Trials and tribulations: student approaches and difficulties with proposing mechanisms using the electron-pushing formalism
Gautam
Bhattacharyya†
Department of Chemistry, Clemson University, 363 Hunter Labs, Clemson, SC 29634-0937, USA. E-mail: gautamb@clemson.edu; Tel: +1-864-656-1356
First published on 4th June 2014
Abstract
The skill of proposing mechanisms of reactions using the electron-pushing formalism (EPF) is not only of value to practicing organic chemists but it is also emphasized to students enrolled in organic chemistry courses at all levels. Several research studies in the past decade have documented the difficulties that undergraduate, and even graduate students, encounter when trying to propose mechanisms using the EPF. An examination of this work suggests the emergence of a preliminary, but coherent, picture of students' strategies and difficulties with using electron-pushing to solve a variety of organic chemistry tasks. The first two sections of this paper, I present (1) two factors that may underlie several of the students' difficulties as presented in the organic chemistry education research literature; and (2) a model of how students approach solving mechanism tasks using the EPF. This paper concludes with a section on potential implications for instruction and a set of research questions arising from this analysis that have yet to be answered.
Introduction
Ever since Morrison and Boyd (1959) published their ground-breaking text, using mechanisms to teach organic chemistry has become widespread (Ferguson and Bodner, 2008). Organic chemistry reaction mechanisms are most frequently represented diagrammatically using the electron-pushing formalism (EPF).‡ In this formalism, a double-headed curved arrow is drawn with its tail at the electron source – usually a lone pair or a bond – to the electron sink – usually an electron-deficient atom. An example of a mechanism using the EPF is shown in Fig. 1. | ||
| Fig. 1 Sample electron-pushing diagram. Note that the curved, double-headed arrows denote the movement of an electron pair from source to sink. | ||
Practicing organic chemists, especially those specializing in synthetic organic chemistry, use this formalism as their primary tool to explain and/or predict reaction outcomes including the generation of side products, regiochemistry, and stereochemistry (Bhattacharyya, 2013). Although these mechanisms are rarely based on empirical data, chemists lend a measure of rigor to them by basing their diagrams on established mechanistic patterns in addition to a variety of chemical concepts and theories. As such, electron-pushing is an efficient and effective mode of communication for expert organic chemists.
Several research studies in the past decade have documented the difficulties that undergraduate, and even graduate students, encounter when trying to propose mechanisms using the EPF (Bhattacharyya and Bodner, 2005; Anderson and Bodner, 2008; Ferguson and Bodner, 2008; Anderson, 2009; Kraft et al., 2010; Grove et al., 2012a, 2012b). An examination of this work suggests the emergence of a preliminary, but coherent, picture of students' strategies and difficulties with using electron-pushing to solve a variety of organic chemistry tasks. In this paper I present the following aspects of these developments:
• two factors that may underlie several of the students' difficulties as presented in the organic chemistry education research literature; and
• a model of how students approach solving mechanism tasks using the EPF
I conclude with a section on some research questions arising from this analysis that are yet to be answered in addition to potential instructional implications.
Part I: students' difficulties with reactions and reactivity
Meta-analysis procedure
Our studies reported in this paper are a part of an ongoing program of research in the development of conceptual expertise in organic chemistry. With the exception of one study the research reported here centered on organic chemistry graduate students working towards a PhD degree. Qualitative methodologies were used in all of these studies to offer us the opportunity to develop rich descriptions of the participants.Table 1 contains brief descriptions of the research studies used for the meta-analysis described below. Methodological details of these studies are summarized in appendices. All of the data were collected at large, publically-funded research-intensive Universities in the United States. All of the participants were recruited on a voluntary basis without any compensation for their assistance. As approved by the respective institution's Internal Review Board (IRB), the possibility of a later meta-analysis was made clear to the participants during the process of obtaining their informed consent for each study. At the time of the data collection all of the graduate student participants had the intention of obtaining their PhD degrees; it is possible that some of these students may have left their respective programs with a MSc degree instead.
| Study number | Study title | Number of participants | Data collection instrument | Ref. |
|---|---|---|---|---|
| 1 (Appendix A) | Proposing reaction mechanisms in the absence of traditional cues | 14 graduate students enrolled in an advanced organic chemistry course | 8 standard EPF tasks with starting material, reagents, and products provided | Bhattacharyya and Bodner (2000, 2005) |
| 2 (Appendix B) | Organic synthesis problem-solving | 4 1st-year and 2 3rd-year organic chemistry graduate students | Participants' proposals of synthetic routes to complex molecules | Bhattacharyya (2004) |
| 3 (Appendix C) | Conceptions of Brønsted acidity | 10 advanced organic chemistry graduate students | Model-eliciting activity of alcohols and phenols | Bhattacharyya (2006) |
| 4 (Appendix D) | Cueing in mechanistic problem-solving | 4 1st-year and 12 senior organic chemistry graduate students | 2 sets of mechanistic reasoning tasks | Kraft et al. (2010) |
| 5 (Appendix E) | Mental models of reactions and reactivity | 4 1st-year and 12 senior organic chemistry graduate students | 7 EPF diagrams of complex synthetic transformations | Strickland et al. (2010) |
| 6 (Appendix F) | Structural representations of physical and chemical characteristics | 8 senior chemistry majors | Several sets of tasks in verbal and diagrammatic representations of chemical and physical properties | DeFever et al. (2014) |
As can be the case with qualitative research, each study in Table 1 produced one or more thematic categories that had to be set aside because they were not highly relevant to the focus of the respective study. However, these unpublished results were noteworthy at the time because they either led students to incorrect solutions or to abandon fruitful ones. Furthermore, some of these “previously-unused” themes – such as, misunderstanding of chemical equilibrium – were identified in multiple studies (Bhattacharyya and Bodner, 2005; Kraft et al., 2010). Consequently, a meta-analysis seemed appropriate to determine the extent to which these themes were prevalent across the studies.
Two criteria were initially used to select the categories for this meta-analysis. First, each category had to have been already identified in more than one of the studies listed in Table 1. Second, the category had to have negatively affected the students' ability to successfully complete a task. Five themes resulted from this step: chemical reactions always yield the lowest energy substance, polyfunctional compounds react only at single groups in chemical reactions, products of chemical reactions can be accurately predicted using a single parameter, nucleophilicity is equivalent to basicity, and reactivities of individual functional groups are preserved even when those groups are combined.
With these codes or categories in hand, all of the transcripts from each of the studies were reviewed using content analysis (Patton, 1990). Evidence for each of these categories was found in more than half of the transcripts in at least four of the six studies. Additionally, in most cases the codes were identified multiple times in each transcript. As such, none of the categories needed to be discarded. However, the category about predicting reactions using a single parameter was divided into sub-categories representing the two main forms by which this phenomenon manifested itself.
Although the codes or categories on which this meta-analysis is based appeared in multiple studies in multiple institutions with multiple samples, it was important to further determine whether they, or phenomena similar to them, had been identified in other research studies on the learning of organic chemistry. To carry out this part of the analysis, I searched Education Resources Information Center (ERIC), PsycInfo, and Medline using the keywords, “organic chemistry”, “chemical reactions”, and “chemical reactivity”. Since Medline covers a broader set of disciplines than the other two databases, I used the Boolean operator “AND” along with the keywords “education research” or “education practice” to narrow each search. Additionally, the websites for Chemical Education Research and Practice (also University Chemistry Education), Journal of Chemical Education, The Chemical Educator, Journal of Research in Science Teaching, International Journal of Science Education, Science Education, Science & Education, Research in Science Education, and Journal of College Science Teaching, were also searched using the keywords, “organic chemistry”, “chemical reactions”, and “chemical reactivity”. To exclude a multitude of reports on laboratory experiments, the Boolean “AND” and keyword “research” were used in the search for Journal of Chemical Education. To further narrow the scope, the search was limited to publications from 2000 to 2013.
