Water-soluble multi-cage super tetrahedral uranyl peroxide phosphate clusters†
Jie
Qiu
a,
Jie
Ling
a,
Laurent
Jouffret
a,
Rebecca
Thomas
a,
Jennifer E. S.
Szymanowski
a and
Peter C.
Burns
*ab
aDepartment of Civil and Environmental Engineering and Earth Sciences, University of Notre Dame, Notre Dame, IN 46556, USA
bDepartment of Chemistry and Biochemistry, University of Notre Dame, Notre Dame, IN 46556, USA
First published on 23rd October 2013
Abstract
Combination of uranyl, peroxide, phosphate, and counter ions under ambient conditions in aqueous solution over the pH range 5.5–6.5 results in the self-assembly of a large super tetrahedral cluster containing 124 uranyl hexagonal bipyramids and 32 phosphate tetrahedra designated U124P32. With a diameter of 4 nm, U124P32 consists of four symmetrically identical cage clusters, each built from 23 uranyl hexagonal bipyramids and eight phosphate tetrahedra, as well as a central cage defined by 52 uranyl hexagonal bipyramids and 32 phosphate tetrahedra. It is the first uranyl-based cluster that contains multiple cages and it also has the highest number of uranyl ions. U124P32 contains 152 peroxide groups that are bidentate bridges between uranyl ions. Crystals of U124P32 provided structural details, and were dissolved for subsequent small-angle X-ray scattering and electrospray ionization mass spectrometry characterization that indicated U124P32 remains intact upon dissolution in water. Whereas uranyl phosphates are generally insoluble in water, prompting interest in their applications in remediating contaminated water, U124P32 is highly soluble, reflecting the impact of the nanoscale structure on solubility.
Introduction
The intensely studied transition metal polyoxometalates exist because “yl” O atoms that are double bonded to metal cations truncate them,1–9 preventing growth into extended solids. The pentavalent and hexavalent oxidation states of the early actinides dominantly form linear dioxo cations in aqueous solution,10 in contrast to the transition metals that normally bond to only one “yl” O atom, or two in a cis configuration. Assembly of actinyl ions into polyoxometalates therefore provides a unique opportunity to truncate the cluster both inside and outside with “yl” O atoms, giving cage clusters.11,12Uranyl ions readily self-assemble into nanoscale cage clusters in aqueous solution under ambient conditions when they are bridged through bidentate peroxide ligands.11,12 The bonding between the peroxide and uranyl units includes covalent interactions that favor a bent configuration consistent with curvature of the cage,13–16 and “yl” oxygen atoms truncate the cage on both the inside and outside. Although peroxide is important for formation of uranyl cage clusters, other uranyl bridges in addition to peroxide include hydroxyl,11,17–25 pyrophosphate,18,20,25,26 phosphite,27 nitrate,18,28 methylenediphosphonate,20 and oxalate groups.19,29 More than 40 uranyl peroxide clusters have been reported that consist of single cages, and core–shell cages in two cases. Such clusters are of interest for possible applications in an advanced nuclear energy system, for example as intermediates in manufacturing of materials and for novel approaches to radionuclide separations.
The details of metal speciation in aqueous solution impact the solubility of solid phases containing the metal,30–33 and in uranium geochemistry the available ligands and pH largely control the distribution of uranium between solid phases and groundwater.34,35 Uranyl phosphates, such as (UO2)3(PO4)2(H2O)4 and members of the autunite mineral group, are very sparingly soluble in water and retard the mobility of uranium in contaminated sites.36–53 Strategies for remediating subsurface groundwater uranium contamination by phosphate amendment have been considered,54,55 as precipitation of uranyl phosphates may lower uranium concentrations below drinking water standards.
Here we report the synthesis, structure, and properties of the first multi-cage uranyl peroxide phosphate cluster and designate it U124P32. U124P32 contains 124 uranyl ions, is 4 nm in diameter, and has a mass of ∼45 kDa. The cluster presents several novel features, including the largest number of uranyl ions in a cluster, the presence of five cages within a single cluster, phosphate bridges, and a remarkably high solubility in aqueous solution.
The largest number uranyl ions in a cluster core reported previously is for U60, with 60 uranyl ions and a diameter of 2.7 nm,22 and the somewhat larger and topologically identical U60Ox30 with oxalate bridges.29 U60 consists of a single cage with a fullerene topology, and the counterions used in the synthesis are Li and K. U28U40R and U120Ox90, consisting of 68 and 120 uranyl ions, respectively, are core–shell configurations in which the cores contain either 28 or 60 uranyl ions.19,28 The general design strategy for obtaining cage clusters with large numbers of uranyl ions has involved a combination of different counterions to template various rings of uranyl polyhedra, together with utilization of different chemical bridges between uranyl ions.12 A rather exhaustive search indicates that attainment of clusters with more than 60 uranyl ions will either require formation of core–shell structures, such as U28U40R and U120Ox90, or clusters in which the core consists of multiple cages, such as U124P32.
