Traditional dyeing – an educational approach
H.
Alves
a,
A.
Manhita
b,
C.
Barrocas Dias
b and
T.
Ferreira
*b
aEscola Secundária Padre António Macedo, Vila Nova de Santo André, Portugal
bCentro de Química de Évora & Laboratório HERCULES, Universidade de Évora, Évora, Portugal. E-mail: tasf@uevora.pt
First published on 16th June 2014
Abstract
This paper describes a mini-project developed with 10th grade Portuguese students where, by using an experimental activity involving the use of natural dyes to colour wool, students acquired a better understanding of the concepts and relationship between the colour, the electromagnetic spectrum, and chemical bonding. As demonstrated by the results of a laboratory activity interest survey, the interdisciplinary nature of the mini-project contributed to raise student awareness to the existing relationship between science, culture and daily life, promoting their overall interest in scientific topics.
Introduction
Natural dyes have become part of human life from time immemorial. The alchemy of colours started its use from an early time (Alam et al., 2007), with the textile dyeing industry being in existence for more than 4000 years. For all but the last 150 years, dyes were obtained from natural sources such as plants, lichens, insects and shellfish. These compounds can be found either directly in the crude extracts or gain colour from extracted colourless precursors as a result of reactions, such as hydrolysis, oxidation, condensation, etc. Nevertheless, not every shade is available directly from a natural source. The range of colours can be greatly extended by overdyeing the major components, which have blue, red and yellow hues. Nowadays, in a world full of colours produced by bright, fast, inexpensive synthetic dyes, it is hard to imagine a time when a good quality dye was as valuable as gold or silver (Surowiec et al., 2003 and Ferreira et al., 2004). However, nowadays the increasing use of natural dyes in colouration of textile materials and other purposes is just a consequence of the greater environmental awareness, since they exhibit better biodegradability, have better compatibility with the environment and possess lower toxicity and allergic reactions than synthetic dyes (Alam et al., 2007).But what is a dye? Dyes are generally organic compounds that are soluble in a solvent. Colour is obtained by applying a chemical compound called a chromophore, something that brings or creates colour. When used as a textile dye, the chromophore must also be captured as strongly as possible into the fibres (Melo, 2009). The vast majority of natural red and yellow dyes are mordant dyes. Textiles have been treated for centuries with these inorganic-based solutions of metal salts. The solution is absorbed by the fibre allowing the metal ion to become complexed to appropriate functional groups in the structure of the fibre. The mordant, attached to receptor sites on the surface of the fibres, makes a chemical bridge between the dyestuff and the fibre. The mordant ensures the brightness and wash fastness of the dye and also has a great influence on the final colour obtained. The most common mordant was alum (KAl(SO4)2·12H2O); iron or copper salts (e.g. FeSO4 or CuSO4) were also employed as mordants for the dyes. For different cultures and periods, different mordanting systems and procedures were used to produce a wide variety of hues (Dussubieux and Ballard, 2005; Ferreira et al., 2004; Joosten et al., 2006).
From an educational point of view, several activities which intend to show the potentialities of natural dyes in explaining concepts that are taught at secondary school or undergraduate university levels have been proposed. Terci and Rossi (2002) used fruit extracts containing anthocyanins as pH indicators. Natural dyes present in different plants and vegetables were also used to identify acidic and basic solutions and to discuss the concept of the pH equilibrium and pH indicator (Ramos et al., 2000; Soares et al., 2001; Dias et al., 2003; Marconato et al., 2004). The influence of the mordant on the resulting colour and fastness of the dyed fabrics was evaluated in several studies proposed to students (Editorial JCE, 1999; Mihalick and Donnelly, 2006, 2007). Some aspects of spectrophotometric concepts and applications and their relationship with colours were also studied in several reports (Couto et al., 1998; Ekrami et al., 2011). Paixão et al. (2006) developed a project that incorporates local art, history, and industry into the curriculum, which are relevant to real life, thus making the chemistry of dyeing more meaningful to students.
These types of activities may play a fundamental role in the learning process of students, since informal and creative learning contexts may provide the necessary space for mastering fundamental concepts in the classroom. In fact, according to Walker et al. (2011), learning is influenced by several factors: the information that students retain and what they attend to depends on existing knowledge and beliefs; knowledge is not an abstraction that can be transferred readily from how it is learned in the classroom to how it needs to be used outside of school, that is why classroom activities need to be designed so they mirror real-world situations and afford students opportunities to share, support, and revise their ideas; and, finally, context and culture affect learning and students often learn best by talking and collaborating with others in addition to more experienced adults.
