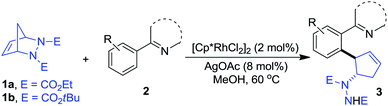Rh(III)-catalyzed C–H activation–desymmetrization of diazabicycles with arenes: facile synthesis of functionalized cyclopentenes†
Yan
Zhang
,
Qifan
Wu
and
Sunliang
Cui
*
Institute of Materia Medica and College of Pharmaceutical Sciences, Zhejiang University, Hangzhou, Zhejiang 310058, China. E-mail: slcui@zju.edu.cn
First published on 27th September 2013
Abstract
A Rh(III)-catalyzed C–H activation–desymmetrization of diazabicycles with arenes as an expedient approach to functionalized cyclopentenes is reported. This protocol provides a novel and efficient strategy for the desymmetrization of diazabicycles, featuring simple starting materials, mild reaction conditions, high efficiency and broad substrate scope. Control experiments and a kinetic isotope effect study were conducted, and a plausible mechanism was proposed.
Introduction
Functionalized cyclopentenes, especially vicinal 2-arylated cyclopentenylamines, are known to be biologically active small molecules, and also key intermediates in the synthesis of pharmaceutically important cyclopentane derivatives (Fig. 1).1 For example, Carbovir is a potential anti-HIV agent, and other trans vicinal cyclopentenylamines act as tachykinin receptor antagonists, CNS stimulating agents, human neurokinin-1 receptor antagonists, and thromboxane A2 inhibitors.2 As a consequence, direct synthetic approaches to vicinal cyclopentenylamines possessing features of simple starting materials and high efficiency, remain of continuous interest and importance.3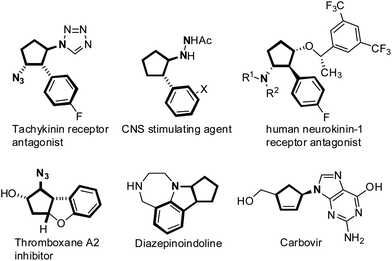 | ||
| Fig. 1 Representative important pharmaceutical scaffolds containing the vicinal 2-arylated cyclopentenylamine unit. | ||
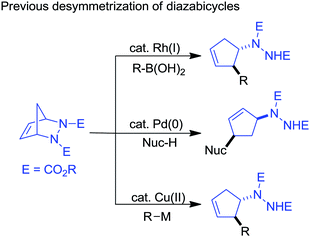
Interest in desymmetrization by ring-opening reactions for access to various building blocks has grown significantly recently.4 In particular, the reactivity of diazabicycles has been investigated with various nucleophiles via desymmetrization because of their stability, ease of preparation, and because the ring-opened hydrazine is a known amine precursor (eqn (1)). For example, the Rh(I)-catalyzed ring-opening reactions (especially the asymmetric desymmetrization form) of diazabicycles with boronic acids have been well developed by Pineschi,5a,5e Lautens5b–d,5f and Radhakrishnan6 to access chiral 3-aryl-4-hydrazinocyclopentenes, while the palladium-catalyzed desymmetrization of diazabicycles (including Pd-allylation–nucleophilic substitution) represents an approach to obtain 3,5-disubstituted cyclopentenylhydrazines,7 and the copper-catalyzed ring-opening reaction of diazabicycles with organometallic reagents is a route to vicinal 2-arylated cyclopentenylhydrazines.8 Recently, an elegant work demonstrating the Pd-catalyzed tandem ring-opening–ring-closing reaction of diazabicycles for the synthesis of cyclopentannulated heterocycles has been performed by Radhakrishnan and Gilbertson.9 Therefore, diazabicycles are versatile synthons for the construction of various functionalized cyclopentenes with extensive structural diversity.
