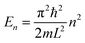Learning quantum chemistry via a visual-conceptual approach: students' bidirectional textual and visual understanding†
Vered
Dangur
a,
Shirly
Avargil
a,
Uri
Peskin
b and
Yehudit Judy
Dori
*ac
aDepartment of Education in Science and Technology, Technion, Israel Institute of Technology, Israel. E-mail: yjdori@technion.ac.il
bSchulich Faculty of Chemistry, Technion, Israel Institute of Technology, Israel
cElectrical Engineering and Computer Science Department, Massachusetts Institute of Technology, USA
First published on 21st February 2014
Abstract
Most undergraduate chemistry courses and a few high school honors courses, which focus on physical chemistry and quantum mechanics, are highly mathematically-oriented. At the Technion, Israel Institute of Technology, we developed a new module for high school students, titled Chemistry – From “the Hole” to “the Whole”: From the Nanoscale to Microelectronics. The module is based on a qualitative approach to teaching quantum chemistry, emphasizing interdisciplinary real-life applications and integration of visualization. While aimed at honors high school chemistry students, the module was also partially implemented and assessed in an undergraduate chemistry course. The research objective was to investigate the effect of the module on the visual and textual understanding of quantum mechanical concepts among 122 honors and 65 volunteer undergraduate chemistry students. The research tools included students' pre- and post-questionnaires. High school honors and undergraduate students, who were exposed to the module, significantly improved their textual and visual understanding of quantum mechanical concepts and their ability to move across illustrations and explanations. Honors and undergraduate students minimized the gap that had existed between them in terms of integrating micro and quantum levels into their post-questionnaire answers. Our findings augment the current set of the four chemistry understanding levels – macro, micro, symbol and process – by adding the quantum mechanical level as a fifth level of chemistry understanding. The study contributes to teaching physical chemistry by providing a tool for learning, assessment, and research of chemistry understanding via both visual and textual modes.
Introduction
The conventional approach to teaching undergraduate physical chemistry and quantum mechanics courses is mathematically-oriented, and often lacks emphasis on qualitative understanding of underlying principles. We introduce a visual-conceptual approach to teaching quantum mechanical principles to honors high school students, which can also benefit undergraduate students. Our approach integrates visual representations with textual explanations that focus on understanding chemical principles and concepts. Research over the past decade has indicated the existence of difficulties in teaching and learning topics related to quantum mechanics in both physics and chemistry courses (Ireson, 2000; Taber, 2005; Papaphotis and Tsaparlis, 2008). These difficulties stem from the fact that quantum mechanics involves abstract concepts and complex theories. Integration of visual representations might alleviate these difficulties by promoting better understanding and meaningful learning of science. In line with this approach, we have developed, implemented, and assessed a new learning module that focuses on quantum chemistry, titled Chemistry – From “the Hole” to “the Whole”: From the Nanoscale to Microelectronics‡ (Sasson et al., 2007). The module includes abstract topics that rely on quantum mechanics theories with an emphasis on a qualitative approach and integration of visualizations. The research described here explores the effect of learning quantum chemistry through this qualitative approach and the integration of visualizations on developing textual and visual chemical understanding among chemistry high school honors and undergraduate students. As we were studying the students' responses, we realized that a fifth chemistry understanding level—the quantum level—can and should be added to the current four levels. We elaborate on this in the discussion section.Theoretical background
Research on difficulties in teaching and learning quantum mechanical concepts calls for strategies that promote students' conceptual understanding and minimize their misconceptions regarding the atomic structure. Our learning module integrates visualization and the current four chemical understanding levels—macroscopic, microscopic, symbol, and process—into learning quantum chemistry; thus, we review the literature concerning the teaching and learning of quantum mechanical concepts as well as literature that emphasizes visualizations and the four chemistry understanding levels as key aspects for teaching and learning chemistry for conceptual understanding.Teaching and learning quantum mechanical concepts
Researchers point out difficulties and obstacles in teaching and learning quantum mechanical concepts at the high school and university levels (Harrison and Treagust, 1996; Stefani and Tsaparlis, 2009). Findings indicate that high school students, college students studying for university entrance level exams, and students at the undergraduate level experience difficulties rethinking their previous knowledge while creating alternative conceptions of the atom model, orbitals, energy levels, and related ideas (Harrison and Treagust, 1996; Petri and Niedderer, 1998; Taber, 2005; Papaphotis and Tsaparlis, 2008).One of the most prevalent reported obstacles is the difficulty in understanding abstract concepts. Students in middle and high school tend to depict atoms and molecules as discrete and concrete structures and thus prefer illustrations of the orbit model and planetary (also referred to as ‘solar system’) model of the atom (Harrison and Treagust, 1996; Cokelez, 2012). They usually use the word ‘orbital’ as a synonym for an ‘orbit’ and think that electrons rotate around the nucleus like the planets rotate around the sun (Tsaparlis and Papaphotis, 2002). Tsaparlis (1997) raised the question whether quantum mechanical concepts should be taught at the secondary school and cited Gillespie (1991, 1996) who recommended emphasizing electron density and VSEPR rather than concepts such as orbitals. These recommendations were for teaching both secondary school students and even introductory courses for students at the college and university level. Taber (2005) argued that even when college students, who are studying for university entrance level exams, embrace the term orbital, some still tend to use it to re-label their existing conceptions of electron ‘path’ as it ‘circles’ the nucleus. Cokelez (2012) showed that some students in junior and high school illustrate what he called ‘composition models’, which are more complex and abstract. Yet he claimed that students' knowledge of and experience with the atomic model is not sufficient, and that the atomic model theory should be part of the curriculum in all the science domains.