Papers were downloaded from search hits that had the words “organic chemistry” or an organic chemistry topic in the title or in the list of keywords. Additionally, abstracts from hits which contained general terms in publication titles or keywords such as “reactivity” or “acid–base” were read, and any papers with instruments using organic substances were also downloaded. All of the articles were scanned for evidence of some attempt to conduct a research study by its authors. This evidence included elements such as guiding questions, hypotheses, quotes from interview data, or statistical tests. The analysis was purposefully broad at this stage to ensure the inclusion of as many studies as possible. A total of 36 publications fit these criteria; 17 of which had at least parts that were devoted to students' understanding of chemical reactions and reactivity. Evidence of one or more of the thematic categories was found in more than half of these manuscripts, further evidence suggesting that these characteristics were not idiosyncratic to any specific study.
Through his extensive work on the alternative conceptions of chemical phenomena held by chemistry students, Talanquer (2006) noted that creating lists of misconceptions, or inventories, is an important first step, but that researchers must also strive to create “explanatory frameworks” by determining the factors underlying these alternative conceptions. As such, inductive analysis of the original thematic categories yielded two larger factors – deterministic conceptualization of chemical processes and difficulty with multi-variate thinking – which are discussed in the following sub-section.
Results
The data suggest that the students' understanding of chemical reactions and reactivity are consistent with a deterministic mindset (Hoefer, 2010). In a deterministic perspective, chemical processes are believed to be driven by specific forces to yield specific products. In actuality, however, chemical processes are not pre-determined in this fashion; they are the result of a probability that is determined by the relative energies of the possible products and of the pathways to those products. This deterministic mindset may be seen as a subset of the students' teleological empirical assumption as previously identified by Talanquer (2006). In our studies, students demonstrated determinism primarily in two ways.First, students expressed their beliefs that chemical reactions always yield the lowest energy substance, i.e., the standard free energy change for the reaction, ΔGorxn, must be negative for any reaction that results in an isolable product. Consider reactions such as acid-catalysed ester hydrolysis or reaction of a ketone and an amine to form an imine (Fig. 2). In the first case, ΔGorxn is roughly zero (Lowry and Richardson, 1987) meaning that neither reactants nor products are favoured at equilibrium. In the second case, the product imine is actually at a higher potential energy than the reactant, i.e. ΔGorxn is positive (Lowry and Richardson, 1987). All of the participants who provided acceptable responses to tasks containing acid-catalysed ester hydrolysis and imine formation in a study of reasoning types cued by mechanism tasks (Kraft et al., 2010) supported their answers – without any prompting from the interviewer – by noting that the products were formed because they had to be thermodynamically more stable than the reactants. For example, a 4th-year graduate student given the pseudonym Steve, stated,
“One thing in this reaction is the; make a carbon–hydrog, nitrogen double bond which is more stable…”
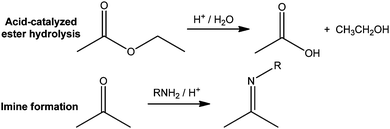 | ||
| Fig. 2 Examples of reactions in which the ΔGorxn is either close to 0 (ester hydrolysis) or positive (imine formation). | ||
The carbon–nitrogen double bond to which Steve referred is actually over 80 kJ mol−1 less stable than the carbon–oxygen double bond it replaced (Lowry and Richardson, 1987). Chemists are able to accomplish this transformation by removing water, a by-product, throughout the course of the reaction; i.e. they push it to completion by mass action.
Several other researchers have also reported students reaching incorrect conclusions based on this belief that reactions must yield products that are more stable than the reactants. In a study of chemistry majors in their final year of their undergraduate degrees, for example, Rushton et al. (2008) found that only 7 out of the nineteen students chose the correct, cis-alkene in a task for which they had to determine the major product of a reaction. The remaining students indicated that the trans-alkene would be the major product because it was lower in energy than the cis (p. 126). Similarly, in his research on organic chemistry graduate students Anderson (2009) found that students believed, “…the driving force of the reaction was explained by the flow of electrons and this flow produced a more energetically stable situation as reactants are transformed into products” (p. 107). In each of these cases, the students' premise that reactions are programmed to produce the lowest-energy substance resulted in ignoring the energetics of the pathways, i.e. reaction kinetics.
Finally, in their study of undergraduate students' conceptions of acidity, McClary and Talanquer (2011) observed
“Four students in this group expressed the belief that acid behavior was somewhat related to the ‘stability’ of the molecule; in particular, the assumption that acids were unstable substances was predominant in some cases. These beliefs allowed some students to generate simple mechanistic explanations for differences in acid strength based on the idea that uneven distributions of charge (as caused by the presence of certain atoms or functional groups, or by their relative positions) induced molecular instability” (p. 404).
The second type of behaviour consistent with a deterministic mindset of chemical processes manifested itself when students considered reactions of compounds containing multiple functional groups. Virtually every participant in each of our studies limited their analysis of the interactions of a polyfunctional compound with reagent(s) to a single location, or functional group, of that compound. In reality, however, some or all of the functional groups in a molecule may react with a single reagent, assuming they had compatible reactivities. Zachary, a 1st-year graduate student who participated in a study on the learning of organic synthesis, for example, proposed the transformation shown in Fig. 3A in his total synthesis proposal (Bhattacharyya, 2004). However, the professor who taught the course indicated in his feedback that the acidic conditions required to effect that transformation would have more likely yielded a by-product (Fig. 3B). Like Zachary's example demonstrates, failure to recognize multiple reaction sites can lead individuals to overlook potential side reactions.
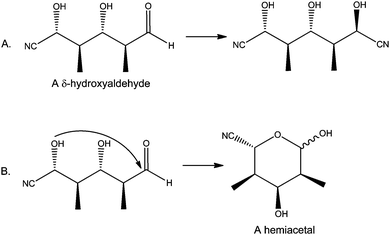 | ||
| Fig. 3 (A) The intended reaction drawn by Zachary in the first draft of his synthetic proposal. (B) A possible side-reaction the compound could undergo suggested in feedback from the professor. | ||
The students' expectation that polyfunctional molecules interact with reagent(s) at a single functional group may be considered a corollary to the first factor since any process producing the lowest energy product would do so through a single reactive center of that molecule. However, this expectation could be, at least in part, a by-product of the students' learning strategy as described by a graduate student in Anderson (2009) case studies
“Eh…I knew that…all you had to do for the exams was…basically…you…you had to know what…if it was an epoxidation you know what it does. It doesn't matter what the R groups are…you know what the reaction itself does. So I just memorized what the reactions themselves do. So…disregarding what else…what else is around it. And then I could just apply it to all the…examples he gives. Just memorized them” (p. 164).
As such, limiting the analysis to a single functional group may have been a cognitive-load-reducing strategy for students (van Merriënboer and Sweller, 2005).
In another example, all of the senior (final-year) undergraduate chemistry majors in a study of transfer between verbal and diagrammatic representations concluded that a complex natural product would react only at single site under highly acidic or basic conditions (DeFever et al., 2014). They reaffirmed these responses even after prompting from the interviewer that the molecules could, and most likely would, react at multiple sites. In another example, students invoked this principle especially when they needed for some part of a molecule to “disappear” while solving mechanism tasks as described by Ferguson and Bodner (2008)
“Jackie provided an interesting example of this category while writing the mechanism for Question #3, when she said: “So, let's see what we can do… well, bromine sometimes, it just leaves 'cause it wants to. So, we'll draw that out and we'll say that bromine leaves” (p. 106).