 | (1) |
 | (2) |
Recently, the Rh(III)-catalyzed functionalization of aryl C–H bonds has enjoyed tremendous advances owing to their wide application in the rapid assembly of various complex molecular structures, particularly in the field of medicinal chemistry.10,11 Direct C–H functionalization has advantages over classical cross coupling reactions, avoiding the preactivation of coupling partners and thus leading to a more atom-economical process.12 For example, Glorius demonstrated a Rh(III)-catalyzed C–H allylation of arenes with easily accessible allyl carbonates, and this protocol provides an unprecedented opportunity for the allylation of electron-neutral arenes.13 The Rh(III)-catalyzed desymmetrization has rarely been investigated. Very recently, Li reported in an elegant work on the Rh(III)-catalyzed coupling of arenes with 7-oxa/azabenzonorbornadienes via a desymmetrization method.14
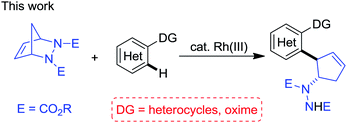
In continuation of our interest in Rh(III)-catalyzed C–H functionalization for the synthesis of biologically interesting small molecules,15 we proposed that the Rh(III)-catalyzed directing group-assisted C–H functionalization of arenes and the desymmetrization of diazabicycles could be integrated to enable access to interesting nitrogen-containing scaffolds. Herein we report a Rh(III)-catalyzed desymmetrization of diazabicycles with arenes toward the construction of functionalized cyclopentenes, with simple starting materials, mild reaction conditions, a broad substrate scope and high efficiency (eqn (2)).
Results and discussion
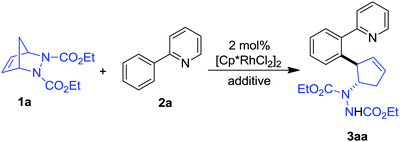
We commenced our study by investigating the coupling of diazabicycle 1a and 2-phenylpyridine 2a using [Cp*RhCl2]2 as a catalyst. When the reaction was conducted in t-amyl alcohol at 100 °C for 6 h with AgSbF6 as an additive, gratifyingly, a trans vicinal 2-aryl cyclopentenylhydrazine 3aa was obtained in 75% yield (Table 1, entry 1). This result encouraged us to further optimize the reaction conditions. We found that changing the solvent to MeOH and decreasing the temperature to 60 °C also led to the formation of the product in a comparable yield (Table 1, entry 2). Next, a survey of the additive and solvent showed that the combination of AgOAc and MeOH was optimal, and that the product could be isolated in 82% yield (Table 1, entry 3). Surprisingly, the reaction was also effective in the absence of any additive to afford the product in good yield with a prolonged reaction time (Table 1, entries 6 and 7). Moreover, the reaction did not proceed in the absence of rhodium, demonstrating its importance as a catalyst.| Entry | Additive | Solvent | Temp (°C) | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (0.22 mmol), [Cp*RhCl2]2 (2 mol%), additive (8 mol%), solvent (1.5 mL). b Yields of isolated products. c 1 Equiv. was used. | |||||
| 1 | AgSbF6 | tert-Amyl alcohol | 100 | 6 | 75 |
| 2 | AgSbF6 | MeOH | 60 | 6 | 73 |
| 3 | AgOAc | MeOH | 60 | 6 | 82 |
| 4 | AgOAc | CH3CN | 60 | 8 | Trace |
| 5 | CsOAcc | MeOH | 60 | 8 | 78 |
| 6 | — | MeOH | 60 | 12 | 79 |
| 7 | — | MeOH | 30 | 12 | 66 |
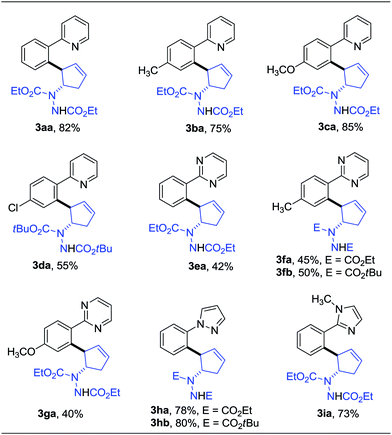
Having determined the optimized conditions, we next tested the generality of this desymmetrization with various heterocyclic arenes. The diazabicycle 1b, which contains a Boc (tert-butoxycarbonyl) group, was also chosen to be subjected to this reaction, due to its easy and practical deprotection. As shown in Table 2, substituents on the aromatic ring of the 2-arylpyridine derivatives were well-tolerated, affording the products in good yields (3ba–da). To expand the potential utility of this reaction for medicinal chemistry, other heterocycles were also subjected to this reaction protocol. For example, 2-arylpyrimidines were successfully applied in this ring-opening transformation to deliver the corresponding products in moderate yields (3ea–ga), and the lower reactivity of these substrates is likely due to the electron-withdrawing nature of the pyrimidine group. Furthermore, we were pleased to find that pyrazole and imidazole, commonly encountered motifs in medicinal chemistry, could also successfully act as directing groups for C–H functionalization to enable the late-stage desymmetrization, and the corresponding ring-opening functionalized 2-arylated cyclopentenylhydrazines were obtained in good yields (3ha–ia).