Tsaparlis and Papaphotis (2002) argued that high school students fail to understand the probabilistic nature of atomic orbitals and retain a deterministic perspective. Students do not fully understand atomic and molecular orbitals. They are confused between the various atomic and orbital representations and misinterpret them. Furthermore, undergraduate students have difficulties identifying the mathematical characteristics of quantum mechanical concepts and the different forms of mathematical equations related to these concepts (Tsaparlis, 1997; Stefani and Tsaparlis, 2009).
One of the problems that lead to students' misconceptions about the atomic model and the quantum mechanical concepts is that students at both high school and undergraduate levels tend to develop hybrid models (Justi and Gilbert, 1999; Taber, 2001a; Stefani and Tsaparlis, 2009). Students merge and use in parallel different models, such as aspects of historical models and aspects of the current acceptable model of the atom. They create a mental model that integrates different models which they treat as coherent (Petri and Niedderer, 1998; Justi and Gilbert, 1999; Justi and Gilbert, 2000, Papaphotis and Tsaparlis 2008).
Learning the atomic model by depicting the planetary model or Bohr model might be useful for middle and high school students, helping them in integrating the idea of shells. However, a learning progression approach should be considered, where a scheme of four stages of conceptual understanding of the atomic structure and electrical forces are introduced. The highest stage of understanding includes quantum mechanics models. Only few students described atomic models and electrons in terms of probability and electron density, while others kept using language that is generally associated with classical mechanics to describe electron motion (Stevens et al., 2010).
Understanding the concepts of ‘probability of finding electrons’ and ‘energy quantization’ is necessary as a basis for comprehension of the quantum mechanics model of the atomic structure when learning at the undergraduate level (Park and Light 2009). However, high school chemistry textbooks usually do not provide learners any reason to accept the quantum mechanics model instead of the Bohr model (Shiland, 1997).
Looking at examples from the literature of teaching quantum chemistry, there are different strategies that were found beneficial to some extent. These include a qualitative teaching approach (Kalkanis et al., 2003), use of simulations and animations for teaching nanotechnology based on quantum theory (Xie and Lee, 2012), and learning with devices whose mechanism can only be explained by conceptually understanding quantum chemistry (Zollman et al., 2002; Green et al., 2009).
Our unique approach integrates extensive use of a variety of visualizations with everyday applications and a visual-conceptual teaching approach. Furthermore, it caters to both high school and undergraduate students. Concepts are taught over a relatively long period of time with multiple experiences with quantum chemistry concepts. This potentially leads to deep conceptual understanding of atomic and molecular structures.
Visualization in learning sciences and chemistry
Visual representations, such as graphs, diagrams, visual models, and inscriptions, play a key role in teaching and learning sciences (Barak and Dori, 2005; Dori and Belcher, 2005; Kozma and Russell, 2005a). ‘Representational competence’ is part of meaningful understanding of chemistry concepts and includes the ability to use a variety of representations or visualizations, including communicating, selecting appropriate representations, using them for explaining chemical phenomena, and integrating new knowledge with existing knowledge (Roth et al., 1999; Kozma and Russell 2005a). Bowen and Roth (2005) claimed that representations that use mathematical equations are highly abstract, and that students find them to be the most difficult feature of science learning (Roth and Bowen, 1994).There are several key principles, suggested by Wu and Shah (2004), that can assist students with understanding chemistry concepts and to develop representational skills and visuospatial thinking. These include (1) exposing students to the same information in a multitude of formats and descriptions, (2) making linked referential connections between representations visible in order to enable students to construct conceptual connections from these multiple representations, (3) presenting the dynamic and interactive nature of chemistry, and (4) encouraging the transformation between 2D and 3D. Mayer (2002) described learning processes in which students use words and pictures based on three assumptions (a) people use the visual-pictorial channel and the auditory-verbal channel, (b) these channels can become overloaded when too many words and pictures are involved in the learning, and (c) meaningful learning occurs when students are actively engaged and process their learning via practicing the two channels.
Researchers also found that computer-based visualization tools and computerized molecular modeling may help students improve their ability to make transformations between 2D and 3D models. In turn, this ability may help students develop understanding of chemical principles (Wu et al., 2001; Kaberman and Dori, 2009). When it comes to quantum theoretical concepts, visualization enables the construction of a richer set of concepts (Gunel et al., 2006). It can also be used to identify students' mental models of the particulate nature of matter (Ben Zvi et al., 1987; Margel et al., 2004; Nyachwayaa, et al., 2011) and of atoms and molecules while investigating students' illustrations. Specifically, visualization can enhance students' understanding of the shape and size of the atom and the probabilistic nature of the atomic orbitals (Papaphotis and Tsaparlis 2008; Park and Light, 2009; Cokelez, 2012).
Chemistry understanding levels
Students' chemical understanding can be evaluated by their comprehension of and transfer between the four levels, which include the macro, micro, symbol, and process levels (Dori and Hameiri, 2003; Kaberman and Dori, 2009). Initially, researchers had argued that chemistry is taught and understood at three levels: the microscopic, also referred to as sub-microscopic – the particulate nature of matter level; the macroscopic – the sensory level; and the symbolic, which is also referred to as the representation level (Johnstone, 1991; Gabel, 1993, 1998). Dori and Hameiri (2003) have added the fourth, process level of chemistry understanding, which accounts for the dynamic nature of chemical reactions and the relationship between the macroscopic, microscopic and symbolic levels. Jensen (1998) proposed a model, which he described as “the logical structure of Chemistry" (p. 680). This model refers to chemists and teachers as they view the logical organization of chemistry, although it can be translated by teachers into their teaching, it does not refer specifically for students. This is unlike the four chemistry understanding levels, which can be used by students as a metacognitive tool for monitoring their understanding of chemistry concepts and processes (Kaberman and Dori, 2009). Jensen's model included three levels: molar, molecular and electricity. The molar level in Jensen's model had similar meaning as the macroscopic level, and the molecular level is what we refer to as the microscopic level. Jensen did not mention the process level (which was added by Dori and Hameiri (2003)) but we can see some resemblance between the process level and the time dimension mentioned in Jensen's article, since they both have the dynamic aspect we discuss when we refer to the process understanding level.Researchers have claimed that understanding microscopic and symbolic representations is especially difficult for students because these representations are invisible and abstract, while students' thinking relies mainly on sensory information (Ben Zvi et al., 1987; Gabel, 1993; Wu et al., 2001). Still, meaningful learning may be enhanced through visualizations, including physical models, computerized visualizations, animations, and drawings. Visualizations contribute to improving students' ability to use the chemistry understanding levels and move across them (Barnea and Dori, 1999; Dori et al., 2003; Kozma and Russell, 2005b).