I believe that these examples provide substantive evidence of a deterministic approach to chemical processes. By believing that reactions take place so that they can always produce the products which are lower in energy than the reactants, students believed the reactants were somehow programmed to interact with each other in a specific manner that would yield the lower-energy products. The use of the phrase “wants to” when describing chemical reactivity is but one of many examples of this mindset. Although none of these studies revealed a specific reason behind this perspective, one possibility is the tendency of humans towards teleological explanations (Talanquer, 2006), i.e. those in which actions are the result of a “specific purpose or need” (p. 814). Garvin-Doxas and Klymkowsky (2008) provide an alternative, but compatible, explanation based on their studies of biology students
“Students carry the underlying belief that random processes are inefficient whereas biological systems are extremely efficient, and are therefore loath to ascribe macroscopic biological phenomena to random underlying processes. They seek alternative rational explanations, the dominant one being the existence of drivers” (pp. 232–233).
In addition to this deterministic approach to chemical reactions and reactivity, the other major obstacle to students' ability to effectively use EPF is managing the complexities associated with multi-variate thinking – i.e. what Kuhn et al. (2009) refer to as “coordinating the effects of multiple variables” (p. 269). We observed that when attempting to explain or predict behaviors of substances in chemical processes, students tended to consider only a single parameter and/or did not account for synergistic interactions between properties. It is common in complex systems for multiple properties to combine synergistically such that the system exhibits emergent properties which cannot be predicted by simple addition of the individual properties (Talanquer, 2006); proverbially, the whole is greater than the sum of its parts.
Regarding their understanding of emergence, the students consistently implied that reactivities of individual functional groups are preserved when those groups are combined. For example, in Study 6, all of the participants – chemistry majors in their final year as undergraduates – were unable to predict how complex natural products would behave under a variety of reaction conditions. For instance, Leah made the following comment when asked how one such compound might react with a strong electrophile:
“With a strong electrophile. Um, it will, double bonds [pointing to the carbon–carbon double bond in conjugation with a carbonyl group] will react with an electrophile. It'd have to be a really strong electrophile to break aromaticity, um, but I guess any point along here could…”
Leah was absolutely correct in all of her comments except for the prediction that the carbon–carbon double bond in conjugation with the carbonyl group would react with an electrophile. Were the carbon–carbon double bond in isolation, Leah would have been correct. However, its conjugation with the carbonyl group gives it a fundamentally different reactivity, making that double bond most likely to react with electron-rich species, or nucleophiles.
This difficulty with emergence has manifested itself in several other studies in the learning of organic chemistry. In their study of students' mental models of Brønsted acidity, for example, McClary and Talanquer (2011)noted:
“For example, the intuitive idea that the properties of chemical compounds are additive (Talanquer, 2006), and thus vary in proportion to the amounts of the components present in the system, was at the core of many of our students' predictions, independently of whether they thought of acids as substances that lost protons or accepted lone electron pairs” (p. 410).
Additionally, in their case study of a student enrolled in a second-year level undergraduate course in organic chemistry, Anderson and Bodner (2008) found that
“Parker applied an approach to understanding organic chemistry that was an extension of a strategy that had been successful in general chemistry: focusing on individual atoms. In doing this, however, he failed to think about molecules as an organized whole. He seemed to view a molecule as a collection of atoms, which reacted more or less independently, rather than as a system of electrons” (p. 98).
Finally, in his case studies of graduate students learning how to propose mechanisms using EPF, Anderson (2009) observed
“What was not clear to him was that these structures are not static; the dynamic nature of the pi system in these molecules was not indicated in the papers he used to construct his synthesis. As a result, he overlooked the importance of this dynamic nature and the overall reactivity of the molecule. Instead, he looked at the isolated reactivity of the functional groups within the molecule” (p. 97).
The key is that in all of these cases, not considering the emergent behavior of the system led students to incorrect answers in their assignments and exams.
In addition to a misunderstanding of emergence the other difficulty students had with multi-variate thinking was using only a single factor to explain or predict outcomes of chemical processes. In our studies this issue was primarily manifested when students dealt with intermediates in which an oxygen or nitrogen atom bore a positive charge. Consider the following comments from two participants from a study in which first-year graduate students were asked to propose mechanisms for tasks without the traditional cues (Bhattacharyya and Bodner, 2000):
Homer: “And then, the oxygen–boron complex strikes me as a kind of an odd leaving group. It's got a positive oxygen.”
Hubert: “Just, so, keeping that in mind, you limit yourself only to pathways that generate positive charges. Oxygen isn't happy with a positive charge so you need to find a way to get it to a carbon which can handle it a little bit better.”
In these and countless other instances in that study the participants were unable to get started with a task or got stuck while generating potentially productive solutions because they were not comfortable with the idea of either an oxygen or nitrogen atom even transiently bearing a positive charge as demonstrated by the following quote from Fred, a second-year graduate student in a study on reasoning processes cued by mechanism tasks (Kraft et al., 2010):
“…because oxygen doesn't like to have a positive charge and… then I got here and I'm like ehh.”
It is particularly noteworthy that the participants had such difficulty with the notion of oxygen or nitrogen bearing positive charges given that they have seen hydronium ions (H3O+) and ammonium ions (NH4+) since their first exposure to chemistry in high school. One of the sources of the difficulties the students may have with positive charges being placed on atoms that have relatively large electronegativities may be that the same symbol, a superscript plus-sign, is used to indicate hypo- and hypervalent cationic species. In the hypervalent cations – such as hydronium ion and ammonium ion – the positively charged atom usually has its full complement of valence electrons. As such, electron-rich substances do not interact with the charged center. The positively charged center of a hypovalent cation, on the other hand, tends to have less than 8 electrons, making it electron-deficient and able to accept a pair of electrons. Carbocations are examples of hypovalent cations because the positively charged carbon only has 6 valence electrons. At a minimum, therefore, the site at which electron-rich species react with hypovalent or hypervalent cations is significantly different – an example of which was captured in the following quote from Ferguson and Bodner (2008):
“Jill demonstrated this confusion when she said, ‘When you have four bonds to nitrogen, it is positively charged. So, if this [a hydrogen] leaves as H + , these electrons stay. So this [alkene] would be bonded to on there [nitrogen] and then the hydrogen would go away.’ … Jill tried to use the alkene to attack a saturated nitrogen the way an alkene might abstract a proton in a reaction with HBr” (p. 106).
A second issue students had with oxygen- and nitrogen-containing compounds – that was the result of not attending to all the factors involved – usually arose in the context of derivatives of aromatic compounds, such as phenols and anisoles and anilines. Students assumed that oxygen and nitrogen always withdraw electron density due to their high electronegativities. This effect holds if one considers only the sigma-bonding framework. However, in the pi-bonding framework, oxygen and nitrogen donate a pair of non-bonding electrons through resonance effects. In the following quote, Eleanor, a 1st-year graduate student in Study 5, mental models of acids, was unable to reconcile her understanding of Brønsted acid–base theory with the relatively high acidity of phenols:
“I wanta say when there's an electron-withdrawing group in close proximity that for some reason, intuitively, I feel like it would be less likely to wanta give a proton, to donate that proton. But maybe I have that backwards, ‘cause I always think of electron, an electronegative group as pulling. So it would be pulling towards. Um, and then just with the last two; I have no explanation for the phenol. But the last two, um, so if you have an electron-donating group and uh, on the other side of phenol and it's delocalized. I have a really hard time explaining why. I guess I have some intuitive feelings and I notice trends.”
In addition to most nitrogen and oxygen aromatics, this phenomenon becomes especially problematic for students when they consider the reactions of carboxylic acids and their derivatives such as esters and amides. All of these classes of compounds play prominent roles in organic chemistry courses from the basic level on up, and in all of these cases the electron-donating behavior of oxygen and nitrogen dominate the chemical outcomes of those species.