| a Reaction conditions: 1 (0.2 mmol), 2 (0.22 mmol), [Cp*RhCl2]2 (2 mol%), AgOAc (8 mol%), MeOH (1.5 mL), 60 °C, 6 h. Isolated yields are given. |
|---|
|
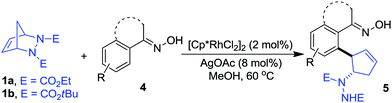
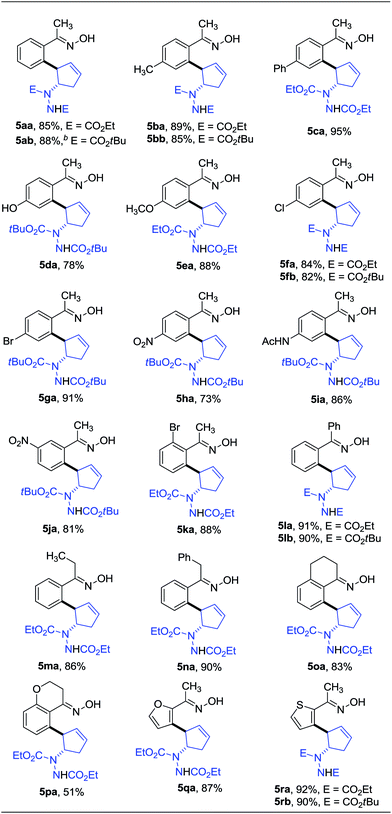
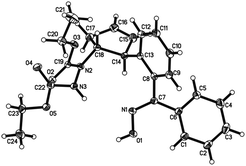
Oximes are popular molecules in C–H functionalization thanks to their good directing group ability, as well as being versatile synthons in organic synthesis. Therefore we extended our protocol to the utilization of diverse oximes as substrates. As shown in Table 3, various oximes could react smoothly in this desymmetrization to furnish the products in good yields (Table 3, 5aa–ka), regardless of the presence of electron-donating or electron-withdrawing groups on the aromatic ring, displaying valuable tolerance to functional groups including phenyl, hydroxyl, methoxy, chloro, bromo, nitro, acetylamino, thus offering ample opportunity for further derivatization. A 5 mmol scale reaction was also carried out to reveal the synthetic potential of this method, and a good yield was obtained (5ab, 88% yield). Additionally, when a meta-substituted oxime was applied, good regioselectivity favoring the activation of the less hindered C–H bond was observed, and the sole product was obtained in good yield (5ja). The more hindered ortho-substituted oxime was also effective in this ring-opening reaction, furnishing the product in moderate yield (5ka). Interestingly, when other functionalized oximes including bicyclic scaffolds were used, this transformation also proceeded smoothly to generate the products in good yields (5la–pa). Notably, the furan and thiophene derived oximes were also applicable in this desymmetrization to produce the heterocyclic substituted cyclopentenylhydrazines in good yields (5qa–rb). Furthermore, the structure of this trans vicinal 2-arylated cyclopentenylhydrazine was unambiguously confirmed by X-ray analysis of compound 5la (Fig. 2).16 It should be noted that the addition of AgOAc is essential in this ring-opening reaction of oximes, and that the omission of AgOAc from the reaction resulted in its failure to proceed.