Chemistry understanding levels also serve as a metacognitive tool for students when they explain chemistry phenomena (Herscovitz et al., 2012). In his review, Taber (2001b) described the difficulty students face when they need to traverse the different levels and the importance of using the macro, representation, and sub-micro levels. When discussing quantum chemistry, Taber (2001b) cited a PhD dissertation (Van Hoeve-Brouwer, 1996) that had suggested adding the quantum level.
In this study, we propose adding a fifth, quantum chemistry understanding level on top of the four we have been using in previous studies (Dori and Hameiri, 2003; Dori and Sasson, 2008; Kaberman and Dori, 2009) and use it for both teaching and assessment.
Research objectives
Considering the difficulties students encounter while learning quantum chemistry and the potential of visualization combined with understanding chemistry at different levels to improve conceptual understanding, we focused on the following research objectives:(1) Investigating the effect of the visual-conceptual approach in the new module on high school honors chemistry and undergraduate students' understanding of quantum mechanical concepts, and
(2) Comparing the learning outcomes of three research sub-groups in terms of visual and textual understanding of chemistry in general and of quantum mechanics in particular.
Context
The new module for honors high school students, Chemistry – From the Nanoscale to Microelectronics, developed at the Technion, includes abstract topics from quantum mechanics theory. It focuses on chemical properties derived from the electronic structure of substances: orbitals in atoms and molecules, and energy bands in bulk semiconductors. The latter topic had never been taught in honors chemistry courses in Israel; therefore, no comparison group could have been established.There are few research articles on teaching semiconductors to high school students. The majority of research articles have appeared in engineering and physics publications, which focus on quantitative, mathematical, and device aspects, rather than chemical aspects (Yeow and Ling, 1999; Santos et al., 2009). When the chemical aspects of a semiconductor are described, the explanations are based on the classic model and apply concepts such as covalent bonds, additional electrons, and “holes” rather than the quantum model (Ren et al., 2008; García-Carmonaa and Criado, 2009). Teaching semiconductors as an application of the electronic structure of solids using quantum theory has received almost no attention in the literature.
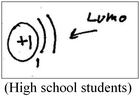
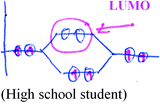
While conventional approaches to teaching undergraduate physical chemistry and quantum mechanics courses are mathematically-oriented, we have adopted a visual-conceptual approach to teaching quantum chemistry. This approach is of a qualitative nature; it emphasizes interdisciplinary real-life applications and integrates ample visualizations. For example, while learning the topic of the electronic structure of molecules and its key concepts, such as molecular orbitals, bonding, anti-bonding, HOMO (Highest Occupied Molecular Orbital), and LUMO (Lowest Unoccupied Molecular Orbital), students investigate questions like “why do organic pigments have colors?”, and the phenomenon of stick lights is explained. The characteristics of the module include numerous visualizations and animations, which are exemplified in Appendix S1 (ESI†).
Since most high school students lack proper background in mathematics at the required level, our qualitative approach helps bypass the introduction of mathematical aspects of quantum theory. While aimed at honors high school students, as noted, this module was also assessed and used during an undergraduate mathematical-oriented quantum chemistry course.
Research participants
The research participants were (1) 12th grade chemistry honors students from eight high schools in Israel over two academic school years (N = 122), who studied the module in its entirety and (2) undergraduate students (N = 65) who volunteered to participate in the research and studied the module in one academic year as part of a quantum chemistry course titled Introduction to Quantum Mechanics and its Applications in Chemistry.The undergraduate students were majors in three domains: chemistry, biochemistry, and electrical engineering. The honors students were divided into two sub-groups: intermediate- and high-academic level. The undergraduate students were also divided into two sub-groups: sub-group 1, “Visual-Conceptual-oriented” (ViCo-oriented) – students who participated in a short-term enrichment course that included the topics of the module, focusing on a visualization and qualitative approach, and sub-group 2, “Mathematical-oriented” (Math-oriented) – students who participated in a short-term mathematical-oriented enrichment course, which included topics taught in the quantum mechanics course.
The undergraduate students took the enrichment course in addition to the regular academic course. The course comprised of six sessions taught over three weeks. The students chose to participate in sub-group 1 or sub-group 2 based on their academic schedules, without being provided with any specific information about the content of the enrichment given to each sub-group.
Research tools
The research tools included pre- and post-questionnaires aimed at assessing thinking skills and chemistry understanding. The high school students responded to the pre-questionnaires prior to the onset of the module, and the post-questionnaire was administered to the students at the completion of the module. The undergraduate students responded to the pre-questionnaire during the first week of the semester and to the post-questionnaire during the semester after they had finished the enrichment course.The pre- and post-questionnaires, which included open-ended questions, were administered for the purpose of assessing two thinking skills: (a) the ability to apply visual and textual modes to explain chemical phenomena in terms of quantum mechanical concepts, and (b) the understanding of various representation and visualization modes, including molecular drawings, graphs, diagrams, and illustrations.