A different way in which students' attempted to solve a multi-variate problem using a single factor was by conflating two behaviours, namely nucleophilicity and basicity. Without further modification, the terms “acid” or “base”, as used in organic chemistry, refer to the Brøsted–Lowry theory of acids and bases (Bruice, 2007). According to this theory, basicity refers to the extent a species will accept a proton from another substance in an acid–base equilibrium. Basicity, therefore, is a “thermodynamic” term since it gives information regarding the position of an equilibrium. Nucleophilicity, on the other hand, refers to the rate at which an electron-rich species, a Lewis base, will react with an electron-deficient carbon center. Nucleophilicity, therefore, is a “kinetic” term because it gives information regarding the rate of a reaction, but does not suggest anything about the position of the equilibrium. Although many substances may exhibit both, basicity and nucleophilicity are two separate behaviors.
In our studies focused on solving EPF tasks and interpreting EPF diagrams (Bhattacharyya and Bodner, 2005; Kraft et al., 2010; Strickland et al., 2010), however, students tended to equate nucleophilicity with basicity. Consider James' comments
“Nucleophile, basic term; it's a base with a lone pair. …Base is like, it should have, it's a, when I think it's like a nucleophile which has lone pair of electrons to give away” (Strickland et al., 2010, p. 297).
One drawback of this lack of differentiation was that if students were able to identify a substance as either a base or a nucleophile, they usually assumed the existence of the other behaviour. This reductionism was most detrimental when participants would attribute basic properties to substances such as triphenylphosphine (Ph3P) or iodide ion (I−), both of which are excellent nucleophiles but very poor bases.
In their study of second-year organic chemistry students, Ferguson and Bodner (2008) also observed a similar phenomenon. In explaining a participant named Ryan's struggles with one of the tasks, they noted
“Yet, for Ryan, the concept of a nucleophile was poorly differentiated from that of a base. Ryan's poor understanding of the content knowledge that would enable him to discriminate between a reactant acting as an base and the same reactant acting as a nucleophile hindered his progress with the mechanism” (p. 107).
Part II: tentative model of students' approach to EPF tasks
While it is amply clear from previous research that the majority of students do not use mechanistic reasoning to reach scientifically-acceptable solutions to mechanism tasks, much less is known about the strategies that students do use to solve such tasks. The attempt to better characterize the students' problem-solving processes began, once again, from our previous studies since I had access to the interview transcripts of thirty research participants (Bhattacharyya and Bodner, 2005; Kraft et al., 2010).For the analysis, I took each transcript and divided it by task. The separated passages from the transcripts, any artifacts the participant produced for that task, and interviewer field notes were examined to create a short description of the problem-solving strategy for each task. (Not all of the descriptions were equally detailed, reflecting differences in utterances by participants during different parts of the interview.) Each participant's strategies for the individual tasks were then examined to establish a flow chart showing that participant's overall problem-solving strategies. (These flow charts were similar to the one in Fig. 4.) Finally, the flow charts for all thirty participants were reviewed so that a more “global” version reflecting trends in problem-solving strategies could be established (Fig. 4). The purpose of this model is not to provide a definitive account; rather, it is to offer a preliminary sketch which can be tested and/or modified in future studies.
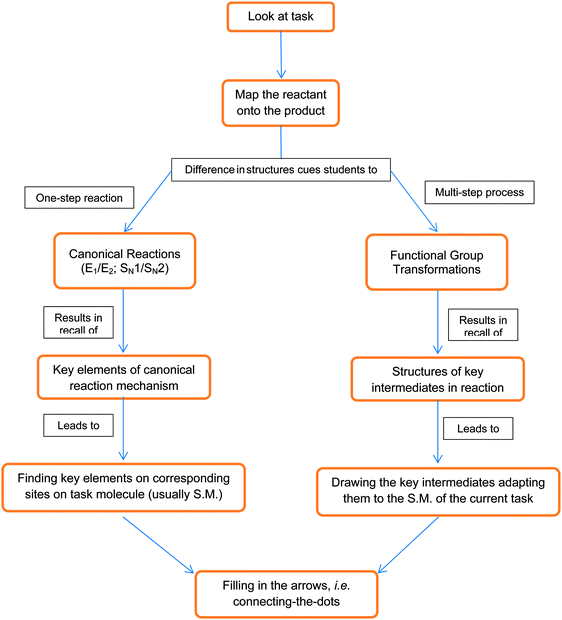 | ||
| Fig. 4 A model of graduate students' approach to solving mechanism tasks. S.M. is “starting material”. | ||
Similar to the meta-analysis described earlier, the organic chemistry education research literature was then reviewed to ascertain whether other researchers had observed similar characteristics in their participants' problem-solving processes. This time, however, only manuscripts reporting mechanistic problem-solving were reviewed. Only four focused on the topic of interest (Anderson and Bodner, 2008; Ferguson and Bodner, 2008; Anderson, 2009; Grove et al., 2012a, 2012b), and a few others had smaller sections devoted to mechanistic problem-solving (Bhattacharyya, 2004; Rushton et al., 2008). Since the participants' behaviours in these reports were consistent with the flow chart in Fig. 4, no further changes were made to it.
As indicated by the flow chart in Fig. 4, students tend to begin proposing a mechanism by trying to identify the atoms of the starting material in the product and the corresponding connectivity as well – a process we and others (Ferguson and Bodner, 2008; Anderson, 2009) have called “mapping”. As a representative example, consider the following comments from Jen, a first-year graduate student from our study on proposing mechanisms in the absence of traditional cues:
“Oh, I'm numbering the carbons, trying to figure out which ones correspond from starting material to product and you figure out how they moved” (Bhattacharyya and Bodner, 2005, p. 1406).
Others also noted similar behaviors in their studies, as evidenced from the following passages:
“We use the term mapping to describe a careful matching, on an atom-by-atom basis, of features of the starting material to the structure of the product. Erika explained how this mapping process worked when she stated, ‘Well, the first thing that I think of is what I need to lose and what I need to get rid of. You know, to form this [product], and what has to become connected, eventually’” (Ferguson and Bodner, 2008, p. 109).
“Overall, Adam's problem-solving approach to the extra credit mechanism problems was to evaluate the connectivity of the product molecule compared to the starting material to work out the details of the mechanism” (Anderson, 2009, p. 91).
One of the results of this mapping process was that students were able to recognize structural differences between the starting materials and products. These structural differences, in turn, cued students to either canonical reactions – nucleophilic aliphatic substitution or elimination, for example – or functional group transformations. The former case is described by Charles from the study of mechanisms in absence of traditional cues
“Well you look back at all the possible reactions you know, and you try to map it onto this reaction and see. Hey, I mean this is a simple hydroboration, simple alkene formation right, so you just think about other mechanisms that involve that” (Bhattacharyya and Bodner, 2000).
Curt, a third-year PhD student in our study of reasoning modes cued by mechanism tasks observed
“And then, sometimes if I don't know how to push arrows, then I just kinda try to recall the reaction and try to match it up with the, with the reaction that is going on” (Kraft et al., 2010, p. 288).
The attempt to use structural changes to identify a general reaction type has also been observed by others such as, Rushton et al. (2008)
“Actually, I'm going to look at the products. I see one of the products ends up with a lone pair on the carbon and I don't know if any reactions end up like that…” (p. 126).
In this quote, the student's comments express difficulty in choosing the correct answer to a multiple-choice mechanism question because s/he could not identify a reaction that would yield her desired product.
When the structural differences between starting material and product did not cue students to a canonical reaction, they often cued them to functional group transformations. These structural cues included details such as relative orientation of substituents in the product as is described by Samuel from the mechanisms without the traditional cues study
“Seeing that there's two products on the bottom, um, I would, in order to get them both on the cis side, I would assume that they both had to be adding at the same time. Um, I guess that's familiar with, like, I think you can use platinum to add two hydrogens on the cis side” (Bhattacharyya and Bodner, 2000).
