Prospective chemistry teachers' mental models of vapor pressure
Halil
Tümay
Department of Science and Mathematics Education – Chemistry Education, Gazi University, Ankara, Turkey. E-mail: tumay@gazi.edu.tr
First published on 16th April 2014
Abstract
The main purpose of this study was to identify prospective chemistry teachers' mental models of vapor pressure. The study involved 85 students in the Chemistry Teacher Training Department of a state university in Turkey. Participants' mental models of vapor pressure were explored using a concept test that involved qualitative comparison tasks. Additionally, 18 participants were interviewed to explore the mental models that emerged more deeply. The researcher analyzed responses using the constant comparative method to document participants' mental models and associated reasoning patterns in sufficient detail to be of practical use to instructors. Initial analysis of the data revealed that participants have many misconceptions about vapor pressure, which are similar to those reported in the literature. A more detailed constant comparative analysis revealed that these misconceptions derived mainly from three faulty mental models of vapor pressure: (1) vapor pressure of a liquid depends on the total number of vapor particles; (2) once the liquid–vapor equilibrium is established, the number of vapor particles is fixed and does not change regardless of the external effects on the system; and (3) vapor pressure is exerted only onto the surface of the liquid. The results have practical implications for teaching vapor pressure and science in general. By eliciting the underlying mental models of vapor pressure, these findings provide valuable insights for effective instructional interventions to address misconceptions.
Introduction
An extensive body of research concerning students' understanding of science concepts has accumulated in recent decades (Pfundt and Duit, 2006). These studies indicated that students' ideas are often different from the scientific concepts they are expected to learn. These ideas have been labeled with different terms by different researchers—preconceptions, alternative frameworks, misconceptions, naive conceptions, and children's science (Smith et al., 1993; Driver et al., 1994; Wandersee et al., 1994; Garnett et al., 1995). In this paper the term “misconception” will be used for scientifically incorrect ideas held by the learners.Whatever label is used, misconceptions are known to be extremely resistant to changes, despite instructions (Vosniadou, 1994; Duit, 1999). Additionally, students' existing conceptions significantly affect their subsequent science learning; flawed ideas can impede future learning (Wandersee et al., 1994; Taber, 2008). Thus, researchers frequently emphasized that students' preconceptions about how the world works should be taken into account when planning and implementing instruction for meaningful learning (Smith et al., 1993).
Studies in chemistry education revealed that many high school and university students experience difficulties in understanding essential concepts in chemistry (for example, Nakhleh, 1992; Garnett et al., 1995; Taber, 2002). Vapor pressure is one of the fundamental concepts of chemistry curriculum taught in high school and university courses. Vapor pressure (or equilibrium vapor pressure) is the pressure of a vapor in equilibrium with its liquid or solid in a closed system. The vapor pressure of a pure substance depends only on temperature. At a certain temperature its value is constant. Understanding vapor pressure is a prerequisite for comprehending many related phenomena, such as boiling, distillation, Raoults' law, and colligative properties. However, the vapor pressure concept has been reported to be a difficult idea for chemistry students (Gopal et al., 2004; Azizoğlu et al., 2006; Canpolat, 2006; Canpolat et al., 2006).
Some of the previous research on learners' conceptions has focused on learners' ideas about evaporation. For example, Osborne and Cosgrove (1983) investigated conceptions held by students aged 8–17 years concerning the state changes of water. Findings of the study showed that younger children have only superficial understanding about evaporation, condensation, and boiling. For example, most students thought that when a substance evaporated it ceased to exist. The distribution of students' conceptions changed along with the age of students, but certain non-scientific ideas were even more common among older students, even though they had received more instruction related to these concepts. This finding was interpreted to argue that the scientific models that teachers teach are very abstract and do not coincide with students' daily lives.
In a similar study, Bar and Travis (1991) examined the conceptual development of primary school children aged 6–14 years concerning boiling, evaporation, and condensation concepts. Students in this study described the matter inside the bubbles coming out of boiling water as water, water vapor, and air. They explained evaporation as water disappeared, water changed to hydrogen and oxygen, and water penetrated solid objects. Bar and Travis (1991) noted that the concept of evaporation requires a high degree of abstraction in order to be understood, because it is essentially an “invisible” phenomenon occurring at the submicroscopic level. They have also found that students have more difficulty in understanding condensation than evaporation.
Bar and Galili (1994) studied the conceptual development of children aged 5 to 14 concerning evaporation. They found that the children had four different conceptions regarding evaporation: (1) water simply disappeared; (2) water was absorbed by the soil or floor; (3) water transferred to another location or medium (i.e., sky, air, clouds, etc.); and (4) water spread out into the air as invisible tiny water droplets, or it was transformed into air.
Following this line of research, Chang (1999) investigated conceptions of prospective teachers studying in different content areas, including science majors. The findings of this research indicated that, although the science major students performed better than the non-science majors, their understanding of the condensation and boiling concepts still needed to be enhanced. For example, most of the students (including 71.2% of the science majors) did not have the saturated vapor concept. Findings of this study indicated that many misconceptions are not limited to school children.
In recent years several researchers have focused on undergraduate students' conceptions of vapor pressure. Gopal et al. (2004) investigated second-year chemical engineering students' understanding of evaporation, condensation, and vapor pressure. During individual interviews, they were questioned on three tasks related to these topics. A key misconception identified in the study was the belief that evaporation and condensation require a temperature gradient in order to take place. According to the authors, students looked for some sort of trigger to cause evaporation to occur, and their initial response was that the temperature gradient triggered the evaporation process. The authors also reported that students had difficulty in defining vapor pressure and pressure changes in equilibrium.
Canpolat et al. (2006) examined prospective primary science teachers' misconceptions related to evaporation and vapor pressure. Open-ended diagnostic questions and semi-structured interviews were used to determine learners' understanding. The results of the study showed that participants had various misconceptions about these topics. Identified misconceptions are as follows: (1) vapor pressure is the pressure exerted onto the surface of a liquid by particles at the vapor phase; (2) vapor pressure is the pressure caused by particles at the vapor phase during boiling; (3) vaporization starts with boiling; (4) a liquid has to be heated for a certain time in order to vaporize; (5) at a constant temperature, vapor pressure changes with changes in the volume of the vapor; (6) a liquid's vapor pressure increases with the amount of liquid; and (7) boiling liquids at atmospheric pressure have different vapor pressures.
In another study, Canpolat (2006) explored undergraduate students' misconceptions related to evaporation, evaporation rate, and vapor pressure. Findings of the study showed that students had superficial understanding of these concepts. The main misconceptions identified in this study were similar to the ones identified by Canpolat et al. (2006). Additional misconceptions were also revealed in this study: (1) at constant temperature, the evaporation rate of water in an open container is higher than that of water in a closed container; (2) in a closed container, the evaporation rate of water decreases as time passes; (3) when liquid–vapor equilibrium is established, evaporation does not occur anymore; (4) the evaporation rate depends on the liquid's surface area; and (5) removal or addition of vapor particles or inert gases to the liquid–vapor equilibrium system changes the vapor pressure.
Azizoğlu et al. (2006) used a concept test to determine 59 pre-service chemistry teachers' misunderstanding about phase equilibrium and related concepts, including vapor pressure. Their findings indicated that the undergraduates' understanding of these concepts was insufficient and that they had some misconceptions about the equilibrium vapor pressure. Approximately half of the pre-service teachers thought that equilibrium vapor pressure depended on the volume of the container, and about one in five believed that, in a closed container, an increase in the amount of the liquid will decrease the volume of vapor, causing an increase in vapor pressure.