Derivatization of the products was also conducted since the trans vicinal 2-arylated cyclopentenylhydrazines represent the core structure of an important scaffold. For example, compound 5fa could be converted to compounds 6 and 7 upon epoxidation and Beckmann rearrangement, respectively (Scheme 1).
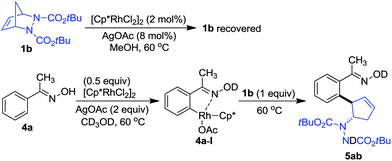
In order to gain insight into the mechanism, control experiments were carried out (Scheme 2). When diazabicycle 1b was subjected to the reaction in the absence of arenes, we found that it was recovered, even after a reaction time of 8 h. On the other hand, when oxime 4a was treated with an equivalent amount of rhodium catalyst at 60 °C in CD3OD, 1H NMR analysis showed that 4a was consumed to form the rhodium species 4a-I. Upon the addition of 1b to 4a-I, a rapid formation of product 5ab was observed. This showed that the desymmetrization proceeded via the reaction of rhodium intermediate 4a-I with the diazabicycle.11o,17
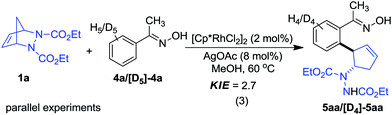
Considering the transformation includes a Rh(III)-catalyzed C–H functionalization step, a kinetic isotope effect (KIE) study was conducted utilizing oxime 4a as a model substrate to probe the mechanism (eqn (3)). A large primary KIE value (2.7) was obtained, suggesting that C–H bond cleavage occurs during the rate-determining step.18
 | (3) |
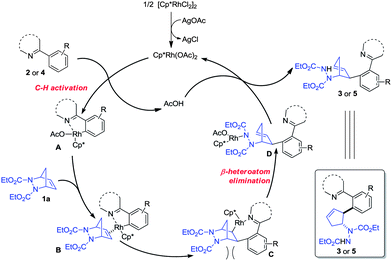
Based on these experiments and literature precedents, a plausible mechanism is proposed in Scheme 3.10–13 The initial rhodium acetate was generated from [Cp*RhCl2]2 and AgOAc. A carboxylate-assisted electrophilic C–H bond cleavage of the arene by rhodium occurs to form intermediate A,19 which coordinates with diazabicycle 1a to give complex B. The subsequent insertion delivers rhodium species C, while a β-heteroatom elimination of C leads to the ring-opening intermediate D. The final protonation of D furnishes the product with concomitant release of the Rh(III) catalyst. The insertion step leading from B to C favors the least steric hindrance, thus affording the trans vicinal 2-arylated cyclopentenylhydrazine products, and the β-heteroatom elimination of C is similar to the oxygen elimination in Glorius' allylation process.13
In terms of desymmetrization of diazabicycles, the Rh(I)-catalytic strategy developed by Pineschi and Lautens revealed the reactivity of diazabicycles with the aryl-rhodium species generated from boronic acids, while this protocol disclosed their reactivity with the in situ generated aryl-rhodium species from the directing group assisted C–H functionalization of arenes. Therefore this protocol represents an alternative tactic for the desymmetrization of diazabicycles and 2-arylated cyclopentene synthesis, in a redox neutral fashion.