The pre-questionnaire was based on topics from chemical structure and bonding, while the post-questionnaire focused on the quantum mechanical content. In order to assess students' knowledge and the above two thinking skills, students were requested to perform the following three assignments.
(A) Choose one of the concepts from the list below and describe it using a drawing, a diagram, or a model.
(B) Choose another concept and explain it textually using an example.
(C) Explain a given illustration (see example in Appendix S2 (ESI†), which relates to the ability to explain the concepts of bonding and anti-bonding molecular orbitals).
Concepts for assignment A and B included atomic orbital, molecular orbital, energy levels, LUMO orbital, and N type semiconductors.
For the purpose of assessing students' ability to describe and explain new concepts using both visual and textual modes, and to understand representations and visualizations, we developed a dedicated assessment tool for each of the three assignments. The tool (see Appendix S3, ESI†) consisted of a detailed rubric designed to assess both the textual and the visual modes. Each mode was graded independently using qualitative content analysis followed by a quantitative score. Although ‘assignment A’ was aimed to assess the visual mode and ‘assignment B’ was aimed to assess the textual mode, students often used both modes in the assignment. In these cases we assessed the two modes used in the same assignment and the extent to which they matched (see Appendix S3, ESI†).
Each assignment set was scored separately according to several categories, some of which were identical across the three assignments.
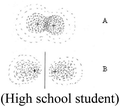
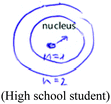
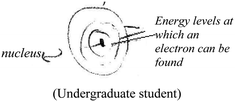
A common criterion to both modes was the correctness of the textual or visual (drawing or illustration) explanation. A non-quantum mechanical illustration, such as depiction of an atom using historical models, the nuclear model (Park and light, 2009), the Rutherford (planetary) type model, (Wheeldon, 2012), or a planetary or solar system model (Harrison and Treagust, 1996; Taber 2001a; Stevens et al., 2010), was categorized as a “naïve illustration”. Additional categories, specific to the visual mode, were the number of illustrations and the complexity (richness) of each illustration. Richer and more detailed illustrations were assumed to reflect deeper understanding of the concept the students had chosen to explain and better visual capability to draw a meaningful representation of that concept.
Understanding of chemistry on both the textual and the visual modes was assessed via the four chemistry understanding levels using a method developed in prior research for textual responses (Dori and Sasson, 2008; Kaberman and Dori, 2009). Analyzing the results from students' pre- and post-questionnaires, we added the quantum-mechanical level as a fifth chemistry understanding level. This level includes the ability to describe the electronic structure of atoms, molecules, and solid state materials in terms such as discrete energy levels, atomic and molecular orbitals, energy bands, and absorption and emission spectra, all stemming from the theory of quantum mechanics.
Each of the assignments A, B, and C was analyzed separately. In each assignment performed by each student, the number of micro explanations, quantum explanations, or the combination of both were counted. In the findings section, we present the distribution of the number of the micro explanations, quantum explanations, and combined micro and quantum explanations as the sum of explanations students in each of the three groups gave in the three assignments. Table 1 presents examples of students' responses for each of the assignments.
The overall score in each assignment was summarized and normalized on a 1-to-100 scale. The average score of the three assignments was statistically analyzed. One-tenth of all the students' responses were scored and validated by four chemistry educational experts, achieving 90% inter-raters reliability.
Findings
The questionnaires were analyzed, first qualitatively and then quantitatively, using the assessment tool presented above. In this section, we present the qualitative findings followed by the quantitative findings of understanding quantum mechanical concepts and principles regarding the textual and visual chemical understanding.Understanding concepts and principles in quantum mechanics – qualitative analysis
The content analysis of students' responses revealed interesting new combinations in describing the concepts. Students used several levels of understanding, particularly the micro and quantum levels. The students used a variety of models, some of which demonstrated deep understanding of the explained concept, while others mixed previous, non-quantum models, indicating incomplete conceptual understanding (Justi and Gilbert, 1999; Taber, 2001a; Stefani and Tsaparlis, 2009).The qualitative analysis exposed a need to teach three subjects: the electronic structure of the atom, the electronic structure of the molecule, and the solid state.The qualitative analysis presented in Tables 2–4 has shown that students' perception of models related to quantum mechanics consisted of different types of models, ranging from naïve models to complete quantum mechanics models. Next, we present the quantitative analysis regarding textual and visual chemical understanding and students' integration of the different levels of chemistry understanding.
Textual and visual chemical understanding – quantitative analysis
Textual and visual chemical understanding skills express students' understanding related to the quantum mechanical content, and their ability to describe and explain these concepts using visual and textual modes. In this section we present findings related to textual and visual chemical understanding of the various research participants. The quantitative analysis included three comparisons: (1) between high school honors and undergraduate students, (2) between high school and undergraduate students who studied the module, where the high school students included high and intermediate academic level honors students, while the undergraduates were the ViCo (Visual-Conceptual)-oriented undergraduate students, and (3) the undergraduate ViCo-oriented students (who studied the module) and the undergraduate math-oriented students (who did not study the module).| Sub-groups | N | Net-gain | S.D. |
|---|---|---|---|
| High-level honors | 73 | 26.5 | 21.2 |
| Intermediate-level honors | 39 | 13.8 | 19.5 |
| ViCo-oriented undergraduates | 34 | 17.4 | 17.5 |
We examined the distribution of chemistry understanding levels that high school and undergraduate students' used in their answers. The responses demonstrate mainly micro and quantum level understanding. We counted and added up the levels of understanding revealed in all three assignments and calculated the frequencies in the pre- and post-questionnaires. The N items were distributed across the micro, quantum, and micro + quantum chemistry understanding levels. Fig. 2a and b present this distribution in pre- and post-questionnaires, respectively, by sub-groups: high-level, N = 73, and intermediate-level, N = 39,§ honors students, and ViCo-oriented undergraduate students, N = 34.