In a more recent study, Yalcin (2012) investigated 103 pre-service primary teachers' understanding of the effect of temperature and pressure on the solid–liquid phase transition of water. She identified nine misconceptions about the phase transition. Regarding the vapor pressure concept, she found that 29.1% of pre-service teachers thought that if the external pressure is lowered at a constant temperature, the vapor pressure of water will raise and it will thus vaporize.
Findings of previous studies on learners' conceptions of vapor pressure and related concepts at different educational levels indicated that learners have many misconceptions about this topic. Although the determination of misconceptions is an important preliminary step in identifying and addressing learning difficulties, that alone is not sufficient. In many studies on students' misconceptions, research findings were presented as a list of common mistakes (Taber, 2000; Talanquer, 2006). This inventory approach was criticized by many science educators; it was argued that science educators should focus on the underlying sources of misconceptions, rather than listing misconceptions (Gilbert and Watts, 1983; Solomon, 1993; Taber, 2000; Talanquer, 2006). As emphasized by Talanquer (2006, p. 811): “Teachers are often unable to identify any consistent patterns in the students' thinking and thus see the vast inventory of students' alternative conceptions as isolated pieces of information. …Every mistake is quickly judged as a misconception, without further reflection on the actual source of the problem or any analysis of the underlying patterns in the students' reasoning that might in fact be used as a resource to promote understanding”.
A mental model is a valuable construct used to understand the underlying source of misconceptions and learners' reasoning patterns. Though definitions and ideas about mental models vary, a mental model can be defined as the internal cognitive representation of a real-world or imaginary situation, event, or process, whose structure reflects the perceived structure of that situation, event, or process (Gentner and Stevens, 1983; Johnson-Laird, 1983; Nersessian, 2008). Through the mental model theory we can “explain human cognitive processes of understanding reality, translating reality into internal representations and utilizing it in problem solving” (Park and Gittleman, 1995, p. 303). The ability to form mental models is a basic characteristic of the human cognitive system and we constantly construct and refine mental representations to interpret our experiences and make sense of the world (Vosniadou, 1994, 2007; Nersessian, 1999; Coll and Treagust, 2003; Clement and Rea-Ramirez, 2008).
Models also play a central role in the production, communication, and acceptance of scientific knowledge (Gilbert and Boulter, 1998; Giere, 1999; Nersessian, 2008). The work of scientists involves cycles of building, testing, and revising mental models (Nersessian, 1992, 2002; Giere, 1999). For example, through a cognitive-historical analysis, Nersessian (1992, 2002) demonstrated the role of constructing and simulating mental models of a situation in the development of Maxwell's electromagnetic theory.
When constructing a mental model of a system, an individual creates mental entities that represent perceived or conceived entities of the system and establishes their properties, relationships, behaviors, or functions (Johnson-Laird, 1983; Nersessian, 2008). There is no complete mental model for any real-world phenomena (Norman, 1983). However, learners frequently fail to construct correct and coherent mental representations, and these faulty mental models give rise to alternative interpretations that differ from the scientific worldview. When the constructed mental model does not include critical entities or when it inaccurately depicts the properties and relationships of some entities, its mental simulation unavoidably leads to various misconceptions. This argument has been supported by research on mental models in many domains, including naïve physics (Borges and Gilbert, 1999), astronomical knowledge (Vosniadou and Brewer, 1992; Vosniadou, 1994), and chemistry (Coll and Treagust, 2003; Lin and Chiu, 2007).
These studies demonstrated that many misconceptions, if not all, are actually generated on the spot as a result of running activated mental models. Therefore, identifying learners' mental models is of central importance in devising strategies to support the construction of scientific concepts. If learners' common mental models are elicited, then instructors can more easily determine the possible causes of learning difficulties and develop more effective instructional practices that support the development of mental models that are closer to the scientific models (Vosniadou 1994; Coll and Treagust, 2003; Lin and Chiu, 2007).
In order to avoid ambiguity about the key terms in this study, mental models should be distinguished from other types of models. Gilbert and Boulter (1998) classified models in science education as mental models, expressed models, consensus models, and teaching models. A mental model is the personal and cognitive representation of a target. It is formed by an individual, either on their own or within a group. When a mental model is expressed by an individual or a group through any mode of representation, such as action, speech, or writing, it becomes an expressed model. A consensus model is an expressed model that has been tested by scientists and which has been socially agreed by some of them as having some merit. A teaching model is a specially constructed model used by teachers to aid learners' understanding of a consensus model (Gilbert and Boulter, 1998).
Particularly, the distinction between mental models and expressed models is critical for this study. As emphasized by Gilbert and Boulter (1998), mental models are private and personal cognitive representations. Since the individuals' mental models cannot be directly accessed by the researcher, they can only be inferred from individuals' expressed models. As a result, research findings related to individuals' mental models do not represent their actual mental models, but rather the researcher's models of the individuals' mental models. Thus, the term “mental model” in this research refers to the model that is inferred by the researcher from learners' expressed models, rather than the learners' actual mental models.
Another important distinction is between mental models and misconceptions. A mental model is a complex conceptual system representing a physical system through mental entities and their properties, relationships, behaviors, or functions (Nersessian, 2008). On the other hand, a misconception is a conception that differs from the accepted scientific conceptions. Mental models and misconceptions differ from each other in several respects. According to Franco and Colinvaux (2000), mental models can be identified and characterized by a set of key features. First, mental models are generative, which means that people can produce new information and make predictions by using mental models. In other words, “mental models not only describe a state of affairs but are also used to infer information which is not explicitly or directly contained in the description itself” (Franco and Colinvaux, 2000, p. 105). Next, mental models involve tacit knowledge—a person is not completely aware of every aspect of his or her mental models or of how she/he makes use of them. Also, mental models are synthetic, which means that they are simplified representations of the target system. Finally, mental models are constrained by worldviews, meaning that the range of possible mental models people will develop and use is constrained by their general belief-systems (Franco and Colinvaux, 2000).
Franco and Colinvaux (2000) argue that these features do not need to be simultaneously present in order to characterize a representation as a mental model. According to them, the only necessary feature to identify a mental model is its generative feature; any of the remaining features may be absent, while the occurring features are sufficient to characterize the representation as a mental model. As a result, Franco and Colinvaux (2000) emphasize that the most distinctive feature of mental models is their generative feature, and that this feature differentiates mental models from other forms of knowledge, such as concepts and schemas.
The generative feature of mental models stems from their structural correspondence to the represented physical system, including entities, their properties and relationships (Gentner, 1983; Johnson-Laird, 1983; Nersessian, 2008). Such a representation provides a dynamic and runnable framework that simulates the represented system. Through this framework an individual can explain and predict the system's structure and behavior in even previously unconsidered situations (Gentner and Stevens, 1983; Johnson-Laird, 1983; Norman, 1983; Markman and Gentner, 2001; Nersessian, 2002, 2008). Because of these features, a mental model is a holistic and generative representation, whereas a misconception is a specific and relatively static notion about the represented system (Franco et al., 1999; Franco and Colinvaux, 2000). Some misconceptions may reflect some aspects of learners' mental models directly or indirectly; however, they may also simply be inferences drawn from the simulation of mental models.
Previous research on learners' understanding of vapor pressure has focused primarily on studying isolated student conceptions rather than investigating learners' common mental models of vapor pressure. However, identifying the mental models that might lead to misconceptions is more crucial in devising instruction to support the construction of scientific concepts. Additionally, since the teachers' understanding of a concept will greatly affect their students' understanding, it is important to identify prospective and practicing teachers' mental models of science concepts. Therefore, the purpose of this study was to investigate prospective chemistry teachers' mental models of vapor pressure.