Conclusions
In summary, we have developed a Rh(III)-catalyzed C–H activation–desymmetrization of diazabicycles with arenes for efficient access to functionalized cyclopentenes, using simple starting materials and mild reaction conditions with high efficiency and a broad substrate scope. Control experiments and a kinetic isotope effect study were conducted, and a plausible mechanism was proposed.Acknowledgements
We are grateful to the NNSFC (21202143), the Fundamental Research Funds for the Central Universities (2012QNA7020, 2013QNA7014), and Zhejiang University for financial support. We thank Mrs Jianyang Pan (Pharmaceutical Informatics Institute, Zhejiang University) for performing the NMR spectrometry.Notes and references
- For reviews, see: (a) M. T. Crimmins, Tetrahedron, 1998, 54, 9229 CrossRef CAS; (b) A. Berecibar, C. Grandjean and A. Siriwardena, Chem. Rev., 1999, 99, 779 CrossRef CAS PubMed.
- (a) P. E. Finke, L. C. Meurer, D. A. Levorse, S. G. Mills, M. MacCoss, S. Sadowski, M. A. Cascieri, K.-L. Tsao, G. G. Chicchi, J. M. Metzger and D. E. MacIntyre, Bioorg. Med. Chem. Lett., 2006, 16, 4497 CrossRef CAS PubMed; (b) K. Ohno, A. Ohtake, S. Nishio, K. Hoshi and S. Tsukamoto, PCT Int Appl. WO 9406785, Toray Industries Inc., 1994; (c) K. Ohno, H. Nagase, K. Matsumoto, H. Nishiyama and S. Nishio, in Advances in Prottaglandin, Thromboxane, and Leukotriene Research, ed. O. Hayashi and S. Yamamoto, Ravan Press, New York, 1985, vol. 15 Search PubMed; (d) G. S. Welmaker and J. E. Sabalski, Tetrahedron Lett., 2004, 45, 4851 CrossRef CAS PubMed; (e) R. Vince, M. Hua, S. Daluge, F. Lee, W. M. Shannon, G. C. Lavelle, J. Qualls, O. S. Weislow, R. Kiser, P. G. Canonico, R. H. Schultz, V. L. Narayanan, J. G. Mayo, R. H. Shoemaker and M. R. Boyd, Biochem. Biophys. Res. Commun., 1988, 156, 1046 CrossRef CAS; (f) R. Vince and M. Hua, J. Med. Chem., 1990, 33, 17 CrossRef CAS.
- For Reviews, see: (a) E. Leemans, M. D'Hooghe and N. Kimpe, Chem. Rev., 2011, 111, 3268 CrossRef CAS PubMed; (b) O. Boutureira, M. I. Matheu, Y. Díaz and S. Castillón, Chem. Soc. Rev., 2013, 42, 5056 RSC.
- (a) S. Cabrera, R. G. Arrayas, I. Alonso and J. C. Carretero, J. Am. Chem. Soc., 2005, 127, 17938 CrossRef CAS PubMed; (b) C. Bournaud, C. Falciola, T. Lecourt, S. Rosset, A. Alexakis and L. Micounin, Org. Lett., 2006, 8, 3581 CrossRef CAS PubMed; (c) Y.-H. Cho, V. Zunic, H. Senboku, M. Olsen and M. Lautens, J. Am. Chem. Soc., 2006, 128, 6837 CrossRef CAS PubMed; (d) J. M. Berlin, S. D. Goldberg and R. H. Grubbs, Angew. Chem., Int. Ed., 2006, 45, 7591 CrossRef CAS PubMed; (e) K. Arai, S. Lucarini, M. M. Salter, K. Ohta, Y. Yamashita and S. Kobayashi, J. Am. Chem. Soc., 2007, 129, 8103 CrossRef CAS PubMed; (f) J. B. Johnson, E. A. Bercot, C. M. Williams and T. Rovis, Angew. Chem., Int. Ed., 2007, 46, 4514 CrossRef CAS PubMed; (g) M. J. Cook and T. Rovis, J. Am. Chem. Soc., 2007, 129, 9302 CrossRef CAS PubMed; (h) G. A. Cortez, R. R. Shrock and A. H. Hoveyda, Angew. Chem., Int. Ed., 2007, 46, 4534 CrossRef CAS PubMed; (i) E. B. Rowland, G. B. Rowland, E. Rivera-Otero and J. C. Antilla, J. Am. Chem. Soc., 2007, 129, 12084 CrossRef CAS PubMed; (j) S. E. Larson, J. C. Baso, G. Li and J. C. Antilla, Org. Lett., 2009, 11, 5186 CrossRef CAS PubMed; (k) M. Ito, C. Kobayashi, A. Himizu and T. Ikariya, J. Am. Chem. Soc., 2010, 132, 11414 CrossRef CAS PubMed; (l) R. Shintani, K. Moriya and T. Hayashi, J. Am. Chem. Soc., 2011, 133, 16440 CrossRef CAS PubMed; (m) Z. Wang, Z. Chen and J. Sun, Angew. Chem., Int. Ed., 2013, 52, 6685 CrossRef CAS PubMed.