As Carriazo (2011) refers, several approaches have been proposed to teach science in the laboratory, but the cooperative construction of knowledge may perhaps be reached through developing mini-projects. In these, students spend several weeks (more than 4–5 weeks with 3–5 h laboratory sessions) doing practical work for accomplishing a proposed topic. The project-based learning allows introducing the students to a scientific literature context, which exposes them to the interdisciplinary nature of modern chemical research.
At the same time, the teachers' role in the learning process of students is fundamental. Teachers themselves need to have a solid knowledge of the subject under study in order to be more capable of helping his/her students achieve a more complete understanding of the matter (Even, 1990). According to Cheung (2009), citing Abell (2007), three types of knowledge are required for teaching a particular topic in science: subject matter knowledge, pedagogical knowledge, and knowledge of the context. Subject matter knowledge is indispensable in teaching, however, and according to Even (1990), it is only a component of the knowledge of a well prepared teacher, nevertheless, an important one.
Being so, Portuguese school teachers usually invest a large deal of effort in upgrading their skills and in deepening their knowledge of the different matters they teach. Additionally, they invest long time, alone or in groups, sometimes with the support of university professors, in designing activities that can help their students to achieve a better comprehension of the curriculum concepts.
Project aims
This paper describes the design and implementation of a mini-project about colour and dyeing procedures involving a Portuguese high school chemistry teacher and her 10th grade (15–16 years old) students and the University of Évora. The aim of the project was, in a teaching and learning context somewhat different from that students were used to, explain the basis of the chemical bonding, develop a better understanding of the chemistry involved in dyeing processes, explore the relationship between the nature of dyes and mordants and the colour obtained for the dyed fibres, and relate the colour to the electromagnetic spectrum. Additionally, the project intended to promote the relationship between science, culture and daily life, being a fruitful interactive activity where students take an active part and contribute to its evaluation, being at the same time evaluated in the context of the activity.Integrating the activity into the secondary school curriculum
The Portuguese educational system at the secondary school level comprises three years of high school (10th to 12th grade). Chemistry is only taught as a separate subject at the 12th grade.Physics and Chemistry are combined into a single subject, called Physics and Chemistry, at the 10th and 11th grades. Chemistry is taught during half of the school year and Physics is studied during the other half. The curriculum is the same for all schools in the country, but teachers from each school choose the textbooks to be used.
Part of the teaching objectives on the chemistry 10th grade National Curriculum (15–16 years old) is the study of the electromagnetic spectrum, particularly, the visible light spectrum and the energy associated with it. Matter and energy and the interactions between the two is another topic, with the formation of ions and ionization energy being considered as subtopics. The covalent bonding model is a particularly important topic and its study includes bond energy, bond length and bond angle as well as molecular geometry. No d-splitting is considered at this level. For these topics of the Portuguese National Curriculum, particularly, for chemical bonding, no experimental activities are adopted to support the theoretical teaching. In spite of enormous efforts, the teaching of chemical bonding goes on being a very complex event. Many students find it dull and unwelcoming.
In this project, besides the chemical bonding subject, other topics may be discussed. The preparation of a natural dye bath is an activity in daily life and also in a chemistry class, since during the procedure, organic compounds are extracted from a substrate, in this case vegetal ones, and form a coloured tea. The analysis of the structures of the chromophore molecules can be used to introduce students into organic chemistry topics and accompany a discussion on the impressive array of chemicals produced by plants. The absorption of visible light by the chromophore solutions and its relationship with the electromagnetic spectrum are the topics for a lesson on spectroscopy. Finally, the interaction between mordant ions and the chromophore molecules and the textile fibres can be used to explain chemical bonding and metal complex formation.
Project design
Students and their families were informed about the research project and its general objectives and design beforehand. Parents were asked to provide a signed consent form before their children were allowed to participate in the study.
Another 90 min class was used for the students to complete an anonymous individual interest survey provided by the teacher. Beforehand, the teacher read the survey aloud, answering any questions the students might have regarding the survey; after that period, the students individually answered it.
Evaluation procedures. In order to evaluate the success of the project, a two-way process was devised: an evaluation by the teacher in charge of the activities, and an evaluation by the students which undertake the activities.
The teacher evaluates the experimental activity and its learning outcomes based on seven parameters (Table 3). The students evaluation is based on an interest survey with a total of 34 questions divided into 5 groups: group A evaluates the teaching methodologies; group B evaluates the attractiveness of the laboratory activities and groups C, D and E evaluate the success of the activity as a vehicle to teach some of the objectives of the chemistry 10th grade National Curriculum (Table 4).
The parameters in the teacher evaluation form were made by the project team members and were based on the 10th grade chemistry and physics curriculum goals. The students' interest survey was designed by the project team members.