Methodology
Sample
The sample in the present study included 85 prospective chemistry teachers (undergraduate students preparing to be high school teachers), of which 58 were female and 27 were male. Their ages ranged from 18 to 21 years, with a mean age of 19.4 years. All of the prospective teachers participated in the study voluntarily. They were informed that the results of the investigation would be used for research purposes only and they were also assured of the confidentiality of their identity.Instructional context
The participants had received instruction on liquid–vapor phase equilibrium and the concept of vapor pressure in the General Chemistry I and II courses at a public university in Turkey. They took these courses sequentially in the first and second semesters of their first year. They also took a General Chemistry Laboratory course in the second semester of the first year, which included some experiments related to vapor pressure, such as distillation and freezing point depression.The instruction in the General Chemistry I and II courses was primarily given through traditional lecturing, and were not designed explicitly to facilitate conceptual changes. Most of the time the lecturer talked as the students listened and took notes. In-class discussions and examinations were mainly focused on algorithmic problem solving. The laboratory course was also a traditional instruction setting, in which experiments were performed in a “cookbook” style.
Data collection
To reveal the participants' mental models of vapor pressure, a concept test consisting of one descriptive question and six qualitative comparison tasks was developed by the researcher (Appendix). Past informal interviews, class observations, and previous research related to learners' conceptions of vapor pressure (Gopal et al., 2004; Azizoğlu et al., 2006; Canpolat, 2006; Canpolat et al., 2006) were taken into account for developing the concept test. Before the administration of the test, a pilot study was conducted to refine the concept test, using a similar group at the same level. Required modifications were made in the light of the pilot study results to ensure that these tasks were effective in uncovering subjects' mental models. Content validity of the test was evaluated by two experienced chemistry educators.Qualitative comparison tasks in the concept test required the participants to make inferences about the vapor pressures of different liquid–vapor equilibrium systems. Different systems were generated by changing either relevant or irrelevant variables with respect to vapor pressure. In all of the tasks participants were asked to compare the vapor pressures of two different systems and explain their reasoning for each answer. Analyzing their responses and the reasoning behind their responses allowed the researcher to identify their reasoning patterns and how they comprehended the entities and their properties and relationships in the liquid–vapor equilibrium systems. In this way, the researcher also attempted to identify possible model triggers and how the features of different tasks trigger a particular model. The concept test was administered to participants at the end of the second semester, after the vapor pressure concept and related experiments had been taught. The test was administered in the class during the regular instructional period without previous warning.
Additionally, to develop a robust picture of the subjects' mental models of vapor pressure, follow-up semi-structured interviews were conducted with 18 participants (11 female and 7 male). For the semi-structured interviews, participants were selected purposively (Lincoln and Guba, 1985) in the light of a preliminary analysis of their test responses, with the aim of identifying the full range of participants' mental models as much as possible. The interviews lasted approximately 30–40 minutes. Participants were interviewed individually and all interviews were tape-recorded with the permission of each participant, then transcribed for analysis. During the interviews, participants were asked to explain their written answers to the concept test. Follow-up probes were used for additional clarification when necessary. The subjects were also encouraged to draw diagrams to show their ideas.
Data analysis
Since mental models are personal, internal representations that reflect the learner's subjective world (Gilbert and Boulter, 1998; Greca and Moreira, 2002), interpretive qualitative methods are appropriate for obtaining the rich descriptions necessary to elicit and understand learners' mental models. Eliciting mental models requires determining consistent patterns in participants' responses; therefore, most researchers have placed stronger emphasis on analytical inductive analysis methods. Mainly, six basic steps are followed in such an analysis (Creswell, 1994): (1) organize and prepare the data for analysis, (2) read through all the data, (3) code the data, (4) generate themes or categories from codes, (5) organize and describe the data in terms of the codes and themes, and (6) interpret the data.In this study, data obtained from the concept test and interviews were carefully analyzed and coded using an iterative, constant comparison technique (Glaser and Strauss, 1967) in which common ideas and reasoning patterns were identified. The constant comparative analysis technique permits researchers to identify the patterns in learners' ideas from different data sources and interpret how learners comprehend the target system. In order to establish the full range of responses, an open coding approach was adopted. Through constant comparative analysis of the data, codes and categories were iteratively refined. Following this process, patterns in participants' responses and in their underlying mental models were elicited. Mental models that were identified in at least 10% of the participants are reported here.
Throughout the analysis, maximal closeness to original data was attempted by checking iteratively that the codes and the categories came from students' statements and were not imposed on them. Additionally, to ensure inter-rater reliability, the author and a chemistry educator discussed and mutually agreed on the description and the scope of each code in a subset of participants' responses. They then independently analyzed the participants' written responses and interview transcripts. They negotiated disagreements in coding results and reanalyzed responses until the coding agreement was above 90%.
Results and discussion
In order to identify participants' mental models of vapor pressure, their conceptions about vapor pressure were first determined. This initial analysis of the participants' responses showed that the majority of the prospective chemistry teachers have misconceptions about vapor pressure that are similar to those documented in previous research (Azizoğlu et al., 2006; Canpolat, 2006; Canpolat et al., 2006; Yalcin, 2012). Identified misconceptions and the percentages of the participants who had each misconception are as follows:• As the surface area of a liquid increases, vapor pressure of the liquid increases (37.6%).
• As the surface area of a liquid increases, vapor pressure of the liquid decreases (10.6%).
• As the amount of a liquid increases, vapor pressure of the liquid increases (25.9%).
• As the external pressure acting on the liquid surface increases, vapor pressure of the liquid decreases (44.7%).
• As the volume of vapor in a closed container increases, vapor pressure of the liquid decreases (58.8%).
• As the volume available for vaporization increases, vapor pressure of the liquid increases (15.3%).
• When the liquid–vapor equilibrium is established, evaporation or condensation does not occur anymore (58.8%).
• Vapor pressure is the pressure exerted by vapor particles onto the surface of the liquid (11.8%).
Following this initial analysis, a more detailed interpretive analysis was conducted using the constant comparison technique to reveal participants' mental models of vapor pressure and associated reasoning paths. Analytical inductive analysis of the participants' responses allowed for the determination of similarities, differences, and emerging themes in their conceptualizations of the entities, their properties, and their relationships in the liquid–vapor equilibrium system. Findings revealed that all of the participants described vapor pressure as the pressure exerted by vapor particles. They explained that vapor pressure results from the force applied by moving vapor particles. However, their conceptualizations about vapor pressure varied with respect to the factors that determine the magnitude of the vapor pressure, existence and the nature of the liquid–vapor equilibrium, and the direction of vapor pressure.
This explorative analysis of the participants' responses and associated explanations revealed that only 14.1% of the participants held the scientific model of vapor pressure. Scientifically, vapor pressure is the pressure of a vapor in equilibrium with its liquid in a closed system. Vapor pressure of a pure liquid depends only on temperature and at a certain temperature its value is constant. The remaining majority of the participants (85.9%) exhibited three main faulty mental models of vapor pressure. These faulty mental models and the percentages of the participants who had each model are as follows:
• Vapor pressure of a liquid depends on the total number of vapor particles (85.9%).
• Once the liquid–vapor equilibrium is established, the number of vapor particles is fixed and does not change (58.8%).
• Vapor pressure is exerted only onto the surface of the liquid (11.8%).
Each of these faulty mental models should not be considered as a whole mental model of vapor pressure, but rather as a simpler mental model related to a specific aspect of the vapor pressure phenomena. Alternative combinations of these faulty mental models and their scientific counterparts give us possible mental models of vapor pressure in a holistic manner. For example, learners can hold all three faulty mental models, or they can hold only one or two faulty mental models, in which case the remaining parts of their whole mental models of vapor pressure can be consistent with the scientific model.