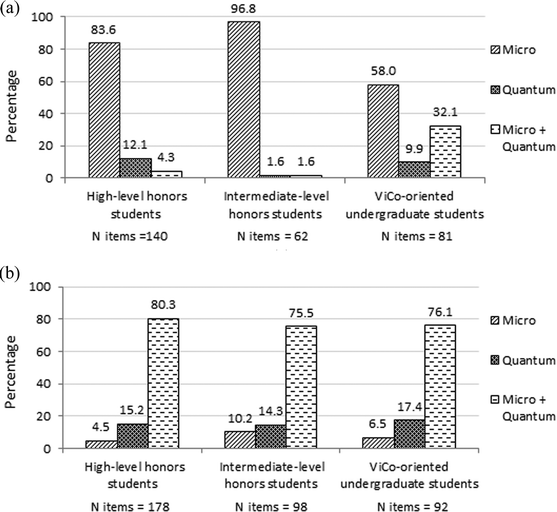 | ||
| Fig. 2 (a) Distribution of chemical understanding levels – pre-questionnaire. (b) Distribution of chemical understanding levels – post-questionnaire. | ||
Findings in Fig. 2a indicate broad differences between the sub-groups of students in the pre-questionnaire. The answers of the intermediate-level honors students were mainly on the micro level, with little use of the quantum level. The same picture was apparent among the high-level honors students; however, the quantum level already appeared. Among the ViCo-oriented undergraduate students, the quantum level already appeared in their answers on the pre-questionnaires (see Fig. 2a), probably since these students had studied the course, Basics of Chemistry, a year or two earlier.
In the post-questionnaire, the number of items increased in all sub-groups and the highest frequency was that of a combination of the micro and quantum levels. High school honors and undergraduate students minimized the gap between them in terms of the integration of micro + quantum levels in their answers. High-level honors students had a higher frequency in integrating the micro + quantum levels than intermediate-level honors students.
Discussion
The Chemistry – From “the Hole” to “the Whole”: From the Nanoscale to Microelectronics module focuses on teaching quantum mechanical topics to honors students using a visual-conceptual approach, which is contrasted with a quantitative, classic approach for teaching most of the undergraduate quantum chemistry courses. In addition to implementing and assessing the module in high school, it was also partially implemented and assessed in an undergraduate quantum chemistry course.The four evolutionary stages of understanding quantum mechanics
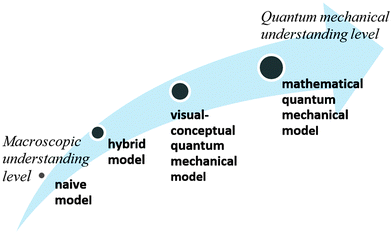
The qualitative analysis reflected four evolutionary stages of understanding quantum mechanics, as presented in Fig. 3.Naïve models, such as the planetary or solar system model (Harrison and Treagust, 1996; Taber, 2001a; Stevens et al., 2010), are based on a macroscopic perspective that describes electron rotation in a deterministic trajectory around the nucleus, resembling that of a planet rotating around the sun. In our qualitative analysis we discovered students' use of hybrid models that mix the terminology of definite orbits of the electron with the probabilistic quantum mechanical concept. We also discovered the use of a hybrid model using a mathematical formula.
These findings are consistent with those of previous studies, which indicated that honors and college students merge concepts from previous historical models with the probabilistic quantum mechanical terminology (Mashhadi and Woolnough, 1997; Taber, 2002, 2005; Papaphotis and Tsaparlis, 2008; Stefani and Tsaparlis, 2009; Stevens et al., 2010).
The visual-conceptual quantum mechanics model, which was taught in the module, is more abstract and required understanding of the quantum level. Honors students restricted their explanations to qualitative and visual terms, which they had learned in the module. Some of the undergraduate student responses referred to the generic mathematical structure of quantum mechanics theory, based on their background knowledge (see Fig. 3).
Historical reconstruction of the atomic model, taught as part of most chemistry and physics curricula, may be a source of confusion to some students. Students need to understand why previous models do not represent the acceptable atomic quantum model, which scientists believe is more accurate. Although some researchers question the need to teach quantum mechanical concepts in secondary school and introduction courses in college (Gillespie, 1991, 1996; Tsaparlis, 1997), to this end, we recommend that high school students, as well as students in college introductory chemistry courses, will be gradually exposed to ever more sophisticated models, culminating in the quantum mechanics model. This approach is in line with recommendations suggested by researchers in previous studies (Shiland, 1997; Coll et al., 2005; Niaz and Coştu, 2009; Stevens et al., 2010), some of whom recommended teaching students the different models, emphasizing their limitations and advantages.
Textual and visual chemical understanding
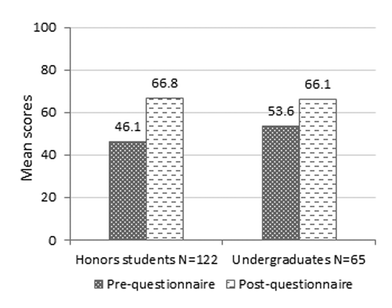
The quantitative analysis has shown that students in our study improved their textual and visual chemical understanding skills. However, the extent of the high school students' improvement (net-gain) was significantly higher than that of the undergraduate students. These results might relate to the shorter duration of the enrichment course to which the undergraduate students were exposed. The higher mean scores of undergraduate students in the pre-questionnaire compared with the honors students is due to the more extensive experience of the undergraduate students in using visual representations and better understanding of quantum mechanical concepts, which they had gained in previous physical chemistry courses. This relatively higher starting point may also explain their lower improvement.In this research, we started evaluating the students' understanding of chemistry via four chemistry understanding levels – macro, micro, symbol and process – using a method developed in earlier research for evaluating textual responses (Dori and Sasson, 2008; Kaberman and Dori, 2009). Analyzing the students' responses, the need to add a fifth level of understanding – the quantum level – became apparent. This level includes the ability to depict the electronic structure of atoms, molecules and solid state in terms of quantum mechanics theory. After learning the module, there was a shift in the honors students' responses from the micro chemistry understanding level to the quantum level and to the combination of the two. This might indicate that most of them had gained better understanding of the quantum model of atoms and molecules while studying the module.