Experimental
Methodology followed in the activity
A set of three experimental protocols for dyeing with madder, onion skins and logwood, according to two different dyeing procedures, was prepared. Each protocol included a historical introduction to the dyestuff and its usage, the chemical structures of the main chromophores present on each dyestuff, the goals of the activity, the materials and reagents, the experimental procedure and a set of questions to be answered by the students. Supplementary material was also prepared and included extended texts about natural dyes, textiles and fibres, chemical bonding and interaction of light and matter. A fourth protocol on spectroscopy was used to obtain the absorption spectra of the solutions of the different chromophores of the dyes used.At the end of each experimental session and in some classes dedicated to it, students presented and discussed the results, establishing a broad discussion on chemical concepts such as those already referred: chemical bonding, metal complexes, organic structures, spectroscopy, and electromagnetic spectrum, among others. They also elaborated a report and answered an interest survey on laboratory activity and the project.
Materials and reagents
Copper(II) sulphate pentahydrate was purchased from Himedia Laboratories, alum (aluminium potassium sulphate dodecahydrate), madder (Rubia tinctorum L.) and logwood (Haematoxylum campechianum L.) pieces were obtained from Kremer Pigmente. Standards alizarin and haematein were purchased from Fluka, quercetin was obtained from Sigma and purpurin was purchased from Eastman Organic Chemicals. Wool was obtained from Aljarraiolos. Yellow onion skins were collected by the students. Deionized water was used in all the experiments.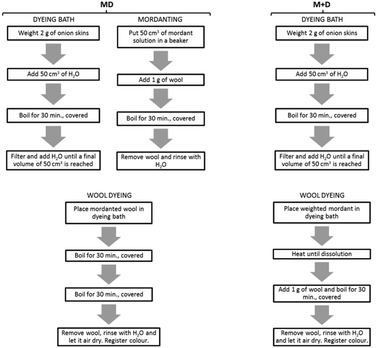 | ||
| Fig. 1 Example of an organogram built by the students and containing all steps involved in the dyeing procedures at the laboratory. | ||
In the first lab period, all dyeing procedures were performed using alum as mordant while copper(II) sulphate was chosen for the second lab period. Madder, logwood and yellow onion skins were used as dyeing sources. Two groups of students used madder as the dyeing source, two other groups dyed wool with onion skins and the fifth group used logwood. Two methods were employed for wool dyeing, which included a pre-mordanting procedure (MD procedure) and a simultaneous mordanting procedure (M + D procedure):
MD procedure. Four mordant solutions (50 cm3) were prepared according to traditional receipts (Ekrami et al., 2011; Manhita et al., 2011): alum at concentrations of 0.0030 mol dm−3 and 0.0085 mol dm−3 (7% wt and 20% wt mordant) and copper(II) sulphate at concentrations of 0.0016 mol dm−3 and 0.0400 mol dm−3 (2% wt and 50% wt mordant; the highest concentration value was chosen for comparison). 1.0 g of wool was mordanted for 30 min in each 50 cm3 of boiling mordant solution, covered. Afterwards, the wool was removed and rinsed with cool water. The dye baths were prepared with 2.0 g of dye immersed in 50 cm3 boiling water for 30 min, covered. The solution was allowed to cool and after simple filtration of the plant material, water was added until a final volume of 50 cm3 was reached. The previously mordanted wool was added to the dye solution bath and boiled for 30 min, covered. Dyed wool was removed, rinsed with water and air dried. Wool final colours were registered.
M + D procedure. Dye baths were prepared as described for the MD procedure. After plant material filtration, the different amounts of mordant salts were added (the same amounts that were calculated to prepare the mordant solutions in the MD procedure) to each solution. When the mordant salts were dissolved, 1.0 g of wool was immersed in each solution that was boiled for 30 min. After the dyeing procedure, all wool samples were thoroughly rinsed with ultrapure water and left to air dry. The obtained colours were registered.
The absorption spectrum of each dye standard solution was recorded. Solutions of the dye standards combined with each of the mordants (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) were also prepared and their spectra were recorded. The absorption spectra measurements were carried out on a Thermo Nicolet Evolution 300 UV/Vis spectrophotometer, using disposable plastic cells of path length 1 cm.
1, v/v) were also prepared and their spectra were recorded. The absorption spectra measurements were carried out on a Thermo Nicolet Evolution 300 UV/Vis spectrophotometer, using disposable plastic cells of path length 1 cm.
Results and discussion
A wide range of colours and hues for the dyed fibres was obtained under the experimental conditions used, varying from orange to red, green, brown, purple and black.Students could observe that the mordant metal ion, the mordant bath concentration and the dyeing procedure have a strong influence on the wool fibre hue. Different metal complex structures were formed according to the dyeing conditions used, leading to the colours observed in the dyed wool.