- (a) F. Bertolini, F. Macchia and M. Pineschi, Tetrahedron Lett., 2006, 47, 9173 CrossRef CAS PubMed; (b) F. Menard, C. F. Weise and M. Lautens, Org. Lett., 2007, 9, 5365 CrossRef CAS PubMed; (c) F. Menard and M. Lautens, Angew. Chem., Int. Ed., 2008, 47, 2085 CrossRef CAS PubMed; (d) J. Panteleev, F. Menard and M. Lautens, Adv. Synth. Catal., 2008, 350, 2893 CrossRef CAS; (e) S. Crotti, F. Bertolini, F. Macchia and M. Pineschi, Chem. Commun., 2008, 3127 RSC; (f) J. Bexrud and M. Lautens, Org. Lett., 2010, 12, 3160 CrossRef CAS PubMed.
- For Pd-catalyzed desymmetrization of diazabicycles with boronic acid, see: J. John, V. S. Sajisha, S. Mohanlal and K. V. Radhakrishnan, Chem. Commun., 2006, 3510 RSC.
- A. P. Luna, M. Cesario, M. Bonin and L. Micouin, Org. Lett., 2003, 5, 4771 CrossRef CAS PubMed.
- (a) M. Pineschi, F. D. Moro, P. Crotti and F. Macchia, Org. Lett., 2005, 7, 3605 CrossRef CAS PubMed; (b) C. Bournaud, C. Falciola, T. Lecourt, S. Rosset, A. Alexakis and L. Micouin, Org. Lett., 2006, 8, 3581 CrossRef CAS PubMed.
- (a) J. John, U. Indu, E. Suresh and K. V. Radhakrishnan, J. Am. Chem. Soc., 2009, 131, 5042 CrossRef CAS PubMed; (b) B. A. B. Prasad, A. E. Buechele and S. R. Gilbertson, Org. Lett., 2010, 12, 5422 CrossRef CAS PubMed; (c) S. S. Chand, E. Jijy, P. Prakash, J. Szymoniak, P. Preethanuj, B. P. Dhanya and K. V. Radhakrishnan, Org. Lett., 2013, 15, 3338 CrossRef CAS PubMed; (d) P. Prakash, E. Jijy, P. Preethanuj, P. M. Pihko, S. S. Chand and K. V. Radhakrishnan, Chem.–Eur. J., 2013, 19, 10473 CrossRef CAS PubMed.
- For reviews, see: (a) T. Satoh, K. Ueura and M. Miura, Pure Appl. Chem., 2008, 80, 1127 CrossRef CAS; (b) T. Satoh and M. Miura, Synthesis, 2010, 3395 CrossRef CAS PubMed; (c) C. Zhu, R. Wang and J. R. Falck, Chem.–Asian J., 2012, 7, 1502 CrossRef CAS PubMed; (d) G. Song, F. Wang and X. Li, Chem. Soc. Rev., 2012, 41, 3651 RSC; (e) F. W. Patureau, J. Wencel-Delord and F. Glorius, Aldrichimica Acta, 2012, 45, 31 CAS.