Experimental section
Caution: Although depleted uranium was used in these experiments, it is toxic and radioactive and should only be handled by qualified personnel in appropriate facilities.Crystals containing cluster U124P32 (Fig. S1†) were synthesized by loading aqueous solutions of UO2(NO3)2·6H2O (0.5 M, 0.1 mL), H2O2 (30%, 0.1 mL), LiOH (2.4 M, 0.1 mL), phosphoric acid (0.5 M, 0.05 mL), and potassium hydrogen phthalate (0.25 M, 0.3 mL) into a 5 mL glass vial. The resulting solution was cloudy and was centrifuged after two weeks. Subsequently, the resulting clear solution was transferred to a new 5 mL glass vial, yielding a solution with pH ∼ 5.9. Yellow acicular crystals of U124P32 formed within 40 days during the slow evaporation of the solution under ambient conditions. The acicular transparent yellow pleochroic crystals reached millimeter lengths and were readily separated from a minor amount of amorphous precipitate. The yield of crystal is ∼5% calculated based on uranium.
Repeated trials showed that crystals of U124P32 can be obtained using combinations of LiOH, H3PO4, and potassium hydrogen phthalate with pH ∼5.5–6.5. The role of potassium hydrogen phthalate in the reaction is to control the solution pH and to provide K cations. The synthesis was also successfully scaled up by a factor of 10 and was readily repeated. Syntheses conducted outside the pH range of ∼5.5–6.5, but under otherwise similar conditions, gave crystals containing different clusters.
A suitable crystal was isolated from the mother solution and mounted on a cryoloop in oil and cooled by a flow of nitrogen gas at 100 K. Data were collected using a Bruker APEX II Quazar diffractometer equipped with graphite monochromated Mo Kα X-radiation provided by a microfocus source combined with Montel optics. A complete sphere of data was collected using frame widths of 0.5 in ω. Data were corrected for Lorentz, polarization, and background effects using the Bruker software APEX II, and an empirical absorption correction was done using SADABS.56 Structure solutions and refinements were done with SHELXTL.57 The final refinement included positional parameters for all atoms, anisotropic displacement parameters for U and K atoms, and isotropic displacement parameters for the remaining atoms. H atoms were not located. Whereas the structure determination provided definitive information concerning the atomic positions of the uranyl peroxide phosphate clusters, the crystal structure contains relatively large non-cluster areas that contain disordered electron density that is attributed to counter ions and H2O. Crystallographic details are provided in the ESI.†
Bond-valence analyses confirmed the oxidation state of U to be U(VI).58 O atoms were assigned as PO43−, HPO42−, H2PO4−, OH−, H2O, or peroxo ligands on the basis of bond-valence sums and O–O bond lengths in the case of peroxo ligands.
An electrospray ionization mass spectrum (ESI-MS)59 was collected in negative-ion mode using a Bruker micrOTOF-Q II high resolution quadrupole time of flight (Q-TOF) spectrometer (3600 V capillary voltage, 0.8 bar nebulizer gas, 4 L min−1 dry gas, 180 °C dry gas temperature). Crystals were isolated from the glass vial in which they had grown and were vacuum filtered using a Whatman 1 filter membrane, followed by rinsing with 10 mL of ethanol. Visually clean crystals were hand-picked from the membrane and were dissolved into 50 μL of ultrapure water. The resulting solution was diluted 10-fold using ultrapure water, giving a solution with ∼50–100 ppm U. The samples were introduced into the spectrometer by direct infusion at 7 μL min−1 and scanned over a 500–5000 m/z range with data averaged over 5 min. Data was deconvoluted using MaxEnt software.
Crystals were isolated from the mother solution, vacuum filtered and rinsed with 100 mL of ethanol in preparation for chemical analysis. Visually clean crystals totaling ∼10 mg were dissolved in 0.5 mL ultrapure water. The resulting solution was diluted using 5% HNO3 solution to four 10 mL dilutions containing ∼20 ppm U, P, and K, and ∼3 ppm Li, respectively. These dilutions were analyzed using a Perkin-Elmer inductively coupled plasma optical emission spectrometer. The measured atomic ratios of U![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P
P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K
K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Li in the crystals is 124
Li in the crystals is 124![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35.4
35.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85.4
85.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51.7.
51.7.
Small-angle X-ray scattering (SAXS) profiles have provided information concerning the size and shape of uranyl peroxide cage clusters in solution.27,28 Crystals for analysis by SAXS were isolated from their mother solution by vacuum filtration and rinsed using ethanol. Visually clean crystals were harvested from the filter membrane and were subsequently dissolved in ultrapure water, giving a solution with ∼0.1 mM U124P32. The solution was placed in a 0.5 mm diameter glass capillary, and both ends were sealed using wax. Water was placed in an identical capillary for background measurement. Samples were measured using a Bruker Nanostar equipped with a Cu microfocus source, Montel multilayer optics, and a HiSTAR multi-wire detector. The sample-to-detector distance was 26.3 cm and the exposure time was 2 h. Reaction solutions after different aged time were also measured to track assemblies of clusters in the solution. These solutions were centrifuged to remove the precipitate and the resulting clear solutions were placed in capillaries for data collections. The collection time was 1 h.
A thermal gravimetric analysis (TGA) was conducted using a Mettler Toledo analyzer for 18 mg of crystals of U124P32 in an alumina crucible. The sample was heated from 25 to 900 °C at a rate of 5 °C min−1 under flowing air. The thermogram is shown in Fig. S2.† The residue from the TGA analysis of crystals of U124P32 was used for the collection of X-ray powder diffraction data with a Bruker D8 ADVANCE diffractometer with DAVINCI design with Cu Kα radiation (Fig. S3†).