At this time, students remembered the electromagnetic spectrum in the UV/Visible region and that low energy corresponds to a lower frequency (longer wavelength) of light being absorbed. Light consisting of rays in the range 380–430 nm looks violet to us, 430–490 nm looks blue, 490–540 nm green, 540–580 nm yellow, 580–650 nm orange, and 650–700 nm red. This is the case when seeing light emitted from a light source. It is different when we see a coloured object, since the effect of colouration is based on taking away colour (certain wavelengths), removing one or more of the colours of white light (Zollinger, 1999). Students were then taught that the absorption of light energy by an organic dye causes an electron to be promoted into a higher energy level, thus bringing the dye molecule into an ‘excited’ state. As the excitation of an electron becomes easier, the required spectral energy moves from the invisible ultraviolet into the longer wavelengths of visible spectrum (Ingamells, 1993).
Being so, the reddish colours correspond to complexes with larger energy gaps, while bluish colours are due to complexes with shorter energy gaps, and in between are the violet colours (Brisdon, 1998). Students recorded the different colours obtained for the dyed wool in Table 1 and interpreted them accordingly.
Afterwards, students considered that a dyestuff is a complex mixture of more than one chromophore; being so, all of the chromophores that are present contribute to the final hue of the dyed fibre. Students then used the UV/Vis spectrophotometer to measure the absorption spectra of coloured solutions of the chromophores alizarin and purpurin (madder dye), quercetin (onion skins) and haematein (logwood), alone or resulting from the addition of the metal salts (mordants) alum or copper(II) sulphate. They also registered the wavelength of maximum absorption (Fig. 2).
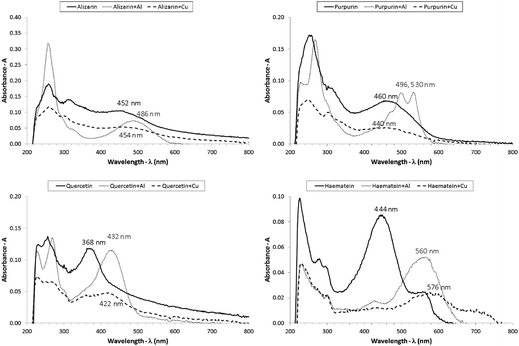 | ||
| Fig. 2 UV/Visible absorption spectra of the chromophores alizarin, purpurin, quercetin and haematein. | ||
A fruitful discussion about the structures of the compounds and the absorption spectra was undertaken and enhanced the understanding of the students about the absorbed colours. Students were then introduced with the idea of complementary colours and the possibility of getting some enlightenment about the colour they see. Colours directly opposite to each other on the colour wheel are said to be complementary. The mixture of two complementary colours of light will result in white light. If a particular colour is absorbed from white light, one will see its complementary colour, resulting from the mixing up of all the other wavelengths (Zollinger, 1999). In Table 2 students registered the observed colour for the solutions prepared and the colour that was absorbed according to the corresponding spectrum. Then they checked and confirmed the relationship of complementarity between these two colours.
| Chromophore | H2O | H2O + alum | H2O + copper(II) sulphate | |||
|---|---|---|---|---|---|---|
| Observed colour | Absorbed colour | Observed colour | Absorbed colour | Observed colour | Absorbed colour | |
| Alizarin | Orange | Blue | Orange | Blue-green | Light yellow-orangish | Blue |
| Purpurin | Orange | Blue | Rose | Blue-green, green-yellowish | Light orange | Violet-blue |
| Quercetin | Light yellow | UV (violet) | Yellow | Violet-blue | Yellow | Violet |
| Haematein | Dark yellow | Blue | Purple | Yellow-green | Violet | Yellow-green |
This step encouraged students to discuss their experimental observations and relate them with the theoretical information they were supplied with. An extended discussion about electromagnetic spectrum and colour was undertaken, as well as metal complexes formation and chemical bonding.
Students concluded that for the same chromophore, the addition of a mordant salt displaces the value of the wavelength of maximum absorption in a different way, depending on the mordant used, the chemical bonding that is established being responsible for that. Each chromophore has a different absorption spectrum since they all differ in their chemical structures. The absorbed colours (wavelengths) of the solutions are complementary to the observed colours.