Rather than listing each participant's different mental model, adapting such a modular approach enabled us to represent findings of this research in a more clear, concise, and practically useful way. Different combinations of the identified faulty mental models and their scientific counterparts can explain the misconceptions about vapor pressure. They also enable researchers and educators to foresee learners' expected responses in different contexts to a variety of vapor pressure related questions, such as “What is vapor pressure and how is it formed?”, “Which factors does the magnitude of the vapor pressure depend on?”, and “What factors affect it and in which ways?”
The three faulty mental models that emerged from the analytical inductive analysis are explained in detail below.
Mental model 1: “Vapor pressure of a liquid depends on the total number of vapor particles.”
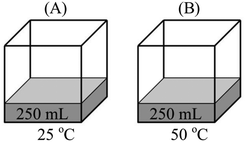
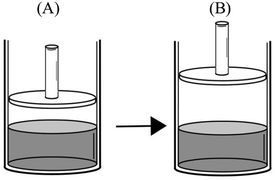
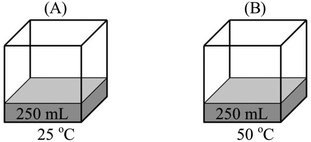
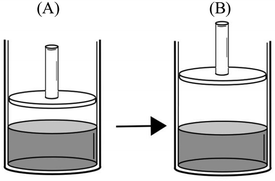
The most common faulty mental model was that “Vapor pressure of a liquid depends on the total number of vapor particles. As the number of vapor particles increases, the force exerted by these particles and therefore the vapor pressure increases.” 85.9% of the participants who had this mental model thought of vapor pressure as an extensive property, like force, rather than an intensive property. Accompanying this mental model was the belief that increasing the temperature, the surface area, the amount of liquid, and the volume available for vaporization, or decreasing the external pressure, will either accelerate or facilitate vaporization. Many participants explicitly stated in response to various questions that as the vaporization accelerated or was facilitated, the number of vapor particles and therefore the vapor pressure of the liquid increases. Participants' exposition of this mental model and its related assumptions can be seen in the following extracts from their responses to qualitative comparison tasks. (Because the study was conducted in Turkish, the quotes from the participants' written responses and interviews were translated into English by the researcher.)Comparison task 1:
“PB > PA – with the increase in temperature, there will be more evaporation. Vapor pressure in container B will be higher as a result of more evaporated particles.”
“PB > PA – evaporation occurs at all temperatures. However, at higher temperatures, liquids have more kinetic energy and higher evaporation rate. Therefore, there will be more particles in the vapor phase and the vapor pressure will increase.”
Comparison task 2:
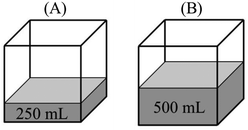
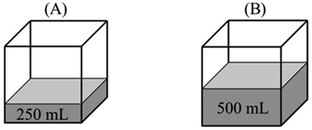
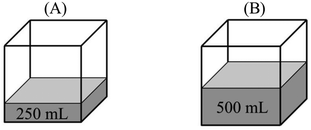
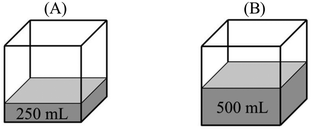
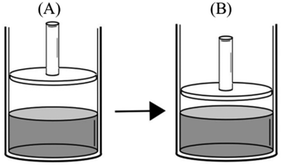
“PA = PB – evaporation only occurs at the surface of a liquid. Since the evaporation surface in both containers is equal, the number of particles that will evaporate is also equal. Therefore, vapor pressure in both containers is the same.”
“PB > PA – because vapor pressure changes with the number of evaporated particles. Increase in the amount of liquid results in an increase in the number of liquid molecules that have sufficient kinetic energy to evaporate. In addition, PV = nRT and nB > nA. Thus, the vapor pressure of B becomes higher.”
Comparison task 3:
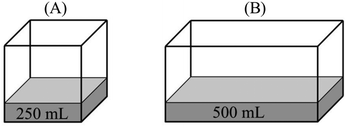
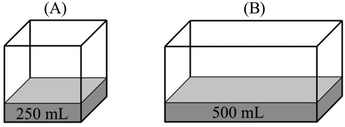
Participant: B has more vapor pressure than A.
Researcher: Why do you think so?
P: Because the surface area of the water in B is larger than A.
R: How is this related to vapor pressure?
P: As the surface area increases, evaporation rate increases. More vapor particles are formed with the increasing vaporization and thus, vapor pressure increases.
“PB > PA – since they have the same temperature, we should compare their evaporation surface. As the surface area increases, it will be easier for liquid molecules to go into the vapor phase. Since the number of particles that evaporated from the surface increases, B has more vapor pressure.”
Comparison task 4:
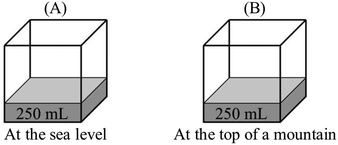
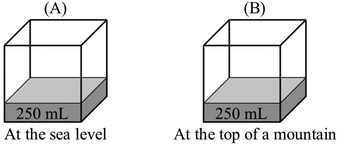
P: P B > PA. Vapor pressure at the sea level will be lower.
R: Could you explain your answer?
P: As the pressure above water increases, fewer water molecules evaporate, and this causes a decrease in vapor pressure.
R: How does external pressure affect evaporation and vapor pressure?
P: At a higher atmospheric pressure, more gas molecules hit the surface of the water. Evaporation of water molecules from the surface becomes difficult, and fewer molecules evaporate. And this causes a decrease in vapor pressure.
“PB > PA – at higher altitudes, atmospheric pressure is lower. Water molecules at the surface encounter less resistance from atmospheric gases. Evaporation of water molecules becomes easier and vapor pressure increases.”
“PB > PA – at higher altitudes, boiling points of liquids are lower. This means they have higher vapor pressure.”
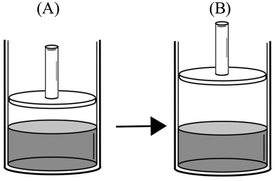
Comparison task 5:
“PB > PA – since the pressure exerted onto the liquid molecules decreases, liquid molecules can enter the gas phase more easily. And this will increase the vapor pressure.”
“PB > PA – when the piston is pulled up, the space above the water increases. More liquid molecules enter into the gas phase and vapor pressure increases.”
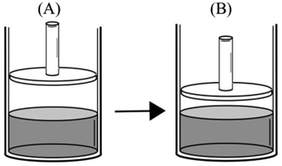
Comparison task 6:
“PA > PB – increase in pressure acting on the water surface causes a decrease in vapor pressure, because evaporation of water molecules becomes difficult.”
“PA > PB – some of the evaporated water molecules will return to the liquid phase. As the evaporation rate decreases, vapor pressure decreases.”
It is clear from these responses that many participants believe that the vapor pressure of the liquid is directly dependent on the total number of vapor particles, instead of the number of vapor particles per unit volume. In other words, participants thought of the vapor pressure as an extensive property, whereas scientifically vapor pressure is an intensive property. What determines the vapor pressure at constant temperature is the number of vapor particles per unit volume.
Why have participants constructed such a mental model? Each learning domain places different constraints on the learner (Vosniadou, 1994) and chemistry is distinguished from many other disciplines because chemical entities, their properties, and their relationships are usually unobservable (Johnstone, 1991; Coll and Treagust, 2003). Because of this constraint, we acquire new information in chemistry primarily by instruction or by reading. It has also been revealed that misconceptions in chemistry generally stem from prior teaching (Taber, 2001). Thus, the analysis of textbooks, instructional practices, and the typical examples used in teaching can shed light on the pathways that learners could follow in constructing faulty mental models. Therefore, examination of the typical instructional practices for teaching vapor pressure can be helpful in determining the possible roots of faulty mental models of vapor pressure.