Infrared spectra for a single crystal of U124P32 and the TGA residue were collected using a SensIR technology IlluminatIR FT-IR microspectrometer. The spectra (Fig. S4†) were collected from 650 to 4000 cm−1 with an ATR objective and a beam aperture of 100 μm. The spectrum of the U124P32 crystal confirmed the presence of uranyl ions, PO43−, H2O, and OH− groups. Uranyl ion stretches occur at ∼850 cm−1, PO43− modes are at ∼1060 cm−1, a H2O bending mode is at ∼1550 cm−1, and O–H bonds are indicated by a broad envelope from about 2600 to 3600 cm−1. The spectrum of the TGA residue confirmed that H2O and OH− groups in the crystals of cluster U124P32 were lost during the TGA measurement.
A Raman spectrum was collected from a single crystal of U124P32 using a Bruker Sentinel system linked via fiber optics to a video assisted probe on a microscope mount, equipped with a 785 nm, 400 mW laser and a high-sensitivity, TE-cooled, 1024 × 255 CCD array. The spectrum was collected for 5 seconds with 5 signal accumulations, in the range of 80 to 3200 cm−1. The spectrum (Fig. S5†) confirmed the presence of uranyl ions by a sharp band located at 808 cm−1,60,61 peroxide groups with a O–O symmetric stretch at 830 cm−1,61 and PO43− ions with the ν3 antisymmetric stretching vibration at 1053 cm−1.60
UV-vis spectra were collected from single crystals using a Craic Technologies microspectrophotometer. Crystals were placed on quartz slides under mineral oil, and the data were collected from 250 to 1400 nm. The spectrum (Fig. S6†) readily confirmed the presence of uranyl ions.
Results and discussion
U124P32 consists of 124 uranyl hexagonal bipyramids and 32 phosphate tetrahedra (Fig. 1), the connectivities of which are described below. All U(VI) cations are present as typical (UO2)2+ uranyl ions, with U–O bond lengths of ∼1.8 Å and O![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) U
U![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O bond angles of ∼180°.58 Each uranyl ion is coordinated by six O atoms that are arranged at the equatorial vertices of hexagonal bipyramids capped by O atoms of the uranyl ion. In each case four of the O atoms correspond to two different peroxide groups. The remaining two O atoms originate from a variety of ligands; specifically, peroxide, hydroxyl, H2O, and phosphate groups. There are 32 phosphate tetrahedra in the cluster, with 16 (PO4)3−, eight (HPO4)2−, and eight (H2PO4)−. The composition of the uranyl and phosphate cluster from the structure determination is [(UO2)124(O2)152(PO4)16(HPO4)8(H2PO4)8(OH)4(H2O)24]132−, with the negative charge balanced by K and Li cations in the crystal structure. Those K cations that are located within the cluster were located in the X-ray diffraction study. The K and Li cations located in void spaces outside the cluster were generally not resolved in the X-ray structure owing to disorder, but were confirmed by chemical compositional analysis. The water content was estimated from the TGA analysis. The composition of U124P32 crystals is assigned as KxLiy[(UO2)124(O2)152(PO4)16(HPO4)8(H2PO4)8(OH)4(H2O)24](H2O)n, where x ∼ 85, y ∼ 52, x + y = 132, and n ∼ 415.
O bond angles of ∼180°.58 Each uranyl ion is coordinated by six O atoms that are arranged at the equatorial vertices of hexagonal bipyramids capped by O atoms of the uranyl ion. In each case four of the O atoms correspond to two different peroxide groups. The remaining two O atoms originate from a variety of ligands; specifically, peroxide, hydroxyl, H2O, and phosphate groups. There are 32 phosphate tetrahedra in the cluster, with 16 (PO4)3−, eight (HPO4)2−, and eight (H2PO4)−. The composition of the uranyl and phosphate cluster from the structure determination is [(UO2)124(O2)152(PO4)16(HPO4)8(H2PO4)8(OH)4(H2O)24]132−, with the negative charge balanced by K and Li cations in the crystal structure. Those K cations that are located within the cluster were located in the X-ray diffraction study. The K and Li cations located in void spaces outside the cluster were generally not resolved in the X-ray structure owing to disorder, but were confirmed by chemical compositional analysis. The water content was estimated from the TGA analysis. The composition of U124P32 crystals is assigned as KxLiy[(UO2)124(O2)152(PO4)16(HPO4)8(H2PO4)8(OH)4(H2O)24](H2O)n, where x ∼ 85, y ∼ 52, x + y = 132, and n ∼ 415.
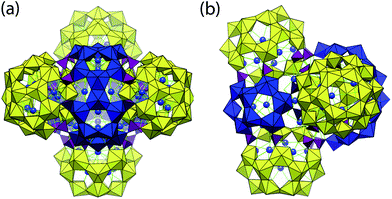
U124P32 is certainly the most complex of the uranyl peroxide clusters described to date, as shown by the representations in Fig. 1. It is the result of the fusion of four symmetrically identical cages, arranged at the vertices of a tetrahedron, through additional polyhedra, resulting also in a central cage. Five membered rings of uranyl ions that are bridged by bidentate peroxide groups are a major feature of U124P32 as in numerous other uranyl peroxide clusters.12 K cations are linked to many of these pentagonal rings, where they occur bonded to five O atoms of uranyl ions on the concave-inwards side of the ring. Seventy-six K cations encapsulated in the cages were located in the crystal structure determination. Eleven of these K cations are encapsulated in each of the four symmetrically identical cages, and the remaining 32 K are encapsulated in the central cage.