Our findings also strengthen the claim of Tasker and Dalton (2006) that textual and visual modes should be intertwined in order to promote conceptual understanding. These findings are consistent with the argument of Gunel and colleagues (2006) that visualizations enable the construction of a richer conceptual understanding of a set of quantum mechanical topics.
However, these findings contradict those of Papaphotis and Tsaparlis (2002, 2008), who claimed that most of the honors students failed to attain understanding of the probabilistic nature of atomic orbitals and retained a deterministic perspective. The differences between their study and ours might be related to the differences in the teaching approach of the new module, which is a less mathematical-oriented approach. The definition of honors in the two countries might be another explanation to the differences in findings between the two studies.
Textual and visual chemical understanding skills of the undergraduate students, who studied the module as enrichment, was significantly higher than their math-oriented peers. This finding points to the benefit that undergraduate students, who had been exposed to the module, gained by improving their conceptual understanding of quantum mechanical concepts and their ability to apply visual and textual modes to explain and describe quantum mechanical concepts. Our finding is consistent with those of Kalkanis and colleagues (2003), and Cataloglu and Robinett (2002), who found that implementation of a qualitative teaching approach, which integrates conceptual and visual understanding in a quantum mechanics course, improved students' understanding. Based on our findings and those of others, we recommend integrating the visual-conceptual approach into both high school and undergraduate quantum chemistry courses in particular and into physical chemistry curricula in general.
Limitations and strengths of the research
Our research had some limitations. One was that we could not find a comparison group for the honors students, because the topics of the module had not been taught in Israel before. Honors students do not have sufficient background in mathematics for learning quantum mechanics. Therefore, we based our module on visual-conceptual explanations. Another limitation is that the undergraduate students were only partially exposed to the module, and the exposure was for a short duration. A future study may investigate the effect of longer exposure to this qualitative approach. Despite these limitations, the qualitative aspect of the assessment method is valuable for a variety of topics in chemistry and science in general.The research findings indicated that using a visual-conceptual approach to teaching quantum mechanical concepts and principles and their integration into daily life applications may help overcome the need to introduce mathematical aspects of quantum theory to honors students. The combination of these aspects is a novel element in our research. Most of the honors student responses after studying the module demonstrated a level of quantum mechanical understanding that enabled them to clearly explain the main concepts in this area.
Our module emphasized the link between quantum-mechanics theory and daily life phenomena, such as the color of pigments and the transition of metal from a conductor to a semi-conductor. Using terms of orbitals, valance and conducting bands, which arise from quantum mechanics theory, enabled the students to understand how microelectronic devices, such as LED (light emitting diode), work without teaching them the mathematical background they lacked. These kinds of daily life applications, which are not represented in most chemistry textbooks (Shiland, 1997), may help students recognize the usefulness of quantum mechanics theory and make it easier for them to shift from the previous models they possess to the quantum model (Stevens et al., 2010). We also found that embedding a visual-conceptual approach in an undergraduate quantum mechanics course broadened students' views and conceptual understanding of key quantum mechanical terms.
One theoretical contribution of this study is the addition of the quantum level as a fifth level of chemistry understanding, augmenting the current set of four chemistry understanding levels – macro, micro, symbol and process. In addition to being a means to understand and explain chemistry, this level provided the basis for our self-developed assessment tool that enables evaluating chemistry understanding in both visual and textual modes. Drawings and illustrations were applied for assessing students' understanding of the atomic structure and chemical bonding (Harrison and Treagust, 2000; Coll and Treagust, 2003; Stevens et al., 2010; Wheeldon, 2012). The tool we developed contains rubric for analyzing and scoring scientific drawings and illustrations (see Appendix S3, ESI†) designed specifically for assessment of quantum mechanics topic understanding, and to the best of our knowledge is the first and so far only such tool. This tool enables characterization of students' chemical understanding of phenomena, such as semiconductors, which can be described at the micro level by illustration of covalent bonds and “holes” (García-Carmonaa and Criado, 2009) or by using quantum-mechanical concepts, such as valance and conducting bands, which constitute the quantum basis of the phenomena. While tailored to quantum mechanics understanding, this tool can be adapted in other research in chemistry, as well.
This research contributes to the body of knowledge on visualization and thinking skills in chemistry, and could potentially enhance the teaching and learning of quantum mechanical concepts at both the honors and undergraduate levels. From the practical perspective, the research may help curriculum developers and teachers to incorporate visual representations in the design and assimilation of new learning materials on the structure of matter at the nanoscale level.
Finally, the approach studied in this work has an additional value in the broader context of chemistry studies. In many fields of chemistry (though not in theoretical quantum chemistry), a visual-conceptual approach is commonly followed. For example, orbitals are discussed in the context of organic reaction mechanisms, at a level, which is far from the formal way taught in undergraduate courses, and from the scope of rigorous mathematical models. Therefore, beyond being an evolutionary stage towards a more rigorous theoretical understanding, the qualitative understanding of quantum mechanics models and concepts, as pursued here, provides a practical thinking tool for all chemists.
References
- Barak M. and Dori Y. J., (2005), Enhancing undergraduate students' chemistry understanding through project-based learning in an IT environment, Sci. Educ., 89, 117–139.