The most common examples used in teaching vapor pressure and its related variables are as follows: comparing the vapor pressures of the same liquid at different temperatures to show the effect of temperature on vapor pressure (while all of the variables except the temperature are the same); and comparing the vapor pressures of two different liquids (generally, water and ethyl alcohol) to show the effect of intermolecular forces on vapor pressure (while all of the variables except the substances are the same). These examples or very similar ones can be found in many general chemistry textbooks (for example, Atkins and Jones, 1997; Petrucci et al., 2002).
When these typical examples are examined with a critical eye, we can see the possible causes of this faulty mental model. In these examples, the general argument chain for explaining the effect of temperature on the vapor pressure is as follows: “As the temperature increases, the kinetic energy of molecules increases. → At higher temperatures more molecules escape into the gas phase. → As the number of vapor particles increases, the force exerted by these particles also increases. → Thus, increase in the temperature causes the vapor pressure of a liquid to increase.” In a similar way, the general argument chain used for explaining the effect of intermolecular forces (or substance type) on the vapor pressure is as follows: “Intermolecular forces in substance B are weaker than A. → Number of evaporated B molecules is more than A molecules at the same temperature. → As the number of evaporated particles increases; the force exerted by these particles also increases. → Vapor pressure of B is higher than the vapor pressure of A at the same temperature.”
Implicit in these comparative examples is the idea that pressure is an intensive property and that changes in vapor pressure result from changes in the number of vapor particles per unit volume, which is vapor concentration. In order to make a valid comparison, only one variable (temperature or intermolecular forces) is changed, while all the other variables (including the volume of the container) are fixed. Thus, changes in the number of vapor particles are directly proportional to the changes in the vapor concentration. This relationship might be obvious for teachers, and furthermore it might be assumed that learners would easily appreciate that. However, what is obvious to instructors might not be so explicit for students. Additionally, it is more likely for learners to focus only on salient features (i.e., the number of vapor particles) and overlook other features of the system (i.e., identical volume, constant temperature, etc.) (Talanquer, 2009; Taber and García-Franco, 2010).
Although this is not their intention, taken together these types of examples and related explanations could easily be comprehended by learners as “the fundamental factor that determines the vapor pressure is the number of vapor particles.” So, learners might reasonably construct an intuitive mental model that “as the number of vapor particles increases, the vapor pressure increases.” It would also be reasonable for learners to think that any factor that can accelerate or facilitate evaporation will increase the number of vapor particles and therefore the vapor pressure. This mental model can easily be rationalized and strengthened by intuitively assuming that “more particles exert more force, and therefore more pressure.” This view was apparent in the participants' responses. Indeed, this false assumption is a common misconception that “pressure is a force” (De Berg, 1992).
It was also observed that many participants intuitively relate atmospheric pressure to the vapor pressure of the liquid. With regard to this finding it should be noted that, although some of the participants (9.4%) offered a mechanism within the framework of this mental model, many participants (35.3%) who thought that there was a causal linkage between atmospheric pressure and vapor pressure could not offer any mechanism. It seems that the direct relationship between atmospheric pressure and the boiling point of the liquid, and the inverse relationship between the boiling point and vapor pressure of the liquid led students to incorrectly infer a direct relational link between atmospheric pressure and the vapor pressure of the liquid. In fact, the boiling point of a liquid is an emergent property that is determined by both the vapor pressure of a liquid and the atmospheric pressure. The boiling point is the temperature at which the vapor pressure of the liquid is equal to the atmospheric pressure. Although there is a relational link between the boiling point and atmospheric pressure on one side, and a relational link between the boiling point and vapor pressure on the other side, this does not necessarily mean that there is a direct relationship between atmospheric pressure and vapor pressure. Learners' tendency to infer this non-existent direct relationship between atmospheric pressure and vapor pressure was also observed in previous studies (Canpolat et al., 2006; Yalcin, 2012).
Mental model 2: “Once the liquid–vapor equilibrium is established, the number of vapor particles is fixed and does not change.”
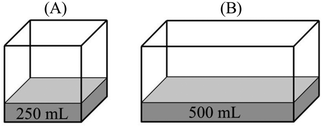
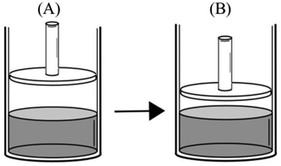
The second most common faulty mental model was related to conceptualizing the liquid–vapor equilibrium as a static rather than a dynamic process. Participants (58.8%) who held this mental model thought that “once the liquid–vapor equilibrium is established, the number of vapor particles is fixed and does not change regardless of the external effects on the system.” This mental model especially emerged in response to comparison tasks that included change in the volume of vapor in a frictionless piston-cylinder device. Participant responses that reflect this faulty mental model are given below:Comparison task 2:
“PB > PA – PV = nRT, and both containers are at the same temperature. So, PV is constant. Since the VBis smaller than VA, pressure in the B will be higher.”
“PB > PA – because empty space above the water in container B is smaller than that in container A. Evaporated water molecules exert more pressure in a smaller volume.”
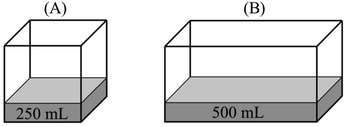
Comparison task 3:
“PA > PB – the number of vapor particles in unit volume in container A is more than that in container B. Therefore, the vapor pressure of A is expected to be greater.”
“PA > PB – both containers have the same amount of vapor. However, vapor spreads into more space in container B and this lowers the pressure. Thus, the vapor pressure of B is smaller than that of A.”
“PA > PB – PV = nRT, since the volume in second container is bigger, its vapor pressure is smaller.”
Comparison task 5:
“PA > PB – PV = nRT, and vapor volume increases in container B. Since the pressure and volume are inversely proportional, PBis lower than PA.”
P: P A >P B , because the volume of B is greater than that of A.
R: How does this affect the vapor pressure?
P: When the volume increases, vapor particles spread out in this space. Fewer molecules hit the surface, and this causes the vapor pressure to decrease.
R: Do you mean that, since the same number of vapor particles spread into more volume, the pressure decreases?
P: Yes, the force exerted onto the unit surface decreases.
Comparison task 6:
“PB > PA – PV = nRT. Since the n, R and T are constant, PV is constant. As we decrease the volume, pressure increases proportionally to balance this effect.”
“PB > PA – When the piston is pushed down, vapor compresses into less space. More particles in less space cause an increase in applied force and therefore, an increase in vapor pressure.”
Liquid–vapor equilibrium in a closed container is a dynamic equilibrium, and a liquid–vapor system tends to reestablish equilibrium when perturbed. When the liquid–vapor equilibrium is reestablished at a different point, the amount of vapor would change with either a change in volume or in temperature. However, quoted participant responses clearly indicate an incorrect assumption that when the liquid–vapor equilibrium is established, the amount of vapor is fixed and does not change. In other words, many participants held a static rather than a dynamic understanding of the liquid–vapor equilibrium. They seemed to treat the vapor in equilibrium with its liquid as an ideal gas in a closed container, without considering the dynamic nature of phase equilibrium. This finding is consistent with the findings of the previous studies on vapor pressure (Azizoğlu et al., 2006; Canpolat, 2006; Canpolat et al., 2006) and research on chemical equilibrium (for example, Andersson, 1990, Garnett et al., 1995), which showed the difficulty in understanding the dynamic nature of equilibrium. This tendency to treat the vapor in equilibrium as an ideal gas and to consider the amount of vapor as constant after the establishment of liquid–vapor equilibrium is more obviously observed in comparison tasks that include a change in volume available to vapor. In these tasks, many participants explicitly tried to apply the ideal gas equation (PV = nRT) to explain how vapor pressure changes.