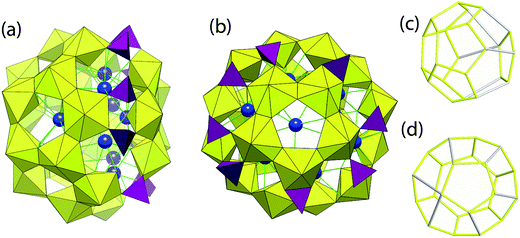
The four symmetrically identical cages in U124P32 are each built from 23 uranyl hexagonal bipyramids that are arranged in a novel topology (Fig. 2). Eighteen of these form a hemisphere for which the graphical representation contains one hexagon and six pentagons, arranged such that the hexagon at the base of the hemisphere shares edges with six different pentagons, and each pentagon shares two edges with other adjacent pentagons (Fig. 2). This topological feature is known from five other uranyl peroxide cage clusters, the smallest of which is U28.12 All of the bridges between the 18 uranyl ions in this unit are bidentate peroxide groups, as is also the case in U28.
The hemisphere consisting of 18 uranyl polyhedra is connected to an additional five membered ring of uranyl ions bridged through bidentate peroxide, resulting in the cage. However, the linkages are through eight phosphate tetrahedra, with six two-connected and two three-connected within the cage (Fig. 2). Four equatorial vertices of uranyl hexagonal bipyramids in the cluster are non-bridging and correspond to H2O groups. This cage is hereafter referred to as U23P8.
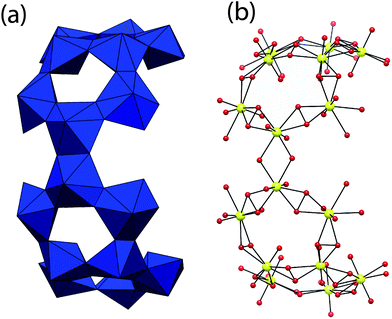
Four symmetrically identical U23P8 cages in U124P32 are linked through two 16-membered units of uranyl hexagonal bipyramids. These are shown in Fig. 3, and they each consist of four five-membered rings of uranyl polyhedra. These are in pairs of five-membered rings that have two polyhedra in common, and these are linked to symmetrically identical units though the sharing of an equatorial edge defined by two hydroxyl groups by uranyl polyhedra.
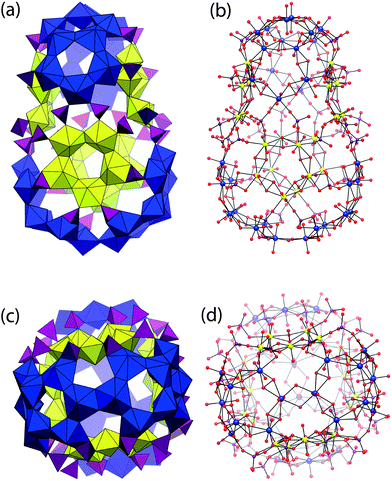
Linkage of four symmetrically identical cages in U124P32 results in a larger cage located at the center of the cluster. This is illustrated in Fig. 4, and is defined by 52 uranyl polyhedra and all 32 phosphate tetrahedra that are contained in the overall cluster. U124P32 has a maximum outer diameter of 39.4 Å, as measured between the edges of two outer uranyl O atoms and assuming an ionic radius of 1.35 Å for O2−, and a maximum inner diameter of 22.0 Å, as measured between the edges of inner uranyl O atoms of the central cage.
The overall cluster contains 16 (PO4)3−, all of which share a tetrahedral edge with a uranyl hexagonal bipyramid, and the other two vertices with two other uranyl hexagonal bipyramids. All of these tetrahedra bridge between the U23P8 cages and the uranyl hexagonal bipyramids that define the central cage of U124P32. The cluster contains eight (HPO4)2− groups, and these share each of three O atoms with different uranyl hexagonal bipyramids, with the fourth O being protonated. As for the (PO4)3− groups, each (HPO4)2− group bridges between uranyl polyhedra of U23P8 cages and those of the central cage. Eight (H2PO4)− are linked to two uranyl hexagonal bipyramids each by sharing single O atoms, with the remaining two O atoms protonated. The (H2PO4)− groups bridge uranyl hexagonal bipyramids that are contained within a single U23P8 cage.
Crystals of U124P32 rapidly dissolve in pure water to form a clear solution, aliquots of which were examined using ESI-MS and SAXS. The mass spectrum (Fig. 5) indicates persistence of U124P32 upon dissolution, and yielded an average weight for clusters in solution of ∼43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 Da, which may be compared to the value of 44
150 Da, which may be compared to the value of 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 882 Da calculated for the cluster including encapsulated K cations and H2O groups. The modest disparity may reflect experimental uncertainty, or that the cluster encapsulates fewer K or H2O subsequent to dissolution.