In his case studies of graduate students learning to solve mechanism tasks, Anderson (2009) wrote the following about one of his participants, Jenny:
“Her method for solving most of these problems was to work the mechanism out based on her understanding of the reactivity of the functional groups by comparing this system to other systems she was familiar with from her previous experience and her organic chemistry content knowledge. She also tried to identify the difference in connectivity between the product and reactants to map out the bonds that were broken and formed during the reaction” (p. 113).
When students were cued to a canonical reaction, they tended to recall from their memory the key elements involved in that mechanism and then confirm the presence of those moieties in the starting material. In the following quote, Hubert explains what he did once he decided the transformation in the task was an elimination reaction:
“I knew I had to get rid of a hydrogen, through an elimination reaction to form this double bond. I knew bromine was a good leaving group so I had to find a way to, you know, to abstract that hydrogen” (Bhattacharyya and Bodner, 2005, p. 1406).
We noticed similar behavior in our study on reasoning types cued by mechanism tasks. Consider the following comment made by Matt, a first-year student:
“We have this compound [points to 1,3-cyclohexandione], we have a base, ketone, so we form this, this attachment. Hopefully we will have enough base so we will subtract this hydrogen and we are going to attack the carbonyl, making this kind of reaction, 1,4-addition, but here it will form, it will be the nucleophile, the electrophile [points to the two reactants]” (Kraft et al., 2010, p. 290).
In this passage, Matt had correctly decided that the transformation was a Robinson Annulation and, as such, showed the interviewer all the necessary components he had identified for the first part of the transformation – Michael addition of the enolate of a β-diketone to methyl vinyl ketone (MVK).
Alternatively, when the students were cued to a functional group transformation, they tried to recall the key intermediates in the reaction, as is explained by Marcus, from the mechanisms without traditional cues study, in the following quote:
“‘Cause even though I may know, I know what's going on. Like I know the bromine's, you're going to get this bridged intermediate and then you're going to have a back, like backside attack from the other bromine” (Bhattacharyya and Bodner, 2005, p. 1406).
Once again, we observed similar behaviors in our study on reasoning types cued by mechanism tasks, as is shown in the following exchange with John:
“Ok, on this one, so I would have…attach the water to this group, so I have an ester here, so the other possible thing to have a different product, so I have an acidic medium here, conditions here, whatever. so we have here what if we use the catalyst, at this point I go back to this water, OH here, so I would go to the starting material, but we are going backwards, so to obtain a different compound… I must remove the hydroxide, to get the acid. Then the problem here is the hydroxide is too much, much more strong than the acid, so it's going to deprotonate the carbonyl and it forms that.”
Interviewer: “Right. So what is it that you interpreted when you saw this, the drawings?”
John: “Um. The hydrolysis of the ester” (Kraft et al., 2010, p. 286).
In this case, once John realized that the overall transformation was acid-catalyzed ester hydrolysis, he recalled the key intermediates in that process.
There is reason to believe that a symptom of this strategy was also observed in Ferguson and Bodner's (2008) study of second-year undergraduate students. In the task that involved the reduction of a ketone with sodium borohydride, for example, many of the participants knew that the ethanol was supposed to appear at the end of the mechanism, but could not recall its exact purpose. Consider the following quote:
“Jim confessed that, ‘I just can't remember what ethanol does. I know that it comes in at the end of the reaction.'” (Ferguson and Bodner, 2008, p. 105).
Like the participants in our studies, Jim and his colleagues were trying to recall specific substances and roles, rather than reasoning mechanistically to arrive at an acceptable answer.
Regardless of the initial process of analysis, the students in our studies tended to use a method which we previously called “connect-the-dots”. Basically, they first drew intermediates and/or products and then drew the relevant arrows in the reactant(s). During this process students may have shown all of the relevant intermediates, the route we and Anderson (2009) have observed with graduate students, or, in the case of students in the undergraduate second-year course, drew all of the curved arrows in a single reaction step, a technique Grove et al. (2012a) termed “cyclic pathway” In the same study, Grove et al. (2012a) reported a variant of the “connect-the-dots” approach, calling it “decorating with arrows.” Whether “connecting the dots, decorating with arrows, or drawing cyclic pathways”, these students did not use EPF as a tool for reasoning mechanistically; rather they just placed the curved arrows as an afterthought because it was required of them.
Conclusion
In the first part of this manuscript, I presented a set of students' conceptions that in one fashion or another hampers their ability to mechanistically reason using the electron-pushing formalism (EPF). These conceptions are consistent with a deterministic approach to chemical processes, and also reflect fundamental difficulties with multi-variate thinking. This array is not meant to be a comprehensive catalogue of student misconceptions in organic chemistry. Rather, it is a set of understandings that we have consistently seen over a decade of studies in multiple settings which resulted in students either abandoning or not considering potentially productive solutions to electron-pushing tasks. A review of the organic chemistry education research literature shows that others have also observed the same or similar phenomena. As such, it would be difficult to dismiss our observations as artifacts of a specific sample or data collection instrument and/or method.The second part of this manuscript contains a tentative “sketch” of student strategies for solving electron-pushing tasks. This model is not meant to be complete or definitive; rather, it should be seen as an initial idea in need of refinement. Although it is tempting to conclude that students adopted their strategies as a result of their underdeveloped conceptions, there is ample evidence that students also approach problem solving algorithmically regardless of their conceptual understanding (Nakhleh and Mitchell, 1993). In fact, Raul a senior who participated in the study on transfer between verbal and diagrammatic representations (DeFever et al., 2014) made the following comment when asked why he did not apply his more-than-adequate conceptual understanding of organic chemistry when problem solving.
“When I see that I have to solve a problem, I go into ‘plug-and-chug’ mode. About the only time I stop to think about what I'm doing is when the word ‘explain’ is used in the directions.”
This quote suggests that one of the obstacles that may constrain students' ability to apply their conceptual knowledge to solving EPF tasks is more a matter of metacognition rather than one of conceptual knowledge (Sandí-Ureña and Cooper, 2009). Regardless, the results presented in this manuscript may help inform the design of new instructional materials and research projects; both of which are discussed in the following sections.
Implications for teaching
Deana Kuhn (2007), a leader in the field of multi-variate thinking, recommends that students need explicit instruction to overcome their deficits in that respect. Similarly, Doerr (2000) concludes the same for helping students to switch from deterministic mindsets towards probabilistic ones. However, there do not appear to be any research-based educational interventions for either probabilistic reasoning or multi-variate thinking. Nonetheless, both Kuhn and Doerr suggest general strategies for training students.As to helping students learn multi-variate reasoning, Kuhn (2007) proposed that students should receive training in metacognition, since it is supposed to help them develop awareness of multiple options in a manner that is transferable to the students' academic activities – e.g. homework and exams. Sandí-Ureña, Cooper and co-workers demonstrated that students are able to increase their metacognitive skills for general chemistry problem solving by cooperatively working on lateral thinking exercises (Sandí-Ureña and Cooper, 2011; Sandí-Ureña et al., 2012). A key characteristic of their finding is that the lateral thinking exercises – essentially, brainteasers – do not have to be domain-specific. One of the most common lateral thinking activities is a crossword puzzle in which the clues often contain double-entendres. The added benefit to helping students increase their metacognitive abilities is that it may also help students monitor their problem-solving activities to the extent that they begin to eschew the “plug-and-chug” mode as Raul had put it.