- For selected articles about other Rh(III)-catalyzed functionalization of arenes, see: (a) A. S. Tsai, M. E. Tauchert, R. G. Bergman and J. A. Ellman, J. Am. Chem. Soc., 2011, 133, 1248 CrossRef CAS PubMed; (b) Y.-F. Wang, K. K. Toh, J.-Y. Lee and S. Chiba, Angew. Chem., Int. Ed., 2011, 50, 5927 CrossRef CAS PubMed; (c) N. Schröder, J. Wencel-Delord and F. Glorius, J. Am. Chem. Soc., 2012, 134, 8298 CrossRef PubMed; (d) J. Y. Kim, S. H. Park, J. Ryu, S. H. Cho, S. H. Kim and S. Chang, J. Am. Chem. Soc., 2012, 134, 9110 CrossRef CAS PubMed; (e) R. Zeng, C. Fu and S. Ma, J. Am. Chem. Soc., 2012, 134, 9597 CrossRef CAS PubMed; (f) W.-W. Chan, S.-F. Lo, Z. Zhou and W.-Y. Yu, J. Am. Chem. Soc., 2012, 134, 13565 CrossRef CAS PubMed; (g) X. Tan, B. Liu, X. Li, S. Xu, H. Song and B. Wang, J. Am. Chem. Soc., 2012, 134, 16163 CrossRef CAS PubMed; (h) H. Wang, C. Grohmann, C. Nimphius and F. Glorius, J. Am. Chem. Soc., 2012, 134, 19592 CrossRef CAS PubMed; (i) J. Wencel-Delord, C. Nimphius, F. W. Patureau and F. Glorius, Angew. Chem., Int. Ed., 2012, 51, 2247 CrossRef CAS PubMed; (j) B.-J. Li, H.-Y. Wang, Q.-L. Zhu and Z.-J. Shi, Angew. Chem., Int. Ed., 2012, 51, 3948 CrossRef CAS PubMed; (k) Z. Shi, C. Grohmann and F. Glorius, Angew. Chem., Int. Ed., 2013, 52, 5393 CrossRef CAS PubMed; (l) C. Wang, H. Chen, Z. Wang, J. Chen and Y. Huang, Angew. Chem., Int. Ed., 2012, 51, 7242 CrossRef CAS PubMed; (m) N. Kuhl, M. N. Hopkinson and F. Glorius, Angew. Chem., Int. Ed., 2012, 51, 8230 CrossRef CAS PubMed; (n) J. Ryu, K. Shin, S. H. Park, J. Y. Kim and S. Chang, Angew. Chem., Int. Ed., 2012, 51, 9904 CrossRef CAS PubMed; (o) W. Zhen, F. Wang, M. Zhao, Z. Du and X. Li, Angew. Chem., Int. Ed., 2012, 51, 11819 CrossRef CAS PubMed; (p) D. Wang, F. Wang, G. Song and X. Li, Angew. Chem., Int. Ed., 2012, 51, 12348 CrossRef CAS PubMed; (q) J. Wencel-Delord, C. Nimphius, H. Wang and F. Glorius, Angew. Chem., Int. Ed., 2012, 51, 13001 CrossRef CAS PubMed; (r) B. Ye and N. Cramer, J. Am. Chem. Soc., 2013, 135, 636 CrossRef CAS PubMed; (s) X. Li, S. Yu, F. Wang, B. Wan and X. Yu, Angew. Chem., Int. Ed., 2013, 52, 2577 CrossRef CAS PubMed; (t) C. Wang, H. Sun, Y. Fang and Y. Huang, Angew. Chem., Int. Ed., 2013, 52, 5795 CrossRef CAS PubMed; (u) G. Liu, Y. Shen, Z. Zhou and X. Lu, Angew. Chem., Int. Ed., 2013, 52, 6033 CrossRef CAS PubMed; (v) D. Zhao, Q. Wu, X. Huang, F. Song, T. Lv and J. You, Chem.–Eur. J., 2013, 19, 6239 CrossRef CAS PubMed; (w) Y. Lian, R. G. Bergman, L. D. Lavis and J. A. Ellman, J. Am. Chem. Soc., 2013, 135, 7122 CrossRef CAS PubMed; (x) D.-G. Yu, M. Suri and F. Glorius, J. Am. Chem. Soc., 2013, 135, 8802 CrossRef CAS PubMed; (y) T.-J. Gong, B. Xiao, W.-M. Cheng, W. Su, J. Xu, Z.-J. Liu, L. Liu and Y. Fu, J. Am. Chem. Soc., 2013, 135, 10630 CrossRef CAS PubMed; (z) S. Yu, B. Wan and X. Li, Org. Lett., 2013, 15, 3706 CrossRef CAS PubMed.