882 Da calculated for the cluster including encapsulated K cations and H2O groups. The modest disparity may reflect experimental uncertainty, or that the cluster encapsulates fewer K or H2O subsequent to dissolution.
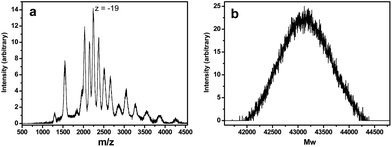 | ||
| Fig. 5 Electrospray ionization mass spectrum (a) and corresponding deconvolution spectrum (b) for a solution prepared by dissolving crystals of U124P32 in pure water. | ||
The SAXS profile (Fig. S7†) gives a radius of gyration, Rg = 14.1 Å, in solution that is comparable to the value of 14.2 Å derived from the crystallographic data using the Crysol method.62 The pair distance distribution function (PDF), p(r), calculated using an indirect Fourier transform of the primary SAXS data, is the probability of finding a vector length in the molecule having a value equal to r; this probability becomes zero at the maximum vector length, rmax, which equals the largest linear dimension in a particle.63 The p(r) plot (Fig. S7†) has rmax = 37.9 Å, similar to the maximum crystallographic radius of U124P32.
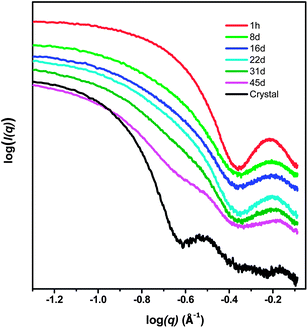
There are several possible routes to the complete assembly of U124P32, ranging from full assembly in aqueous solution prior to crystallization, through assembly only during the crystallization process. SAXS profiles were collected for mother solutions prior to the appearance of crystals (Fig. 6). Data collected one hour after combination of the reactants revealed the presence of nanoscale objects, and the data was readily fit using a core–shell model with inner and outer radii of 4.8 and 9.1 Å (Fig. S8†). These values are consistent with the size of U23P8, as well as with several other uranyl peroxide cage clusters described previously, such as U24 and U28. However, the values are inconsistent with U124P32, thereby indicating that it is not present in solution in detectable quantities after one hour. SAXS data collected for solutions after aging for eight, 16, 22, 31 and 45 days could not be modeled using a monodisperse cluster model. However, insight is gained by comparing the SAXS profile collected after 45 days, at which time crystals containing U124P32 first appeared. The corresponding SAXS profile is compared to that collected one hour after mixing the reactants, as well as that obtained from a solution into which U124P32 had been dissolved in Fig. 6. The profile collected after 45 days indicates larger species are in solution than earlier in the evolution of the system, and although not definitive, the data is consistent with assembly of U124P32 in solution prior to its crystallization.
Approximately 40 uranyl peroxide clusters have been reported to date, but U124P32 is unique in several important features. It is the only uranyl-based cluster that consists of multiple cages, and it contains the largest number of uranyl polyhedral units. Inclusion of phosphate tetrahedra, some of which contain protonated terminal oxygen atoms, provides the possibility of a pH-dependent charge for U124P32. It is also the only super tetrahedral uranyl-based cluster reported to date, although broadly similar clusters have been reported for transition metal polyoxometalates. However, U124P32 appears to be unique in that it assembles into a tetrahedral motif in a one-pot reaction, in contrast to tetrameric transition metal oxide clusters synthesized starting from small clusters such as Wells–Dawson.64,65
The bonds between peroxide bridges and uranyl ions have been shown through computational studies to possess a partially covalent character that favors a bent configuration,15,16 and it has been argued that this fosters curvature in the structure and assembly into cage clusters. Cluster U124P32 is rich in peroxide bridges between uranyl ions. In the U23P8 cages, uranyl ions are bridged by 29 bidentate peroxide groups to form five-membered rings, and uranyl ions are also bridged through phosphate. In the overall cluster U124P32 there are 152 peroxide bridges. A total of four uranyl ions are bridged by pairs of hydroxyl groups. Computations indicate that the dihedral U–O2–U angle for peroxide bridges are ideally ∼140°, whereas U–(OH)2–U dihedral angles tend to be ∼180°. Below we discuss the relationship between the size of a cluster and bridges linking uranyl ions.