Conceptualizing the liquid–vapor phase equilibrium as a static equilibrium and thinking of the amount of the vapor as constant might have originated from the misinterpretation of instructional explanations about the dynamic nature of liquid–vapor equilibrium. In typical instructional practices, it is generally emphasized that liquid–vapor equilibrium is a dynamic process, and since the rate of vaporization is equal to the rate of condensation at equilibrium, amounts of liquid and vapor are stable and do not change. In fact, under equilibrium conditions, amounts of liquid and vapor do not change as long as the equilibrium is not perturbed, but it seems that this explanation is misinterpreted by some participants as “once the equilibrium is established, amounts of the liquid and vapor are fixed and do not change under any circumstances.”
However it should be noted that, while some of the participants did not adequately understand the dynamic equilibrium, it was fairly common that participants who correctly explained the many tasks using the dynamic equilibrium concept surprisingly failed to explain the piston tasks. Additionally, during the interviews with these participants, after being reminded of the liquid–vapor equilibrium, some of them recognized the mistakes in their explanations and constructed more accurate explanations.
This observation implies that context and salient features of the tasks considerably influence which mental models will be activated, and consequently, which responses might be produced. A frictionless piston-cylinder device is typically used in questions related to the ideal gases. In this respect, comparison tasks 7 and 8 are atypical vapor pressure questions and present a different context. Constructing explanations related to vapor pressure in an unfamiliar context requires participants to make a mental simulation by considering the entities, their properties, and their interactions in the system. However, mental simulation is a cognitively demanding process, and repeating simulations in the same context leads people to formulate rules (Markman and Gentner, 2001). Formulating rules will reduce the cognitive load; however, the validity of the rule depends on correctly identifying the relevant entities and their interactions in the context. The frictionless piston-cylinder device might have spontaneously triggered the participants' experiences and mental models related to ideal gas problems and directed them to use the PV = nRT formula. As might be the case in this situation, the activation of inaccurate mental models constrains learners' perceptions and might lead them to focus on irrelevant factors while neglecting the effective ones. Whatever the real reason, what has again emerged with this finding is the fact that many participants have difficulty in identifying the relevant entities, their properties, and their interactions in the liquid–vapor equilibrium system and thinking accordingly.
Mental model 3: “Vapor pressure is exerted only onto the surface of the liquid.”
The least common faulty mental model was about the direction of vapor pressure. 11.8% of the participants thought that “vapor pressure is exerted only onto the liquid surface”. The following quotes exemplify the typical responses of participants who had this mental model.Comparison task 2:
“PA = PB – they have the same vapor pressure, because the surface area of A is equal to that of B.”
“PA = PB – since the surface areas of both are the same, the pressure exerted by the vapor will be the same.”
Comparison task 3:
“PA > PB – vapor pressure is the pressure exerted by the vapor on the liquid. As the surface area increases, vapor pressure decreases.”
“PA > PB – pressure is the force exerted onto unit area (P = F/A). Surface area of B is bigger and therefore, the vapor pressure of B will be smaller.”
Participants who thought that vapor pressure acts only downwards instead of in all directions typically defined vapor pressure as “the pressure exerted by vapor particles onto the surface of the liquid”. We could not obtain enough information from the participants with regard to the origin of this mental model. This mental model may simply emerge from an inaccurate recalling of the definition of vapor pressure, or from typical phrases used in teaching, such as, “as the pressure exerted onto the liquid surface increases, boiling point of the liquid increases”. When we asked these participants to further explain why vapor pressure acts on the surface of the liquid, all of them treated this definition as a fact that does not need any further explanation. Similarly, in a study on prospective chemistry teachers' understanding of vaporization and vapor pressure, Canpolat et al. (2006) revealed that 57% of the students described the vapor pressure as the pressure exerted onto the surface of a liquid by vapor particles in a closed container.
This finding is also congruent with the literature on learners' understanding of pressure, which showed that “the direction of pressure acts downward and not sideways” is a common misconception (Clough and Driver, 1986). As stated in the literature, this faulty mental model may originate from the depictions of atmospheric pressure in textbook illustrations. For example, in many textbook illustrations of Torricelli's experiment on atmospheric pressure, the direction of the pressure is shown as downward (for example, Atkins and Jones, 1997; Petrucci et al., 2002). While instructional illustrations are used for improving comprehension, it is a well-known fact that these aids can also lead to unintended conceptions (Sumfleth and Telgenbüscher, 2001). This finding could be taken as an additional indicator of the necessity of explicitly emphasizing and clarifying the entities and their interactions in the liquid–vapor equilibrium system for the development of scientific understanding.
Conclusions and implications for teaching
The purpose of this study was to identify and describe prospective chemistry teachers' mental models of vapor pressure. To reveal the participants' mental models, their conceptualization of the entities, their properties, and their relationships in the liquid–vapor equilibrium system, and their conceptualization of the system's emergent properties as a result of these interactions were explored using qualitative comparison tasks. Findings of the study revealed that only 14.1% of the participants have constructed a scientific model and used this model successfully in explaining vapor pressure of liquids under different conditions. Findings also revealed that the remaining majority of the participants had three main faulty mental models of vapor pressure. These faulty mental models indicate the participants' alternative conceptualizations of either entities or their properties and relationships in the system. Simulation of mental models that include faulty conceptualizations automatically leads to faulty predictions and explanations that emerge as misconceptions. In other words, identified misconceptions about the vapor pressure were in fact inferences from these faulty mental models.The findings of this study were consistent with the previous research on learners' misconceptions about vapor pressure (Gopal et al., 2004; Azizoğlu et al., 2006; Canpolat, 2006; Canpolat et al., 2006). However, the current study differed from those studies by identifying the prospective chemistry teachers' underlying mental models and associated reasoning patterns about vapor pressure that lead to reported misconceptions. In this respect, this study will make significant contributions to chemistry education. I could not claim that the identified mental models represent the actual mental models of any given student; however, I believe that identified mental models will help instructors better understand learners' difficulties in understanding vapor pressure and subsequently develop more effective ways of supporting the construction of scientific models.
The findings of this study have a number of important implications for teaching vapor pressure. Learning inherently involves building mental models. However, due to limited information processing capability, people cannot construct in their minds a complete blueprint of any system or content domain that is being learned (Johnson-Laird, 1983; Norman, 1983). Rather, they actively select and process certain information or perceptions in order to construct mental representations, which seem to cope with present or anticipated tasks (Johnson-Laird, 1983). Learners' preconceptions and existing mental models influence how they conceptualize, interpret, and think about newly learned domain concepts. Differences in learners' preconceptions will cause learners to build different mental models, even when they have the same curricular experiences. This study revealed that the majority of prospective chemistry teachers constructed three faulty mental models of vapor pressure as a result of traditional instructional practices. This study implies that perceived salient features of instructional examples and associated explanations significantly influence learners' mental representations. This implication naturally leads to a suggestion that the liquid–vapor equilibrium system (including entities and their properties and interactions in this system) and vapor pressure as an emergent property of that system should be explicitly represented and discussed while teaching vapor pressure.
From the perspective of teaching practice, the results of this study particularly suggest that the instructors should explicitly present and emphasize several points:
• Vapor pressure is an intensive property. It is directly proportional to vapor concentration instead of the total number of vapor particles at a constant temperature. Vapor concentration—and consequently vapor pressure of a particular liquid—depends only on the temperature.