Regarding students' tendency towards a deterministic mindset, Doerr (2000) suggests that modeling exercises are one method for teaching probabilistic reasoning. Effective probabilistic reasoning requires students to think in terms of distributions rather than absolute all-or-nothing situations. For example, the classic plots of the fraction of molecules versus kinetic energy that are staples of kinetic molecular theory instruction attempt to convey the notion that at any given temperature the particles of the system are arrayed over a very large distribution of energies rather than all of them being at a single value. As such, an activity that would encourage students to consider the spectrum of kinetic energies rather than a specific one would promote probabilistic thinking. For example, one could require students to verbally explain how water left in an open bowl will evaporate over time even though the temperature of the system and surroundings is always far below the boiling point of water. An attendant epistemic question is whether this type of reasoning requires learners to move from a dualistic worldview to a relativistic one (Perry, 1968)? This issue, which requires further inquiry, may be particularly problematic since Grove and Bretz (2010) found that the students enter the second-year undergraduate organic chemistry sequence largely as dualistic thinkers.
Finally, we can help students use more mechanistic reasoning when solving EPF tasks instead of the strategies in Fig. 4 by using a method from our research (Kraft et al., 2010). In that study we decided to develop a set of tasks in which the product would not be provided since the students in our first study seemed to use the product of a reaction to guide their proposed mechanisms (Bhattacharyya and Bodner, 2005). However, we were concerned that students could answer the conventional predict-the-product tasks – in which a starting material and a set of reagents were provided (Fig. 5A) – by rote recall of memorized reactions. We also did not want to give students something fundamentally new. Thus, we came up with a set of tasks in which the starting material and reagent were provided; however, the major product would result from an intramolecular step, as shown in Fig. 5B. In our research study with organic chemistry graduate students (Kraft et al., 2010), only those who used mechanistic reasoning were able to provide the best answer. Independently, Grove et al. (2012b) demonstrated that those who used mechanistic reasoning in tasks similar to that in Fig. 5B had a statistically better success rate than those who did not, with effect sizes between 0.5 and 0.6. As such, these predict-the-product tasks can be used to help students try strategies different than those in Fig. 4.
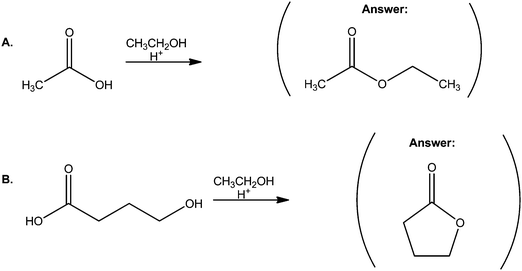 | ||
| Fig. 5 (A) A typical predict-the-product task. (B) A predict-the-product task in which the major product is formed by an intramolecular step. | ||
Implications for research
Although the data presented in Part I are indicative of a deterministic mindset, much more research needs to be done to more fully characterize it, especially the students' underlying conceptions of reaction thermodynamics and kinetics. Since these misconceptions arose during the course of other studies, the interviewer did not stray too far from the topics directly related to the respective research study. At this stage, however, we envision conducting qualitative studies to elicit student conceptions and reasoning as they are asked to explain the type of seemingly-anomalous data presented in Part I – for example, asking students to explain why can a ketone be converted to an imine even though the carbon–nitrogen double bond of the product is significantly weaker than the carbon–oxygen double bond in the reactant. The interviewer in this case would have the critical role of gently and kindly challenging the students' “less-than-optimal” conceptions with additional data in hopes of steering students towards more scientifically-valid explanations. Such a study would also fill a major void in the research literature since a recent review by Bain et al. (2014) shows that there is a gap regarding students' conceptions of thermodynamics outside of the physical chemistry context.As we mentioned previously, the problem-solving model proposed in Fig. 4 is a tentative one requiring more refinement and greater detail about each of the steps. Perhaps the biggest decision point in the flowchart is at the stage where the student must decide whether the transformation is single- or multi-step. Making this judgment would involve, at least in part, a student's assessment of the similarity of the given transformation with one retrieved from long-term memory. We are currently studying this process using multiple paper-and-pencil tasks. A corollary to this research would be to use eye-tracking protocols to gain greater insight into the part(s) of a transformation on which the student fixates while making a similarity judgment. Additionally, eye-tracking research would also yield much more evidence about how students execute each phase of their EPF tasks, especially during the mapping phase.
Appendix A: proposing reaction mechanisms in the absence of traditional cues
(Bhattacharyya and Bodner, 2000, 2005)We investigated how students propose mechanisms to reactions in the absence of the typical cues indicating common classes of reactions. The theoretical framework for this study was phenomenography which Marton (1994) defines as “…the empirical study of the limited number of qualitatively different ways in which various phenomena in, and aspects of, the world around us are experienced, conceptualized, understood, perceived and apprehended” (p. 4424). The participants in this study were recruited from a 1st-semester graduate-level organic chemistry course, “Advanced Organic Chemistry”. Of the fourteen graduate students who participated one of the students had an MS in Chemistry and one was a 4th-year analytical chemistry student; the others were 1st-year chemistry graduate students with BS degrees.
The participants were audiotaped as they used EPF to propose mechanisms of reactions that were two- to four-step variants of traditional reactions, such as SN1 and SN2 substitution, shown in the figure on the following page. The interviewer's role was to remind participants to verbalize their thoughts and to ask follow-up questions to better capture the students' conceptualizations. The three sources of data – interview transcripts, field notes, and written solutions – were carefully and repeatedly examined and coded to generate emergent themes. These themes were further subdivided into categories based on the characteristics of the data (Patton, 1990). The final conclusions were discussed with the professor who taught the course as a validity check, to ensure that the researchers' interpretations of the data were consistent with his experiences with the students.
Appendix B: organic synthesis problem-solving
(Bhattacharyya, 2004)Another important facet of organic chemistry is the synthesis of complex molecules from readily available, simpler starting materials. While the bulk of the work in organic synthesis occurs in a laboratory, there is a significant planning component done on paper, in which chemists propose a stepwise strategy for assembling the target molecule. It was this paper-based exercise which was the focus of this study (Bhattacharyya, 2004). I used ethnomethodology – which strives to understand how members of a group make sense of their routine activities – as the theoretical framework to study four first-year graduate students enrolled in a course focused on organic synthesis and two third-year graduate students who were preparing for the oral component of their PhD candidacy exams. Both sets of participants were in the process of preparing proposals for the synthesis of molecules like those shown in the figure below. (Showing the exact molecules the participants used could compromise their anonymity.)
The data for this study included transcripts of audio-taped individual interviews with the students, the written artifacts generated by the participants during the interviews, and the interviewer's field notes. Each first-year participant was interviewed between eight and ten times and each third-year participant between six and eight times. The goal of these interviews was to get the participants to account for each and every decision, thereby revealing their sense-making processes as the participants became acculturated into the organic synthesis community of practice. The final interview, which was conducted after participants had submitted their work and received feedback either from the course professor, in the case of the 1st-year students, or the respective PhD examining committees, in the case of the 3rd-years, also served as a member-checking interview to obtain one measure of validity for this research (Patton, 1990).
The three sources of data – transcripts, researcher notes, and artifacts – for each participant were repeatedly examined to establish a chronology of events for each individual. Chronology was deemed important because the analysis of when an individual acts can be as important as the act itself in an ethnomethodological approach (Brandt, 1992). From those chronologies, emergent themes were generated for each of the sample populations – the 1st-year and 3rd-year students – and these themes were then compared to find similarities and differences in the problem-solving approaches of the two groups. An overarching theme emerged from the comparison of the two data sets that summarized the experiences of all participants, leading to the final conclusions of the study.