- For reviews, see: (a) R. Giri, B.-F. Shi, K. M. Engle, N. Maugel and J.-Q. Yu, Chem. Soc. Rev., 2009, 38, 3242 RSC; (b) T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1147 CrossRef CAS PubMed; (c) L. McMurray, F. O'Hara and M. J. Gaunt, Chem. Soc. Rev., 2011, 40, 1885 RSC; (d) J. Wencel-Delord, T. Dröge, F. Liu and F. Glorius, Chem. Soc. Rev., 2011, 40, 4740 RSC; (e) K. M. Engle, T.-S. Mei, M. Wasa and J.-Q. Yu, Acc. Chem. Res., 2012, 45, 788 CrossRef CAS PubMed; (f) J. Wencel-Delord and F. Glorius, Nat. Chem., 2013, 5, 369 CrossRef CAS PubMed.
- H. Wang, N. Schröder and F. Glorius, Angew. Chem., Int. Ed., 2013, 52, 5386 CrossRef CAS PubMed.
- Z. Qi and X. Li, Angew. Chem., Int. Ed., 2013, 52, 8995 CrossRef CAS PubMed.
- (a) S. Cui, Y. Zhang and Q. Wu, Chem. Sci., 2013, 4, 3421 RSC; (b) S. Cui, Y. Zhang, D. Wang and Q. Wu, Chem. Sci., 2013, 4, 3912 RSC.
- ESI†.
- (a) P. Zhao, F. Wang, K. Han and X. Li, Org. Lett., 2012, 14, 3400 CrossRef CAS PubMed; (b) M. E. Tauchert, C. D. Incarvito, A. L. Rheingold, R. G. Bergman and J. A. Ellman, J. Am. Chem. Soc., 2012, 134, 1482 CrossRef CAS PubMed; (c) M. Brasse, J. Cámpora, J. A. Ellman and R. G. Bergman, J. Am. Chem. Soc., 2013, 135, 6427 CrossRef CAS PubMed; (d) G. Zhang, L. Yang, Y. Wang, Y. Xie and H. Huang, J. Am. Chem. Soc., 2013, 135, 8850 CrossRef CAS PubMed.
- E. M. Simmons and J. F. Hartwig, Angew. Chem., Int. Ed., 2012, 51, 3066 CrossRef CAS PubMed.
- For reviews, see: (a) K. Fagnou, Top. Curr. Chem., 2010, 292, 35 CrossRef CAS; (b) M. Livendahl and A. M. Echavarren, Isr. J. Chem., 2010, 50, 630 CrossRef CAS; (c) L. Ackermann, Chem. Rev., 2011, 111, 1315 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization of data for all new compounds. CCDC 940835. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3sc52524d |
| This journal is © The Royal Society of Chemistry 2014 |