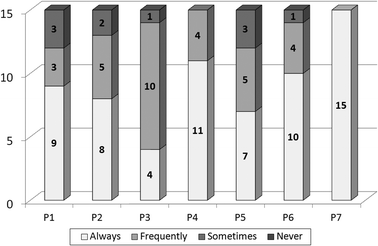
The teacher created a qualitative evaluation grid (see in Table 3 and in more detail in the Appendix) to evaluate the behaviour/degree of interest of the students in the laboratory. Each parameter is subdivided into three indicators which evaluate each student individual and relational skills. Other researchers considering adoption of this observation schedule should first investigate its validity and reliability in their own research context. The teacher's results of the qualitative evaluation grid are presented in Fig. 3. Based on the subjective impression of the teacher, the students' general behaviour in the laboratory improved. They became more autonomous and worked better in groups and they understood the importance of taking notes for experimental work. Additionally, they developed consciousness about security rules and self- and hetero-evaluation. The evaluation revealed that at least 80% of the students were given the qualitative assessment of always or frequently in the indicators of the different parameters (see Fig. 3).
| # | Parameter | # | Parameter |
|---|---|---|---|
| P1 | The student is autonomous and follows the experimental protocol | P5 | The student is familiarised with the experimental work objectives |
| P2 | The student cooperates with his colleagues and discusses progress of work | P6 | The student takes notes of the experimental observations and results |
| P3 | The student selects and properly handles the laboratory material | P7 | The student makes self- and hetero-evaluation |
| P4 | The student complies with safety rules and follows instructions |
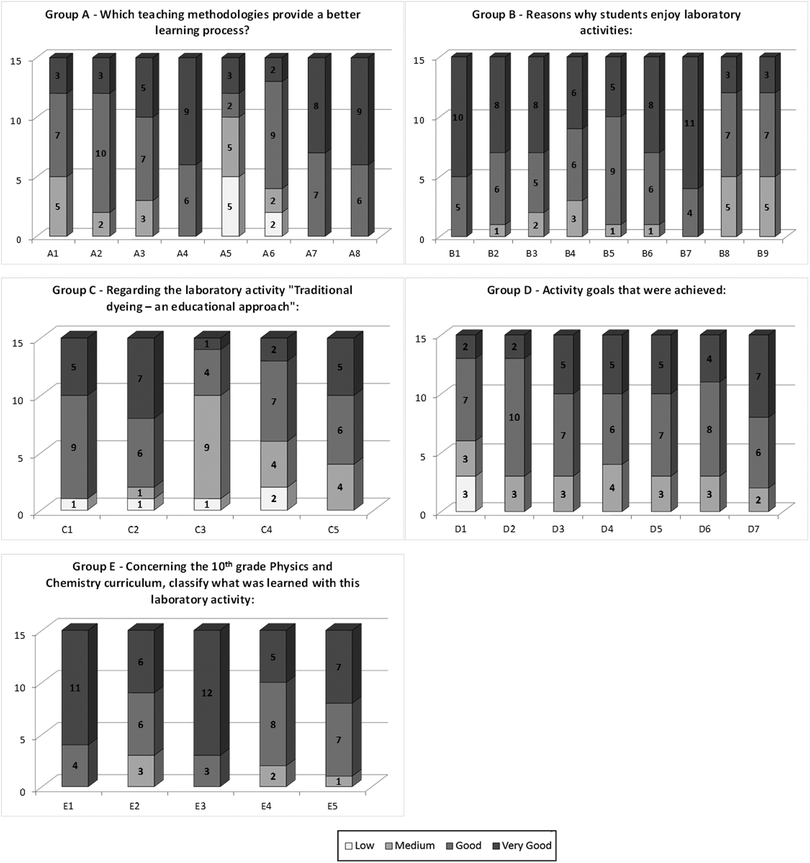
Students were then invited to evaluate the project and express their feelings about it by answering a laboratory activity interest survey. The parameters referring to the interest survey are presented in Table 4. Students are asked to assess each individual question (group A–E) considering the evaluation of each parameter under those questions for which they have four possibilities of answer: low, medium, good and very good. The answers given by the students are presented in Fig. 4.
