99, 124–133 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3sm50947h |
| This journal is © The Royal Society of Chemistry 2014 |