• Liquid–vapor equilibrium is a dynamic equilibrium. When a system at equilibrium is perturbed, the equilibrium is reestablished at a different point. During this change the number of vapor particles could change, but the vapor concentration would not change as long as the temperature of the system remains constant.
• Vapor particles move freely in all directions and therefore the vapor pressure acts in all directions, not only downwards.
• There is no direct causal relationship between atmospheric pressure and the vapor pressure of a liquid. Although the boiling point of a liquid is determined and affected by both of them simultaneously, this does not necessitate a direct relationship between atmospheric pressure and vapor pressure of the liquid.
Experimental comparisons of the vapor pressure of different systems can be used to challenge and inform learners' mental models of vapor pressure. However, these practical experiences should be supported by animated or static microscopic illustrations to represent the dynamic nature of the liquid–vapor equilibrium system; entities and their properties and interactions in this system; and vapor pressure as an emergent property of this system. Appropriate microscopic representations can significantly facilitate the construction of more accurate mental models that are closer to scientific models (Schnotz and Bannert, 2003). Additionally, concept maps or causal maps can be useful in representing relevant entities in the system and relationships between them (Novak, 1990).
Despite the above suggested interventions, learners can still construct and maintain faulty mental models, or simply have difficulties in identifying the relevant entities and interactions in the system under consideration. In this study, some participants who understand and use the dynamic liquid–vapor equilibrium concept in their explanations then incorrectly employ the static equilibrium model in response to frictionless piston-cylinder questions. This finding showed that perceived salient features of the questions apparently triggered different mental models. This finding may also imply that these participants are in the transitional stage in the continuum from novice to expert. As seen in research on expertise (Chi et al., 1981; Kozma and Russell, 1997), novices often concentrate on surface features, whereas experts concentrate on conceptually relevant entities and their interactions. The ability to identify the relevant entities and underlying mechanisms and act accordingly in different contexts (despite distracting surface cues) is one of the important characteristics of experts (Chi et al., 1981). This implies that one of the important responsibilities of instructors is to develop learners' proficiency in selecting conceptually relevant entities and interactions and thinking accordingly in varying contexts (Taber, 2000).
Learners' faulty mental models can and do change over time, but often the original perceptions and beliefs are not easily changed, even in the face of contradictory evidence (Duit, 1999). Additionally, learners' alternative conceptualizations could be coherent and sensible from the learners' perspective (Gilbert and Watts, 1983; Vosniadou and Brewer, 1992; Vosniadou, 1999). When we consider the faulty mental models of vapor pressure identified in this study, it can be seen that these faulty mental models will generate correct predictions at least for some time. If we only asked learners to predict what will happen to vapor pressure if the temperature is changed, these models will generate a correct answer; however, when we change irrelevant factors (such as the surface area of the liquid or the volume of vapor) at a constant temperature, these models no longer yield a correct answer.
Very often, we create our mental models without metaconceptual awareness; we are generally unaware of inconsistencies and inadequacies in our mental models (Vosniadou, 1999). Therefore, it can be argued that helping learners develop awareness of the limitations of their own ideas is crucial for promoting conceptual change (Vosniadou, 1999). In order for the reconstruction of faulty mental models into scientific models, it is essential that the learners have opportunities to be aware of their mental models, continually reflect on them, and correct or refine those models as needed. In this respect, engaging learners in metacognitive reflection through dialogic argumentation can be especially useful for supporting the development of mental models that are closer to the scientific models (Driver et al., 2000).
Engaging learners in argumentation can help them to be aware of their own mental models, as well as others' mental models, and the strengths and limitations of these mental models. Particularly in contexts that are different from those used in teaching, learners need to rethink their models and see alternative conceptualizations of entities, their interactions, and the emergent results. Thus, engagement in a dialogical argumentation in which learners share, justify, and critique alternative conceptualizations will serve as the catalyst for the emergence of the scientific model as the shared mental model (van Zee and Minstrell, 1997).
Both the identification of the prospective chemistry teachers' common mental models of vapor pressure and the explanation of possible sources of the faulty mental models and instructional suggestions based on these findings are among the contributions of this study. Through the mental models identified in this study, science educators and researchers can better understand learners' conceptualizations and learning difficulties about vapor pressure, and can subsequently design more effective teaching approaches that promote the construction of scientific models.
Appendix: vapor pressure concept test
– What is vapor pressure? Please explain your answer.– Please compare the vapor pressures of systems A and B in the following questions, and please justify your answer. (If not stated otherwise, the volume of the closed container is 1 L and the temperature of the liquid is 25 °C.)
(1)
(2)
(3)
(4)
(5)
After the liquid–vapor equilibrium was established at 25 °C in a frictionless piston-cylinder (A), the piston is pulled upwards and fixed at that position (B).
(6)
After the liquid–vapor equilibrium was established at 25 °C in a frictionless piston-cylinder (A), the piston is pushed down and fixed at that position (B).
Acknowledgements
I would like to thank the reviewers for their critical review and valuable suggestions which helped to improve the manuscript. In addition, I would like to thank Dr Keith S. Taber for his constructive suggestions and helpful comments.References
- Andersson B., (1990), Pupil's explanations of some aspects of chemical reactions, Sci. Educ., 70, 549–563.
- Atkins P. and Jones L., (1997), Chemistry: molecules, matter, and changes, 3rd edn, Oxford: Oxford University Press.
- Azizoğlu N., Alkan M. and Geban Ö., (2006), Undergraduate pre-service teachers' understandings and misconceptions of phase equilibrium, J. Chem. Educ., 83, 947–953.
- Bar V. and Galili I., (1994), Stages of children's views about evaporation, Int. J. Sci. Educ., 16, 157–174.
- Bar V. and Travis A. S., (1991), Children's views concerning phase changes, J. Res. Sci. Teach., 28, 363–382.
- Borges A. T. and Gilbert J. K., (1999), Mental models of electricity, Int. J. Sci. Educ., 21, 95–117.
- Canpolat N., (2006), Turkish undergraduates' misconceptions of evaporation, evaporation rate, and vapour pressure, Int. J. Sci. Educ., 28, 1757–1770.
- Canpolat N., Pinarbasi T. and Sözbilir M., (2006), Prospective teachers' misconceptions of vaporization and vapor pressure, J. Chem. Educ., 83, 1237–1242.
- Chang J. Y., (1999), Teacher college students' conceptions about evaporation, condensation, and boiling, Sci. Educ., 83, 511–526.
- Chi M. T. H., Feltovich P. J. and Glaser R., (1981), Categorization and representation of physics problems by experts and novices, Cognitive Sci., 5, 121–152.
- Clement J. J. and Rea-Ramirez M. A., (2008), Model based learning and instruction in science, New York: Springer.
- Clough E. and Driver R., (1986), A study of consistency in the use of students' conceptual framework across different task contexts, Sci. Educ., 70, 473–496.
- Coll R. K. and Treagust D. F., (2003), Investigation of secondary school, undergraduate, and graduate learners' mental models of ionic bonding, J. Res. Sci. Teach., 40, 464–486.
- Creswell J. W., (1994), Research design qualitative and quantitative approaches, Thousand Oaks: Sage Publications, Inc.
- De Berg K. C., (1992), Students' thinking in relation to pressure–volume changes of a fixed amount of air: the semiquantitative context, Int. J. Sci. Educ., 14, 295–303.
- Driver R., Newton P. and Osborne J., (2000), Establishing the norms of scientific argumentation in classrooms, Sci. Educ., 84, 287–312.
- Driver R., Squires A., Rushworth P. and Wood-Robinson V., (1994), Making sense of secondary science: Research into children's ideas, London: Routledge.