| # | Parameter | # | Parameter | ||
|---|---|---|---|---|---|
| Group A – Which teaching methodology provides a better learning process? | A1 | Listening to the teacher's lesson complement to home study | Group C – Regarding the laboratory activity “Traditional dyeing – an educational approach”: | C1 | The activity was easy to run |
| A2 | Solving exercises | C2 | The activity was interesting | ||
| A3 | Running a laboratory activity helps understanding the subjects | C3 | The activity stimulated the curiosity about chemical phenomena in everyday life | ||
| A4 | Running a laboratory activity in groups of 2 or 3 | C4 | The laboratory activity helped understanding the concept of chemical bonds lectured in classes | ||
| A5 | Running a laboratory activity individually | C5 | The laboratory activity helped understanding the relationship between the concept of chemical bonds lectured in theoretical classes and its application in everyday life | ||
| A6 | Planning a laboratory activity and carrying it out | Group D – Activity goals that were achieved: | D1 | Distinguish between covalent and ionic bonds | |
| A7 | Running a laboratory activity guided by an experimental protocol | D2 | Verify the existence of colour in solutions containing organic molecules (chromophores), which exhibit conjugated covalent double bonds | ||
| A8 | Listening to the teacher's lesson together with demonstrations and questions | D3 | Explain the colour phenomenon through energy absorption in the visible region of the electromagnetic spectrum | ||
| Group B – Reasons why students enjoy laboratory activities: | B1 | They make classes more interesting | D4 | Interpreting the absorption spectrum of chemical species in coloured solutions | |
| B2 | Motivation to scientific subjects is improved | D5 | Relating the wavelength (λ) of the absorbed colour to the observed colour | ||
| B3 | They enable us to relate theory and practice | D6 | Relating absorbance to different concentrations of the same solution | ||
| B4 | They contribute to better understand the theoretical subjects | D7 | Check for different colours/hues in solutions of different concentrations | ||
| B5 | Cooperation habits are promoted | Group E – Concerning the 10th grade Physics and Chemistry curriculum, classify what was learned with this laboratory activity | E1 | Handling laboratory equipment and safety rules | |
| B6 | Development of the handling skills of laboratory equipment | E2 | Make calculations for determining the concentration of solutions | ||
| B7 | Classes are more motivating | E3 | Prepare solutions | ||
| B8 | The laboratory obtained results are usually consistent with the theoretical learning | E4 | Distinguish between organic and inorganic materials | ||
| B9 | Everyday life subjects are related to the lectured subjects | E5 | Interpretation of graphics |
The answers to the interest survey showed that students clearly enjoy running a laboratory activity to consolidate the topics they learn at the theoretical classes, since this procedure motivates them, improves cooperation and development of different types of skills (answers to groups A and B). Students also agreed that the project was successful in reaching its different goals, especially those related to chemical bonding and colour and the electromagnetic spectrum (answers to groups C and D). Additionally, according to the students, the activity was particularly useful for acquiring skills in some other general topics present in the 10th grade Physics and Chemistry curriculum, such as reading graphics, distinguishing organic and inorganic compounds, handling laboratory equipment and materials, and making calculations to determine the concentration of solutions (answers to group E). This was in fact observed in the results obtained for their final exams when compared to the students from the three previous school years (data not shown).
Both the evaluation of the students by their teacher and their own evaluation of the project are entirely encouraging. In fact, students were in general quite motivated to learn these topics included in the 10th grade of secondary school chemistry Portuguese National Curriculum, usually considered uninteresting, when using the innovative approach followed in this project. In the evaluation of the topics related to chemical bonding and the electromagnetic spectrum, all the students which undertook the practical laboratory experiment passed them, 80% of them with a grade of very good or excellent, demonstrating the importance of the implemented project in their learning process. During the former three school years, and using tests with similar difficulty, only an average of 15% of the students attained the very good and excellent marks, and around 40% of the students failed their exams.
Conclusions
The use of natural dyes in the chemistry classes is not new (Soares et al., 2001; Paixão et al., 2006; Dias et al., 2013), and has already proved to help convey chemical concepts to students. However, it was never used in the teaching of chemical bonding and the relationship between the colour and the electromagnetic spectrum.This project was done with a group of students enrolled in a 10th grade physics and chemistry class and highlights the importance of using creative methodologies in the teaching of chemistry. Based on the student's interest survey and on the teacher evaluation, one can conclude that the project activities proved to be an effective way to convey some of the topics of the 10th grade chemistry National Curriculum, namely, the chemical bonding, general fundamentals of absorption spectroscopy and an introduction to organic chemistry.
The project also enabled students to relate daily life issues and activities to scientific facts and concepts.
The project design based on theoretical classes, laboratory sessions and classes dedicated to open discussion and integration of the laboratory results enabled a more interactive attitude of the students towards their own learning process. Extensively discussing their doubts and conclusions with their colleagues and the teacher provided a better and more comprehensive understanding of the basic concepts as demonstrated by the students' self-evaluation.
Additionally, the laboratory sessions positively contributed to the development of students' practical skills, an important parameter in a chemistry course. The importance of using laboratory experiments and the use of daily life concepts in the improvement of the students' learning process observed in this project has already been proved in other studies. (Soares et al., 2001; Paixão et al., 2006; Bopegedera, 2011; Dias et al., 2013).
The project received a very positive evaluation from the students, as the results of the interest survey revealed. This comes in agreement with the general comments the students had made during the laboratory and discussion sessions, where they vocally expressed their excitement about the experiments being carried out and how they were helping them understand things that are usually considered quite dull and unwelcoming.
Appendix
High School Padre António MacedoLaboratory Session Evaluation Grid
Class: ______________________________________________ Year: _____ 2010/2011
Activity: ____________________________________________ Date: _____/_____/____
| Student name | The student is autonomous and follows the experimental protocol | The student cooperates with the colleagues and discusses work progress | The student selects and properly handles the laboratory material | The student complies with safety rules and follows instructions | The student is familiarised with the experimental work objectives | The student takes notes of the experimental observations and results | The student makes self- and hetero-evaluation | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | c | Psc | a | b | c | Psc | a | b | c | Psc | a | b | c | Psc | a | b | c | Psc | a | b | c | Psc | a | b | c | Psc | |
| Indicators | Indicators | ||
|---|---|---|---|
| The student is autonomous and follows the experimental protocol |
(a) Uses the spectrophotometer after reading the instructions on how to use the equipment.