The average U–X–U dihedral angles, where X = O2 or (OH)2, for all published uranyl peroxide cage clusters are summarized in Fig. 7, with full details including ranges in the ESI (Table S1†). The topologies of these cage clusters are dominated by squares, pentagons, and hexagons, and the dihedral angles associated with each type of graphical polygon are indicated in Fig. 7. The U–O2–U dihedral angles associated with topological squares are generally the smallest, in the range of 132.5–139.1°. Those associated with topological pentagons and hexagons are somewhat larger, but are still less than 150° in all but one case. However, U–O2–U dihedral angles associated with all three kinds of polygons are almost independent of the number of uranyl ions in the cluster, and thus the size of the cluster. In contrast, the dihedral angles of U–(OH)2–U bridges, which only occur in pentagons and hexagons, range from 150 to 180°. Most importantly, those associated with hexagons are larger for larger clusters, indicating incorporation of the flexible U–(OH)2–U bridge is important for increasing the size of a cluster. Larger uranyl-peroxide cage clusters all contain a mixture of peroxide and other bridges with “side-on” configurations that are topologically analogous to the direct sharing of equatorial edges between uranyl polyhedra,66 as peroxide bridges alone would provide too much curvature. The largest cluster with both peroxide and hydroxyl bridges between uranyl ions reported previously is U60, which contains nearly flat U–(OH)2–U bridges.22
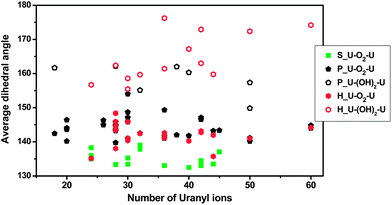 | ||
| Fig. 7 Average U–X–U dihedral angles in published uranyl peroxide cage clusters, where X = O2 or (OH)2. The symbol codes represent uranyl bridges that are within different topological features of the clusters, specifically squares, pentagons and hexagons. Details are in the ESI.† | ||
In the case of the synthesis of U124P32, the availability of hydroxyl groups for incorporation into the cluster is limited by the slightly acidic conditions. One might then expect a cluster such as U28, which utilizes only peroxide bridges between uranyl ions, to form. In U28 and all of the cage clusters we have reported that contain only uranyl polyhedra, the outer surface of the cage corresponds to relatively unreactive uranyl ions. As such, these clusters can only aggregate into larger units through linkages with lower-valence cations, most commonly alkali and alkaline earth cations. However, the synthesis of U124P32 provided an alternative: U23P8 cage clusters are linked into the larger unit through phosphate tetrahedra. In a system dominated by peroxide bridges between uranyl ions, smaller cage clusters such as U28 are favored by the degree of curvature imposed by the U–O2–U dihedral angles, as seen in the U23P8 cages, but formation of the larger U124P32 cluster is favored by providing phosphate bridges as well.
Although we have not rigorously measured the aqueous solubility of U124P32, we have observed that crystals placed in a drop of pure water immediately dissolve, and SAXS data indicate that U124P32 clusters remain intact in solution. The aqueous solubility of the nanometer-scale anionic U124P32 cluster is substantial, in stark contrast to other species typical of the uranyl phosphate system. Of the wide range of uranyl minerals known, uranyl phosphates are amongst the least soluble, and have been identified retarding the movement of uranium in contaminated subsurface environments.37,43,46,47 Compositionally, some of these minerals are very similar to U124P32. Meta-ankoleite has composition K2(UO2)2(PO4)2(H2O)6, and the solubility of this phase was reported to be 0.002 grams per liter at pH = 5.24.67 In contrast, the solubility of U124P32 appears to be in the range of tens or hundreds of grams per liter in ultrapure water with initial pH = 5.8. Although the anionic charge state of U124P32 in aqueous solution under various pH conditions is currently unknown, its low charge to radius ratio is likely an important consideration in determining its high solubility.
Conclusions
Cluster U124P32 is the largest cluster of uranyl peroxide polyhedra reported, both in terms of its 4 nm diameter and ∼45 kDa mass. It spontaneously self-assembles in aqueous solution as the solution evaporates under ambient conditions. Its multi-cage super-tetrahedral polyhedral connectivity is also unique, and is a consequence of multiple stages of assembly and the abundance of peroxide bridges between uranyl ions. The cluster remains intact upon dissolution in water, and is highly soluble in comparison to various forms of uranyl phosphate solids including minerals, demonstrating the importance of the nanoscale structure in determining the properties of uranyl phosphates.Acknowledgements
This material is based upon work supported as part of the Materials Science of Actinides Center, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Award Number DE-SC0001089.Notes and references
- M. Nyman and P. C. Burns, Chem. Soc. Rev., 2012, 41, 7354–7367 RSC.
- P. B. Duval, C. J. Burns, D. L. Clark, D. E. Morris, B. L. Scott, J. D. Thompson, E. L. Werkema, L. Jia and R. A. Andersen, Angew. Chem., Int. Ed., 2001, 40, 3357–3361 CrossRef CAS.
- D. L. Long, E. Burkholder and L. Cronin, Chem. Soc. Rev., 2007, 36, 105–121 RSC.
- D. L. Long, R. Tsunashima and L. Cronin, Angew. Chem., Int. Ed., 2010, 49, 1736–1758 CrossRef CAS PubMed.
- X. Lopez, J. J. Carbo, C. Bo and J. M. Poblet, Chem. Soc. Rev., 2012, 41, 7537–7571 RSC.
- H. N. Miras, J. Yan, D.-L. Long and L. Cronin, Chem. Soc. Rev., 2012, 41, 7403–7430 RSC.
- A. Muller, F. Peters, M. T. Pope and D. Gatteschi, Chem. Rev., 1998, 98, 239–271 CrossRef PubMed.
- M. T. Pope and A. Muller, Angew. Chem., Int. Ed. Engl., 1991, 30, 34–48 CrossRef.
- Y.-F. Song and R. Tsunashima, Chem. Soc. Rev., 2012, 41, 7384–7402 RSC.
- L. R. Morss, N. M. Edelstein, J. Fuger and J. J. Katz, The chemistry of the actinide and transactinide elements, Springer, Dordrecht, 2006 Search PubMed.