- Barnea N. and Dori Y. J., (1999), High school chemistry student's performance and gender differences in a computerized molecular modeling learning environment, J. Sci. Educ. Technol., 8, 257–271.
- Ben Zvi R., Eylon B. and Silberstein Y., (1987), Student's visualization of a chemical reaction, Educ. Chem., 24, 117–120.
- Bowen G. M. and Roth, W.-M., (2005), Data and graph interpretation practices among pre-service science teachers, J. Res. Sci. Teach., 42, 1063–1088.
- Cataloglu, E. and Robinett, R. W., (2002), Testing the development of student conceptual and visualization understanding in quantum mechanics through the undergraduate career, Am. J. Phys., 70, 238–251.
- Cokelez A, (2012), Junior high school students' ideas about the shape and size of the atom, Res. Sci. Educ., 42, 673–686.
- Coll, R. K., France, B. and Taylor, I., (2005), The role of models and analogies in science education: implications from research, Int. J. Sci. Educ., 27(2), 183–198.
- Coll R. K. and Treagust D. F., (2003), Investigation of secondary school, undergraduate, and graduate learners' mental models of ionic bonding, J. Res. Sci. Teach., 40(5), 464–486.
- Dori Y. J., Barak, M. and Adir, N., (2003), A web-based chemistry as means to freshmen learning, J. Chem. Educ., 80, 1084–1092.
- Dori Y. J. and Belcher J. W., (2005), Learning electromagnetism with visualizations and active learning, in Gilbert J. K. (ed.), Visualization in Science Education, Dordrecht, The Netherlands: Springer, pp. 187–216.
- Dori Y. J. and Hameiri M., (2003), Multidimensional analysis system for quantitative chemistry problems – symbol, macro, micro and process aspects, J. Res. Sci. Teach., 40(3), 278–302.
- Dori Y. J. and Sasson I., (2008), Chemical understanding and graphing skills in an honors case-based computerized chemistry laboratory environment: The value of bidirectional visual and textual representations, J. Res. Sci. Teach., 45(2), 219–250.
- Gabel D. L., (1993), Use of the particle nature of matter in developing conceptual understanding, J. Chem. Educ., 70(3), 193–194.
- Gabel D. L., (1998), The complexity of chemistry and implications for teaching, in Fraser B. J., Tobin K. J., (ed.), International Handbook of Science Education, Great Britain: Kluwer Academic Publishers, pp. 233–248.
- García-Carmonaa A. and Criado A. M., (2009), Introduction to Semiconductor Physics in Secondary Education: Evaluation of a teaching sequence, Int. J. Sci. Educ., 31(16), 2205–2245.
- Gillespie R. J., (1991), What is wrong with the general chemistry courses? J. Chem. Educ., 68(3), 192–194.
- Gillespie R. J., Spencer J. N. and Moog R. S., (1996), Demystifying introductory chemistry. Part 2. Bonding and molecular geometry without orbitals- the electron domain model, J. Chem. Educ., 73(7), 622–627.
- Green W., Trotochaud A., Sherman J., Kazerounian K. and Faraclas E., (2009), Using LEDs and phosphorescent materials to teach high school students quantum mechanics. A guided-inquiry laboratory for introductory high school chemistry, J. Chem. Educ., 86(3), 340–342.
- Gunel M., Hand B. and Gundus S., (2006), Comparing student understanding of quantum physics when embedding multimodal representations into two different presentation format versus writing formats: Presentation format versus summary report format, Sci. Educ., 90, 1092–1112.
- Harrison A. G. and Treagust D. F., (1996), Secondary students' mental models of atoms and molecules: implications for teaching chemistry, Sci. Educ., 80(5), 509–534.
- Harrison A. G. and Treagust D. F., (2000), Learning about atoms, molecules, and chemical bonds: A case study of multiple-model use in grade 11 chemistry, Sci. Educ., 84, 352–381.
- Herscovitz O., Kaberman Z., Saar L. and Dori, Y. J., (2012), The relationship between metacognition and the ability to pose questions in chemical education, in Zohar A. and Dori Y. J. (ed.), Metacognition in science education: Trends in current research, Dordrecht, The Netherlands: Springer, pp. 165–195.
- Ireson G., (2000), The quantum understanding of pre-university physics students, Phys. Educ., 31(1), 15–21.
- Jensen W. B., (1998), Logic, history, and the chemistry textbook. I. Does chemistry have a logical structure? J. Chem. Educ., 75(6), 679–687.
- Johnstone A. H., (1991), Why is science difficult to learn? Things are seldom what they seem, J. Comput. Assist Learn., 7, 75–83.
- Justi R. and Gilbert J. K., (1999), A cause of ahistorical science teaching: Use of hybrid models, Sci. Educ., 83, 163–177.
- Justi R. and Gilbert J. K., (2000), History and philosophy of science through models: Some challenges in the case of ‘the atom', Int. J. Sci. Educ., 22(9), 993–1009.
- Kaberman Z. and Dori Y. J., (2009), Metacognition in chemical education: Question posing in the case-based computerized learning environment, Instr. Sci., 37(5), 403–436.
- Kalkanis J., Hadzidaki P. and Stavrou D., (2003), An instructional model for a radical conceptual change towards quantum mechanics concepts, Sci. Educ., 87, 257–280.
- Kozma R. and Russell J., (2005a), Student becoming chemist: Developing representational competence, in Gilbert J. K. (ed.), Visualization in Science Education, Dordrecht, The Netherlands: Springer, pp. 121–146.
- Kozma R. and Russell J., (2005b), Assessing learning from the use of multimedia chemical visualization software, in Gilbert J. K. (ed.), Visualization in Science Education, Dordrecht, The Netherlands: Springer, pp. 299–324.