Appendix C: conceptions of Brønsted acidity
(Bhattacharyya, 2006)Brønsted acid–base theory is one of the core models used by practicing organic chemists. One of the unique uses of this construct is that it can help determine the relative stabilities of basic species, which are typically anionic. This information, in turn, can be used to predict the outcomes of a wide variety of reactions, not just simple acid–base ones. For this study, therefore, we used the model-eliciting activity (MEA) shown in the figure below to investigate ten advanced organic chemistry graduate students' conceptualizations of Brønsted acidity. The choice of the specific molecules in the MEA was based on several considerations. First, I wanted to use this construct to also investigate students' ability to engage in multi-variate thinking. It was important, therefore, to choose molecules from a single functional group so that students could not attribute differences in acidity to differences in the atom to which the molecule's most acidic proton was bonded. Second, I chose alcohols because a familiar, and relatively simple, set of molecules could be used to showcase the effects of the main variables: steric, resonance, and inductive (polar) effects.
The participants were interviewed using the think-aloud protocol as they worked on the MEA (Ericsson and Simon, 1984). No resources were made available during the interviews as the goal was to elicit the participants' personal models of organic acids, although the interviewer told the participants that he would answer specific content-related questions. Since the object of this research was to elicit the participants' mental models, the unit analysis was the individual. After using the data sources – verbatim transcripts of interviews, interviewer's notes and observations, and written artifacts – to create individual models there was a striking similarity between them. As such, a composite model was generated and subsequently used to guide the remainder of the data analysis, which was further examination of the data sources to determine the concepts and principles invoked by the participants in support of their models. All of the results were discussed with the participants' research mentors as one form of a validity check.
The model-eliciting activity (MEA) that was used to probe the participants' conceptualizations of acids| Substance | pKa in H2O |
|---|---|
| Water | 15.7 |
| Methanol | 15.1 |
| Ethanol | 15.9 |
| Propanol | 16.2 |
| Butanol | 16.1 |
| iso-Propanol | 17.1 |
| t-Butanol | 19.2 |
| F3C–CH2–OH | 12.4 |
| F3C–CH2–CH2–OH | 14.6 |
| F3C–CH2–CH2–CH2–OH | 15.4 |
| Phenol | 9.95 |
| para-Nitrophenol | 7.14 |
| para-Methoxyphenol | 10.20 |
“One evening, while you are working in the lab, a fellow graduate student in the organic division calls and asks you to explain the trends in the acidities of these compounds. To help her out, create a set of rules that could explain acidities of organic molecules from these data” (Bhattacharyya, 2006, p. 241).
Appendix D: cueing in mechanistic problem-solving
(Kraft et al., 2010)In this study we wanted to investigate the cues students use to solve mechanism tasks. Some of our previous work suggested that expert organic chemists are cued to a models-based reasoning approach when solving unfamiliar mechanism tasks. As used by the experts, models-based reasoning is a way of reducing the number of variables dictating the outcome of the reaction. As such, we were interested in understanding the reasoning processes students use when solving similar tasks. This project, therefore, combined our ongoing interests in multi-variate reasoning and mechanistic problem-solving.
Sixteen graduate students – four 1st-year students in an organic synthesis course and twelve advanced students – completed two tasks each with multiple parts. In Task 1 students were given six partially-completed mechanisms and asked to complete the remaining steps (Fig. 6). We purposely chose to limit the information in these tasks because one of the goals of this project was to assess the students' ability to interpret information contained in electron-pushing diagrams. In Task 2 students were asked to predict the major product(s) of three reactions (Fig. 7). Our goal was to see whether students could apply mechanistic reasoning to solve a problem. To help ensure that students could not answer these questions by simple recall, each of these reactions involves at least one intramolecular step.
Using the think-aloud protocol (Ericsson and Simon, 1984), each student participated in a single audio taped, semi-structured interview lasting between 60 and 75 minutes. Although there were four different sets of tasks in the interview, only two, Tasks 1 and 2, are discussed here. (The other two are discussed in Appendix E.) The data for each participant – verbatim interview transcripts, field notes in the form of the interviewer's observations during the interview, and student written artifacts – were partitioned by each part of each task.
Using a rubric, two of the researchers independently scored the students' written responses and identified one of three reasoning modes based on the participant's utterances about that particular part of a task. When they met to compare their assessments, any disagreements on either score or reasoning mode were discarded, leaving 125 out of 144 responses for further analysis. The remaining data were inductively analysed for trends in use of reasoning mode and the students' success as a function of said reasoning mode. These trends were further grouped into a set of assertions, which were then discussed with a random sample of four participants as a form of member-checking.
Appendix E: mental models of reactions and reactivity
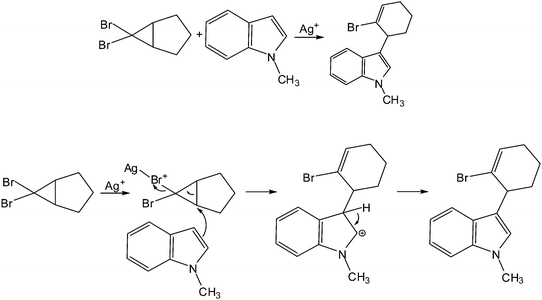
(Strickland et al., 2010)As a companion to the study described in Appendix D the goal of this part of the data was to investigate what students understand from diagrams of reactions and their mechanisms. The same 16 students who took part in the previous study participated in this one. The main instrument of this study was a set of diagrams in which students were given an overall reaction followed by the electron-pushing mechanism, an example of which is shown in the figure below. The students were asked to verbally describe each step of the mechanism and then the overall transformation for a total of 7 reactions. To help the students understand the directions the interviewer used the transformation in the figure shown as an example and described the steps as: (1) Lewis acid–base reaction; (2) Addition followed by elimination; and (3) elimination; and the overall reaction as electrophilic aromatic substitution of the indole ring. Before the students were given these reactions, however, they were asked to define the terms, functional group; nucleophile/electrophile; and acid/base, since the participants would be likely to use these terms during their descriptions of the diagrams.
For this study, the data consisted of verbatim interview transcripts and interviewer field notes. The chemical terminology were grouped by term for all the participants and were independently analyzed for emergent trends by two members of the research team. The same two members independently analyzed each participant's descriptions of the electron-pushing diagrams. After meeting to discuss their findings, only trends that were identified by both researchers, or could be agreed upon by both researchers, were considered for further analysis. The combined codes were further analyzed for assertions that could describe the overall data.
Appendix F: structural representations of physical and chemical characteristics
(DeFever et al., 2014)Based on the results of our previous work indicating that the participants had impoverished understandings of the representations in the electron-pushing diagrams, this project was an attempt to determine the extent to which this issue was due to their knowledge of the types of information that could be inferred from chemical representations. Thus, we investigated the structural features students attribute to physical and chemical characteristics by interviewing eight senior chemistry majors about a month from graduation with their Baccalaureate degrees. In the first interview students were asked what structural features they attributed to the following terms or characteristics: solid, liquid, gas, polar molecule, soluble in water, high boiling point, high melting point, nucleophilicity, electrophilicity, acidity, and basicity. They were also, subsequently, asked to provide specific examples of a hydrocarbon, oxygen-containing compound, and nitrogen-containing compound for each of these terms. The results of the first interview were analyzed much like the terminology in the study described in Appendix E.
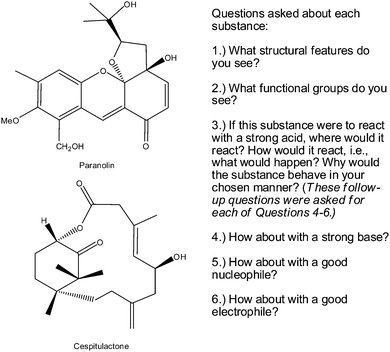
We followed up the results of the first interview with a second in which we asked students to work on two different tasks, which are shown in Fig. 8 and 9, respectively. In the first task we asked students to identify the structural features and functional groups of each of the molecules (Fig. 8). We distinguished the terms “structural feature” and “functional group” because it could have been possible that a student may have noticed the large ring in the second molecule but not have identified it as a functional group, per se. We also asked the students to predict how the molecule would interact if placed in the following conditions: strong acid, strong base, strong nucleophile, and strong electrophile. We did not give them specific reagents because we did not want memory of specific content knowledge to be a major determinant of the students' ability to answer the questions. In the second task, we asked students to provide compounds that would likely fit the given characteristics (Fig. 9). Note that the parameters were purposely chosen so that they appear to be almost contradictory, at least with respect to the typical compounds students tend to associate with the individual characteristic.