- P. C. Burns, K. A. Kubatko, G. Sigmon, B. J. Fryer, J. E. Gagnon, M. R. Antonio and L. Soderholm, Angew. Chem., Int. Ed., 2005, 44, 2135–2139 CrossRef CAS PubMed.
- J. Qiu and P. C. Burns, Chem. Rev., 2013, 113, 1097–1120 CrossRef CAS PubMed.
- P. Miro and C. Bo, Inorg. Chem., 2012, 51, 3840–3845 CrossRef CAS PubMed.
- P. Miro, J. Ling, J. Qiu, P. C. Burns, L. Gagliardi and C. J. Cramer, Inorg. Chem., 2012, 51, 8784–8790 CrossRef CAS PubMed.
- P. Miro, S. Pierrefixe, M. Gicquel, A. Gil and C. Bo, J. Am. Chem. Soc., 2010, 132, 17787–17794 CrossRef CAS PubMed.
- B. Vlaisavljevich, L. Gagliardi and P. C. Burns, J. Am. Chem. Soc., 2010, 132, 14503–14508 CrossRef CAS PubMed.
- T. Z. Forbes, J. G. McAlpin, R. Murphy and P. C. Burns, Angew. Chem., Int. Ed., 2008, 47, 2824–2827 CrossRef CAS PubMed.
- J. Ling, M. Ozga, M. Stoffer and P. C. Burns, Dalton Trans., 2012, 41, 7278–7284 RSC.
- J. Ling, J. Qiu and P. C. Burns, Inorg. Chem., 2012, 51, 2403–2408 CrossRef CAS PubMed.
- J. Ling, J. Qiu, G. E. Sigmon, M. Ward, J. E. S. Szymanowski and P. C. Burns, J. Am. Chem. Soc., 2010, 132, 13395–13402 CrossRef CAS PubMed.
- G. E. Sigmon and P. C. Burns, J. Am. Chem. Soc., 2011, 133, 9137–9139 CrossRef CAS PubMed.
- G. E. Sigmon, D. K. Unruh, J. Ling, B. Weaver, M. Ward, L. Pressprich, A. Simonetti and P. C. Burns, Angew. Chem., Int. Ed., 2009, 48, 2737–2740 CrossRef CAS PubMed.
- G. E. Sigmon, B. Weaver, K.-A. Kubatko and P. C. Burns, Inorg. Chem., 2009, 48, 10907–10909 CrossRef CAS PubMed.
- D. K. Unruh, A. Burtner, L. Pressprich, G. E. Sigmon and P. C. Burns, Dalton Trans., 2010, 39, 5807–5813 RSC.
- D. K. Unruh, J. Ling, J. Qiu, L. Pressprich, M. Baranay, M. Ward and P. C. Burns, Inorg. Chem., 2011, 50, 5509–5516 CrossRef CAS PubMed.
- J. Ling, J. Qiu, J. E. S. Szymanowski and P. C. Burns, Chem.–Eur. J., 2011, 17, 2571–2574 CrossRef CAS PubMed.
- J. Qiu, K. Nugen, L. Jouffret, J. E. S. Szymanowski and P. C. Burns, Inorg. Chem., 2013, 52, 337–345 CrossRef CAS PubMed.
- J. Qiu, J. Ling, A. Sui, J. E. S. Szymanowski, A. Simonetti and P. C. Burns, J. Am. Chem. Soc., 2012, 134, 1810–1816 CrossRef CAS PubMed.
- J. Ling, C. M. Wallace, J. E. S. Szymanowski and P. C. Burns, Angew. Chem., Int. Ed., 2010, 49, 7271–7273 CrossRef CAS PubMed.
- M. McBride, S. Sauve and W. Hendershot, Eur. J. Soil Sci., 1997, 48, 337–346 CrossRef CAS.
- C. N. Mulligan, R. N. Yong and B. F. Gibbs, Eng. Geol., 2001, 60, 193–207 CrossRef.
- S. C. B. Myneni, S. J. Traina, G. A. Waychunas and T. J. Logan, Geochim. Cosmochim. Acta, 1998, 62, 3285–3300 CrossRef CAS.
- L. P. Weng, E. J. M. Temminghoff, S. Lofts, E. Tipping and W. H. Van Riemsdijk, Environ. Sci. Technol., 2002, 36, 4804–4810 CrossRef CAS.
- J. R. Bargar, R. Reitmeyer, J. J. Lenhart and J. A. Davis, Geochim. Cosmochim. Acta, 2000, 64, 2737–2749 CrossRef CAS.
- R. J. Silva and H. Nitsche, Radiochim. Acta, 1995, 70–71, 377–396 Search PubMed.
- J. M. Astilleros, A. J. Pinto, M. A. Goncalves, N. Sanchez-Pastor and L. Fernandez-Diaz, Environ. Sci. Technol., 2013, 47, 2636–2644 CrossRef CAS PubMed.
- E. C. Buck, N. R. Brown and N. L. Dietz, Environ. Sci. Technol., 1996, 30, 81–88 CrossRef CAS.
- K. J. Cantrell, K. C. Carroll, E. C. Buck, D. Neiner and K. N. Geiszler, Appl. Geochem., 2013, 28, 119–127 CrossRef CAS PubMed.