- Margel H., Eylon B. and Scherz Z., (2004), “We actually saw atoms with our own eyes”: conceptions and convictions in using the scanning tunneling microscope in Junior-High School, J. Chem. Educ., 81, 558–566.
- Mashhadi A. and Woolnough B., (1997), Dualistic thinking underlying students' understanding of quantum physics, Paper presented at the Annual International Conference of Thinking, Singapore, June, 1997.
- Mayer R. E., (2002), Cognitive theory and the design of multimedia instruction: an example of the two-way street between cognition and instruction, New Dir. Teach. Learn., 89, 55–71.
- Niaz M. and Coştu B., (2009), Presentation of atomic structure in Turkish general chemistry textbooks, Chem. Educ. Res. Pract., 10(3), 233–240.
- Nyachwayaa J. M., Mohameda A.-R., Roehriga G. H., Woodb N. B., Kernc, A. L. and Schneider, J. L., (2011), The development of an open-ended drawing tool: an alternative diagnostic tool for assessing students' understanding of the particulate nature of matter, Chem. Educ. Res. Pract., 12, 121–132.
- Papaphotis G. and Tsaparlis G., (2008), Conceptual versus algorithmic learning in high school chemistry: the case of basic quantum chemical concepts, Part 2. Students' common errors, misconceptions and difficulties in understanding, Chem. Educ. Res. Pract., 9, 332–340.
- Park E. J. and Light G., (2009), Identifying atomic structure as a threshold concept: Student mental models and troublesomeness, Int. J. Sci. Educ., 31(2), 233–258.
- Petri J. and Niedderer H., (1998), A learning pathway in high school level quantum atomic physics, Int. J. Sci. Educ., 20(9), 1075–1088.
- Ren W., Bradley K. D. and Lumpp, J. K., (2008), Applying the rasch model to evaluate an implementation of the Kentucky Electronics Education Project, J. Sci. Educ. Technol., 17, 618–625.
- Roth W. M. and Bowen G. M., (1994), Mathematization of experience in a grade 8 open-inquiry environment: An introduction to the representational practices of science, J. Res. Sci. Teach., 31(3), 293–318.
- Roth W. M., Bowen G. M. and McGinn, M. K., (1999), Differences in graph-related practices between high school biology textbooks and scientific ecology journals, J. Res. Sci. Teach., 36, 977–1019.
- Santos R., Chibante R., Araujo A. and Carvalho, A., (2009), Educational tool for teaching of semiconductors. Power Electronics and Applications, EPE ‘09 13th European Conference, September 2009.
- Sasson I., Stanger R., Dori Y. J. and Peskin, U., (2007), From Nanoscale to Microelectronics, Holon, Israel: Yessod Publishing House, p. 112, (in Hebrew).
- Shiland T. W., (1997), Quantum mechanics and conceptual change in high school chemistry texts, J. Res. Sci. Teach., 34(5), 535–545.
- Stefani C. and Tsaparlis, G., (2009), Students' levels of explanations, models, and misconceptions in basic quantum chemistry: A phenomenographic study, J. Res. Sci. Teach., 46(5), 520–536.
- Stevens S. Y., Delgado C. and Krajcik, J. S., (2010), Developing a hypothetical multi-dimensional learning progression for the nature of matter, J. Res. Sci. Teach., 47(6), 687–715.
- Taber K. S., (2001a), When the analogy breaks down: modeling the atom on the solar system, Phys. Educ., 36, 222–226.
- Taber K. S., (2001b), Building the structural concepts of chemistry: Some considerations from educational research, Chem. Educ. Res. Pract., 2(2), 123–158.
- Taber K. S., (2002), Compounding quanta: probing the frontiers of student understanding of molecular orbitals, Chem. Educ. Res. Pract. Eur., 3(2), 159–173.
- Taber K. S., (2005), Learning quanta: Barriers to stimulating transitions in student understanding of orbital ideas, Sci. Educ., 89, 94–116.
- Tasker R. and Dalton R., (2006), Research into practice: Visualization of the molecular world using animations, Chem. Educ. Res. Pract., 7(2), 141–159.
- Tsaparlis G., (1997), Atomic orbitals, molecular orbitals and related concepts: Conceptual difficulties among chemistry students, Res. Sci. Ed., 27(2), 271–287.
- Tsaparlis G. and Papaphotis G., (2002), Quantum-chemical concepts: are they suitable for secondary students, Chem. Educ. Res. Pract., 3, 129–144.
- van Hoeve-Brouwer G. M., (1996), Teaching structures in chemistry: An educational structure for chemical bonding, CD-β Press.
- Wheeldon R., (2012), Examining pre-service teachers' use of atomic models in explaining subsequent Ionisation Energy values, J. Sci. Educ. Technol., 21, 403–422.
- Wu H.-K., Krajcik J. S. and Soloway E., (2001), Promoting understanding of chemical representations: Students' use of a visualization tool in the classroom, J. Res. Sci. Teach., 38(7), 821–842.
- Wu H. K. and Shah P., (2004), Exploring visuospatial thinking in chemistry learning, Sci. Educ., 88, 465–492.
- Xie C. and Lee H., (2012), A visual approach to nanotechnology education, Int. J. Eng. Educ., 28(5), 1–17.
- Yeow Y. T. and Ling Y. T., (1999), Teaching semiconductor device physics with two-dimensional numerical solver, IEEE Trans. Educ., 42(1), 50–58.
- Zollman D. A., Rebello N. S. and Hogg K., (2002), Quantum mechanics for everyone: Hands-on activities integrated with technology, Am. J. Phys., 70(3), 252–259.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4rp00025k |
| ‡ In what follows, we refer to the module as Chemistry – From the Nanoscale to Microelectronics. |
| § N = 112 in the honors sub-groups due to missing teachers' grades which were needed in order to assign each student to a sub-group. |
| This journal is © The Royal Society of Chemistry 2014 |