Once again, the interviews were audiotaped, with the interviewer recording salient observations and post-interview notes. Since content knowledge can be a confounder when students work on problem-solving tasks, the participants were given a table of atomic weights and one of the pKas of the common organic chemistry functional groups. Students were also provided with a copy of an organic chemistry textbook and Aldrich catalog as in the first interview, along with paper and writing implements.
The data – verbatim interview transcripts, any written work produced by the participants, and interviewer notes – were independently analyzed by two members of the research team by participant for problem-solving strategy in case of the design problems and for correctness for both parts of the interview tasks. To see if there were any common trends in students' constructions of these ideas, the data were also analyzed by task, just as in the previous interview. Once again, trends only common to both researchers' independent analysis were further grouped into larger categories, which were used to generate final assertions.
References
- Anderson J. P., (2009), Learning the language of organic chemistry: how do students develop reaction mechanism problem-solving skills? Doctor of Philosophy, West Lafayette, Indiana: Purdue University.
- Anderson T. L. and Bodner G. M., (2008), What can we do about Parker? A case study of a good student who didn't “get” organic chemistry, Chem. Educ. Res. Pract., 9, 93–101.
- Bain K., Moon A., Mack M. and Towns M., (2014), A review of research on the teaching and learning of thermodynamics at the university level, Chem. Educ. Res. Pract., DOI: 10.1039/c4rp00011k.
- Bhattacharyya G., (2004), A recovering organic chemist's attempts at self-realization: how students learn to solve organic synthesis problems, Doctor of Philosophy, West Lafayette, Indiana: Purdue University.
- Bhattacharyya G., (2006), Practitioner development in organic chemistry: how graduate students conceptualize organic acids, Chem. Educ. Res. Pract., 7, 240–247.
- Bhattacharyya G., (2013), From source to sink: mechanistic reasoning using the electron-pushing formalism, J. Chem. Educ., 90, 1282–1289.
- Bhattacharyya G. and Bodner G. M., (2000), Unpublished data.
- Bhattacharyya G. and Bodner G. M., (2005), “It gets me to the product”: how students propose organic mechanisms, J. Chem. Educ., 82, 1402–1407.
- Brandt D., (1992), The cognitive as the social: an ethnomethodological approach to writing process research, Writ. Comm., 9, 315–355.
- Bruice P. Y., (2007), Organic Chemistry, 5th ed, Prentice Hall: Boston.
- DeFever R., Bruce H. and Bhattacharyya G., (2014), manuscript in review.
- Doerr H., (2000), How can I find a pattern in this random data? The convergence of multiplicative and probabilistic reasoning, J. Math. Behav., 18, 431–454.
- Ericsson K. A. and Simon H. A., (1984), Protocol analysis: verbal reports as data, Cambridge, MA: The MIT Press.
- Ferguson R. and Bodner G. M., (2008), Making sense of the arrow-pushing formalism among chemistry majors enrolled in organic chemistry, Chem. Educ. Res. Pract., 9, 102–113.
- Garvin-Doxas K. and Klymkowsky M., (2008), Understanding randomness and its impact on student learning: lessons learned from building the Biology Concept Inventory (BCI), Cell Biol. Educ. (CBE) – Life Sci. Educ., 7, 227–233.
- Grove N. and Bretz S. L., (2010), Perry's scheme of intellectual and epistemological development as a framework for describing student difficulties in learning organic chemistry, Chem. Educ. Res. Pract., 11, 207–211.
- Grove N., Cooper M. and Rush K., (2012a), Decorating with arrows: Toward the development of representational competence in organic chemistry, J. Chem. Educ., 89, 844–849.
- Grove N., Cooper M. and Cox E., (2012b), Does mechanistic thinking improve student success in organic chemistry? J. Chem. Educ., 89, 850–853.
- Hoefer C., (2010), “Causal Determinism”, in Zalta E. N. (ed.), The Stanford Encyclopedia of Philosophy, Spring 2010 edn, http://plato.stanford.edu/archives/spr2010/entries/determinism-causal/.
- Kraft A., Strickland A. and Bhattacharyya G., (2010), Reasonable reasoning: multi-variate problem-solving in organic chemistry, Chem. Educ. Res. Pract., 11, 281–292.
- Kuhn D., (2007), Reasoning about multiple variables: control of variables is not the only challenge, Sci. Educ., 91, 710–726.
- Kuhn D., Pease M. and Wirkala C., (2009), Coordinating the effects of multiple variables: a skill fundamental to scientific thinking, J. Exp. Child Psychol., 103, 268–284.
- Lowry T. and Richardson K., (1987), Mechanism and theory in organic chemistry, 3rd edn, New York: Harper and Row.
- Marton F., (1994), Phenomenography, in Husen T. and Postlethwaite T. N. (ed.), The international encyclopedia of education, Oxford: Pergamon.
- McClary L. and Talanquer V., (2011), College chemistry students' mental models of acids and acid strength, J. Res. Sci. Teach., 48, 396–413.
- Morrison R. T. and Boyd R. N., (1959), Organic chemistry, Boston: Allyn and Bacon.
- Nakhleh M. B. and Mitchell R. C., (1993), Concept learning versus problem solving: there is a difference, J. Chem. Educ., 70, 190–192.
- Patton M., (1990), Qualitative research and evaluation methods, 2nd edn, Thousand Oaks, CA: Sage.
- Perry W. G. Jr., (1968), Forms of intellectual and ethical development in the college years: a scheme, New York: Holt, Rinehart, and Winston.
- Rushton G., Hardy R. C., Gwaltney K. and Lewis S., (2008), Alternative conceptions of organic chemistry topics among fourth year chemistry students, Chem. Educ. Res. Pract., 9, 122–130.
- Sandí-Ureña S. and Cooper M., (2009), Design and validation of an inventory to assess metacognitive skillfulness in chemistry problem solving, J. Chem. Educ., 86, 240–245.
- Sandí-Ureña S. and Cooper M., (2011), Enhancement of metacognition use and awareness by means of a collaborative metacognitive intervention, Int. J. Sci. Educ., 33, 323–340.
- Sandí-Ureña S., Cooper M. and Stevens R., (2012), Effect of cooperative problem based lab instruction on metacognition and problem solving skills, J. Chem. Educ., 89, 700–706.
- Strickland A., Kraft A. and Bhattacharyya G., (2010), What happens when representations fail to represent? Graduate students' interpretations of organic chemistry diagrams, Chem. Educ. Res. Pract., 11, 293–301.
- Talanquer V., (2006), Commonsense chemistry: a model for understanding students' alternative conceptions, J. Chem. Educ., 83, 811–816.
- van Merriënboer J. and Sweller J., (2005) Cognitive load theory and complex learning: recent developments and future directions, Educ. Psychol. Rev., 17, 147–177.
Footnotes |
| † Present address: Department of Chemistry, Missouri State University, 901 S. National Avenue, Springfield, MO 65897, USA. E-mail: gautamb@missouristate.edu |
| ‡ Although the terms electron-pushing formalism, arrow-pushing formalism, and curved-arrow formalism may have different meanings for some readers they are assumed to be synonyms for the purposes of this manuscript. |
| This journal is © The Royal Society of Chemistry 2014 |