- D. Gorman-Lewis, T. Shvareva, K. A. Kubatko, P. C. Burns, D. M. Wellman, B. McNamara, J. E. S. Szymanowski, A. Navrotsky and J. B. Fein, Environ. Sci. Technol., 2009, 43, 7416–7422 CrossRef CAS.
- E. S. Ilton, J. M. Zachara, D. A. Moore, J. P. McKinley, A. D. Eckberg, C. L. Cahill and A. R. Felmy, Environ. Sci. Technol., 2010, 44, 7521–7526 CrossRef CAS PubMed.
- A. J. Pinto, M. A. Goncalves, C. Prazeres, J. M. Astilleros and M. J. Batista, Chem. Geol., 2012, 312–313, 18–26 CrossRef CAS PubMed.
- T. Y. Shvareva, J. B. Fein and A. Navrotsky, Ind. Eng. Chem. Res., 2012, 51, 607–613 CrossRef.
- D. M. Singer, J. M. Zachara and G. E. Brown, Environ. Sci. Technol., 2009, 43, 630–636 CrossRef CAS.
- A. G. Sowder, S. B. Clark and R. A. Fjeld, J. Radioanal. Nucl. Chem., 2001, 248, 517–524 CrossRef CAS.
- J. E. Stubbs, J. E. Post, D. C. Elbert, P. J. Heaney and D. R. Veblen, Am. Mineral., 2010, 95, 1132–1140 CrossRef CAS.
- J. E. Stubbs, L. A. Veblen, D. C. Elbert, J. M. Zachara, J. A. Davis and D. R. Veblen, Geochim. Cosmochim. Acta, 2009, 73, 1563–1576 CrossRef CAS PubMed.
- W. Um, J. P. Icenhower, C. F. Brown, R. J. Serne, Z. M. Wang, C. J. Dodge and A. J. Francis, Geochim. Cosmochim. Acta, 2010, 74, 1363–1380 CrossRef CAS PubMed.
- D. M. Wellman, J. G. Catalano, J. P. Icenhower and A. P. Gamerdinger, Radiochim. Acta, 2005, 93, 393–399 CrossRef CAS.
- D. M. Wellman, K. M. Gunderson, J. P. Icenhower, S. W. Forrester and S. W. Forrester, Geochem., Geophys., Geosyst., 2007, 8, article number Q11001 CrossRef.
- D. M. Wellman, J. P. Icenhower, A. P. Gamerdinger and S. W. Forrester, Am. Mineral., 2006, 91, 143–158 CrossRef CAS.
- C. R. Armstrong, A. R. Felmy and S. B. Clark, Radiochim. Acta, 2010, 98, 549–554 CrossRef CAS.
- M. F. Fanizza, H. Yoon, C. Y. Zhang, M. Oostrom, T. W. Wietsma, N. J. Hess, M. E. Bowden, T. J. Strathmann, K. T. Finneran and C. J. Werth, Water Resour. Res., 2013, 49, 874–890 CrossRef.
- K. E. Wright, T. Hartmann and Y. Fujita, Geochem. Trans., 2011, 12, article number 8 CrossRef PubMed.
- J. L. Jerden and A. K. Sinha, Appl. Geochem., 2003, 18, 823–843 CrossRef CAS.
- Z. Q. Shi, C. X. Liu, J. M. Zachara, Z. M. Wang and B. L. Deng, Environ. Sci. Technol., 2009, 43, 8344–8349 CrossRef CAS PubMed.
- G. M. Sheldrick, SADABS-Bruker AXS area detector scaling and adsorption, version 2008/1, University of Gottingen, Germany, 2008 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- P. C. Burns, R. C. Ewing and F. C. Hawthorne, Can. Mineral., 1997, 35, 1551–1570 CAS.
- J. B. Fenn, M. Mann, C. K. Meng, S. F. Wong and C. M. Whitehouse, Science, 1989, 246, 64–71 CAS.
- R. L. Frost and J. Cejka, J. Raman Spectrosc., 2009, 40, 591–594 CrossRef CAS.
- S. Bastians, G. Crump, W. P. Griffith and R. Withnall, J. Raman Spectrosc., 2004, 35, 726–731 CrossRef CAS.
- D. Svergun, C. Barberato and M. H. J. Koch, J. Appl. Crystallogr., 1995, 28, 768–773 CrossRef CAS.
- P. B. Moore, J. Appl. Crystallogr., 1980, 13, 168–175 CrossRef CAS.
- A. J. Gaunt, I. May, D. Collison, K. T. Holman and M. T. Pope, J. Mol. Struct., 2003, 656, 101–106 CrossRef CAS.
- U. Kortz, S. S. Hamzeh and N. A. Nasser, Chem.–Eur. J., 2003, 9, 2945–2952 CrossRef CAS.
- P. C. Burns, Mineral. Mag., 2011, 75, 1–25 CrossRef CAS PubMed.
- L. VanHaverbeke, R. Vochten and K. VanSpringel, Mineral. Mag., 1996, 60, 759–766 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Crystallographic parameters, SAXS patterns, Raman, infrared and UV-vis spectra, X-ray powder diffraction patterns, and an analysis of bond angles in uranyl peroxide cage clusters. CCDC 956787. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3sc52357h |
| This journal is © The Royal Society of Chemistry 2014 |