(b) Consults the activity organogram previously built. (c) Coordinates the activities: 1 – simultaneous preparation of the mordant and dyeing baths; 2 – filtration of the dyeing baths; 3 – wool dyeing (procedures MD and M + D). |
The student is familiarised with the experimental work objectives |
(a) In the beginning of the class the student presents the requested activity organogram.
(b) During the laboratory experiment, the student evaluates the results obtained. (c) Critically evaluates the obtained laboratory results taking into account the theoretical concepts learned. |
| The student cooperates with the colleagues and discusses work progress |
(a) Divides the tasks with colleagues, optimizing team effort
(b) Analyses and discusses with colleagues the laboratory experiment procedure, taking into consideration their opinion. (c) Together with colleagues, cleans the laboratory bench, stores reagents and laboratory materials used. |
The student takes notes of the experimental observations and results |
(a) Makes a record of the laboratory observations made: qualitative record of the colour of dyeing bath solutions, dyed wool, dyeing standard solutions; data obtained from absorption spectra.
(b) Makes the required calculations to prepare the laboratory solutions. (c) Whenever needed, the student presents the data using the rules for the significant figures with associated errors. |
| The student selects and properly handles the laboratory material |
(a) Selects the adequate laboratory materials for the laboratory procedure, taking special care with the handling of the glass materials.
(b) Carefully uses the balance and the spectrophotometer. (c) Is able to autonomously prepare the solutions of the mordant. |
The student makes self- and hetero-evaluation |
(a) Assumes and self-evaluates his/her attitudes and responsibilities.
(b) Self-evaluates his/her cooperation with his/her colleagues (c) Evaluates the attitudes and responsibilities of his/her colleagues. |
| The student complies with safety rules and follows instructions |
(a) Recognizes and obeys the laboratory overall safety rules, and recognises the warning symbols in the labels of the chemical reagents used.
(b) Uses the hot plate in an adequate manner. (c) At the end of the laboratory session uses the adequate procedure to discard unused reagents and materials. |
||
The individual indicators (see next page) are scored according to the following scale:
A – Always (3 points) F – Frequently (2 points) S – Sometimes (1 point) N – Never (0 points)
Each Parameter is scored (Psc) calculating the average, rounded to the units, obtained with the scores for the three individual indicators:
A – Always (3 points) F – Frequently (2 points) S – Sometimes (1 point) N – Never (0 points)
Teacher ___________________________________
Methodology used by the teacher in student assessment
Three periods of 135 min are used for the laboratory activities. In each period, the teacher should give detailed instructions orally, or on the board for 10 min. Students undertake the practical activity following the organogram previously constructed, the protocol previously supplied and the notes obtained during the previous theoretical class where the integration of the practical activity has already been done.The teacher observes each student for a period of about 8 min, either individually or in his/her relationship with the rest of the group, and takes notes, following the evaluation grid presented above. Whenever necessary, the teacher interrupts the process to answer a question or to clarify some aspects of the work. The process is repeated in three laboratory classes, and an average score for each of the three individual indicators of each of the seven parameters is obtained by the teacher.
Acknowledgements
The authors acknowledge the financial support of Project REMATAR, FCOMP-01-0124-FEDER-010482 (FCT PTDC/HAH/64045/2006) from the Portuguese Foundation for Science and Technology, FCT. A. Manhita also acknowledges FCT for the PhD fellowship (SFRH/BD/22411/2005).Notes and references
- Abell S. K., (2007), Research on science teacher knowledge, in Abell S. K. and Lederman N. G. (ed.), Handbook of research on science education, Mahwah, NJ: Lawrence Erlbaum Associates, pp. 1105–1149.
- Alam M. M., Rahman M. L. and Haque M. Z., (2007), Extraction of Henna Leaf Dye and its Dyeing Effects on Textile Fibre, Bangladesh J. Sci. Ind. Res., 42, 217–222.
- Bopegedera A. M. R. P., (2011), Putting the laboratory at the center of teaching chemistry, J. Chem. Educ., 88, 443–448.
- Brisdon A. K., (1998), Inorganic spectroscopic methods, New York, USA: Oxford University Press.
- Carriazo J. G., (2011), Laboratory projects using inquiry-based learning: an application to a practical inorganic course, Quím. Nova, 34, 1085–1088.
- Cheung D., (2009), Using think-aloud protocols to investigate secondary school chemistry teachers' misconceptions about chemical equilibrium, Chem. Educ. Res. Pract., 10, 97–108.
- Couto A. B., Ramos L. A. and Cavalheiro E. T. G., (1998), Aplicação de pigmentos de flores no ensino de química, Quím. Nova, 21, 221–227.
- Dias C. B., Miranda M., Manhita A., Candeias A., Ferreira T. and Teixeira D., (2013), Identification of Onion Dye Chromophores in the Dye Bath and Dyed Wool by HPLC-DAD: An Educational Approach, J. Chem. Educ., 90, 1498–1500.
- Dias M. V., Guimarães P. I. C. and Merçon F., (2003), Corantes Naturais: Extracção e Emprego como Indicadores de pH, Quím. Nova Esc., 17, 27–31.
- Dussubieux L. and Ballard M., (2005), Using ICP-MS to Detect Inorganic Elements in Organic Materials: A New Tool to Identify Mordants or Dyes on Ancient Textiles, Mater. Res. Soc. Symp. Proc., 852, OO4.1.1–OO4.1.6.
- Editorial staff of Journal of Chemical Education, (1999), Colors to dye for: preparation of natural dyes, J. Chem. Educ., 76, 1688A–1688B.
- Ekrami E., Mafi M. and Motlagh M. S., (2011), Dyeing of Wool Using Olive Oil Fruit Waste, World Appl. Sci., 13, 996–999.
- Even R., (1990), Subject matter knowledge for teaching and the case of functions, Educ. Stud. Math., 21, 521–544.
- Ferreira E. S. B., Hulme A. N., McNab H. and Quye A., (2004), The natural constituents of historical textile dyes, Chem. Soc. Rev., 33, 329–336.
- Ingamells W., (1993), Colour for textiles - A user's handbook, West Yorkshire, England, UK: Society of Dyers and Colorists.
- Joosten I., van Bommel M. R., Keijzer R. and Reschreiter H., (2006), Micro Analysis on Hallstatt Textiles: Colour and Condition, Microchim. Acta, 155, 169–174.
- Manhita A., Ferreira V., Vargas H., Ribeiro I., Candeias A., Teixeira D., Ferreira T. and Dias C. B., (2011), Enlightening the influence of mordant, dyeing technique and photodegradation on the colour hue of textiles dyed with madder - A chromatographic and spectrometric approach, Microchem. J., 98, 82–90.
- Marconato J. C., Franchetti S. M. M. and Pedro R. J., (2004), Solução-Tampão: Uma Proposta Experimental Usando Materiais de Baixo Custo, Quím. Nova Esc., 20, 59–62.
- Melo M. J., (2009), History of Natural Dyes in the Ancient Mediterranean World, in Bechtold T. and Mussak R. (ed.), Handbook of Natural Colorants, West Sussex, England, UK: John Wiley & Sons, pp. 3–20.
- Mihalick J. E. and Donnelly K. M., (2006), Using Metals To Change the Colors of Natural Dyes, J. Chem. Educ., 83, 1550.
- Mihalick J. E. and Donnelly K. M., (2007), Cooking Up Colors from Plants, Fabric, and Metal, J. Chem. Educ., 84, 96A–96B.
- Paixão M. F., Pereira M. M. and Cachapuz A. F., (2006), Bridging the Gap: From Traditional Silk Dyeing Chemistry to a Secondary-School Chemistry Project, J. Chem. Educ., 83, 1546–1549.
- Ramos L. A., Lupetti K. O., Cavalheiro E. T. G. and Fatibello-Filho O., (2000), Utilização do extrato bruto de frutos de Solanum nigrum L. no ensino de química, Eclet. Quím., 25, 229–240.
- Soares M. H. F. B., Silva M. V. B. and Cavalheiro E. T. G., (2001), Aplicação de corantes naturais no ensino médio, Eclet. Quím., 26, 225–234.
- Surowiec I., Orska-Gawrys J., Biesaga M., Trojanowicz M., Hutta M., Halko R. and Urbaniak-Walczak K., (2003), Identification of natural dyestuff in archeological coptic textiles by HPLC with fluorescence detection, Anal. Lett., 36, 1211–1229.
- Terci D. B. L. and Rossi A. V., (2002), Indicadores naturais de pH: usar papel ou solução?, Quím. Nova, 25, 684–688.
- Walker J. P., Sampson V. and Zimmerman C. O., (2011), Argument-Driven Inquiry: An Introduction to a New Instructional Model for Use in Undergraduate Chemistry Labs, J. Chem. Educ., 88, 1048–1056.
- Zollinger H., (1999), Color: A Multidisciplinary Approach, Zürich: Verlag Helvetica Chimica Acta.
| This journal is © The Royal Society of Chemistry 2014 |