- Duit R., (1999), Conceptual change approaches in science education, in Schnotz W., Vosniadou S. and Carretero M. (ed.), New perspectives on conceptual change, Oxford, England: Elsevier, pp. 263–282.
- Franco C. and Colinvaux D., (2000), Grasping mental models, in Gilbert J. K. and Boulter C. J. (ed.), Developing models in science education, Dordrecht, The Netherlands: Kluwer Academic Publishers, pp. 93–118.
- Franco C., Lins de Barros H., Colinvaux D., Krapas S., Queiroz G. and Alves F., (1999), From scientists' and inventors' minds to some scientific and technological products: relationship between theories, models, mental models, and conceptions, Int. J. Sci. Educ., 21, 277–291.
- Garnett P. J., Garnett P. J. and Hackling M., (1995), Students' alternative conceptions in chemistry: a review of research and implications for teaching and learning, Stud. Sci. Educ., 25, 69–95.
- Gentner D., (1983), Structure-mapping: a theoretical framework for analogy, Cognitive Sci., 7, 155–170.
- Gentner D. and Stevens A. L. (ed.), (1983), Mental models, Hillsdale, NJ: Lawrence Erlbaum.
- Giere R. N., (1999), Using models to represent reality, in Magnani L., Nersessian N. J. and Thagard P. (ed.), Model-based reasoning in scientific discovery, New York: Kluwer Academic/Plenum Press, pp. 41–57.
- Gilbert J. K. and Boulter C. J., (1998), Learning science through models and modeling, in Fraser B. J. and Tobin K. G. (ed.), International handbook of science education, Amsterdam: Kluwer Academic Publishers, pp. 53–66.
- Gilbert J. K. and Watts D. M., (1983), Concepts, misconceptions and alternative conceptions: changing perspectives in science education, Stud. Sci. Educ., 10, 61–98.
- Glaser B. and Strauss A. L., (1967), The discovery of grounded theory: strategies for qualitative research, Chicago: Aldine Publishing Company.
- Gopal H., Kleinsmidt J., Case J. and Musonge P., (2004), An investigation of tertiary students' understanding of evaporation, condensation and vapor pressure, Int. J. Sci. Educ., 26, 1597–1620.
- Greca I. M. and Moreira M. A., (2002), Mental, physical, and mathematical models in the teaching and learning of physics, Sci. Educ., 86, 106–121.
- Johnstone A. H., (1991), Why is science difficult to learn? Things are seldom what they seem, J. Comput. Assist. Learn., 7, 75–83.
- Johnson-Laird P. N., (1983), Mental models, Cambridge, MA: Harvard University Press.
- Kozma R. and Russell J., (1997), Multimedia and understanding: expert and novice responses to different representations of chemical phenomena, J. Res. Sci. Teach., 43, 949–968.
- Lin J.-W. and Chiu M.-H., (2007), Exploring the characteristics and diverse sources of students' mental models of acids and bases, Int. J. Sci. Educ., 29, 771–803.
- Lincoln Y. S. and Guba E. G., (1985), Naturalistic inquiry, Newbury Park, CA: Sage.
- Markman A. B. and Gentner D., (2001), Thinking, Annu. Rev. Psychol., 52, 223–247.
- Nakhleh M. B., (1992), Why some students don't learn chemistry, J. Chem. Educ., 69, 191–196.
- Nersessian N. J., (1992), In the theoretician's laboratory: thought experimenting as mental modeling, in Hull D., Forbes M. and Okruhlik K. (ed.), PSA 1992, Philosophy of Science Association, pp. 291–301.
- Nersessian N. J., (1999), Model-based reasoning in conceptual change, in Magnani L., Nersessian N. J. and Thagard P. (ed.), Model-based reasoning in scientific discovery, New York: Kluwer Academic/Plenum Press, pp. 5–22.
- Nersessian N. J., (2002), The cognitive basis of model-based reasoning in science, in Carruthers P. and Stich S. (ed.), Cognitive basis of science, New York: Cambridge University Press, pp. 133–153.
- Nersessian N. J., (2008), Creating scientific concepts, Cambridge: MIT Press.
- Norman D. N., (1983), Some observations on mental models, in Gentner D. and Stevens A. L. (ed.), Mental models, Hillsdale, NJ: Erlbaum, pp. 7–14.
- Novak J. D., (1990), Concept mapping: a useful tool for science education, J. Res. Sci. Teach., 27, 937–950.
- Osborne R. J. and Cosgrove M. M., (1983), Children's conceptions of the changes of state of water, J. Res. Sci. Teach., 20, 825–838.
- Park O. and Gittleman S. S., (1995), Dynamic characteristics of mental models and dynamic visual displays, Instr. Sci., 23, 303–320.
- Petrucci R. H., Harwood W. S. and Herring F. G., (2002), General chemistry: Principles and modern applications, 8th edn, London: Prentice Hall International.
- Pfundt H. and Duit R., (2006), Bibliography – Students alternative frameworks and science education, Kiel, Germany: University of Kiel Institute for Science Education.
- Schnotz W. and Bannert M., (2003), Construction and interference in learning from multiple representation, Learn. Instr., 13, 141–156.
- Smith J. P., diSessa A. A. and Roschelle J., (1993), Misconceptions reconceived: a constructivist analysis of knowledge in transition, J. Learn. Sci., 3, 115–163.
- Solomon J., (1993), The social construction of children's scientific knowledge, in Black P. J. and Lucas A. M. (ed.), Children's informal ideas in science, London: Routledge, pp. 85–101.
- Sumfleth E. and Telgenbüscher L., (2001), Improving the use of instructional illustrations in learning chemistry, in Behrendt H., Dahncke H., Duit R., Gräber W., Komorek M., Kross A. and Reiska P. (ed.), Research in Science Education – Past, Present, and Future, Dordrecht, Boston, London: Kluwer, pp. 289–294.
- Taber K. S., (2000), Multiple frameworks?: evidence of manifold conceptions in individual cognitive structure, Int. J. Sci. Educ., 22, 399–417.
- Taber K. S., (2001), Building the structural concepts of chemistry: some considerations from educational research, Chem. Educ. Res. Pract., 2, 123–158.
- Taber K. S., (2002), Chemical misconceptions—Prevention, diagnosis and cure: Vol. I: Theoretical background, London: Royal Society of Chemistry.
- Taber K. S., (2008), Exploring conceptual integration in student thinking: evidence from a case study, Int. J. Sci. Educ., 30, 1915–1943.
- Taber K. S. and García-Franco A., (2010), Learning processes in chemistry: drawing upon cognitive resources to learn about the particulate structure of matter, J. Learn. Sci., 19, 99–142.
- Talanquer V., (2006), Commonsense chemistry: a model for understanding students' alternative conceptions, J. Chem. Educ., 83, 811–816.
- Talanquer, V., (2009), On cognitive constraints and learning progressions: the case of “structure of matter”, Int. J. Sci. Educ., 31, 2123–2136.
- van Zee E. H. and Minstrell J., (1997), Reflective discourse: Developing shared understandings in a high school physics classroom, Int. J. Sci. Educ., 19, 209–228.
- Vosniadou S., (1994), Capturing and modeling the process of conceptual change, Learn. Instr., 4, 45–69.
- Vosniadou S., (1999), Conceptual change research: state of the art and future directions, in Schnotz W., Vosniadou S. and Carretero M. (ed.), New perspectives in conceptual change, Amsterdam: Pergamon, pp. 3–14.
- Vosniadou S., (2007), The conceptual change approach and its re-framing, in Vosniadou S., Baltas A. and Vamvakoussi X. (ed.), Reframing the conceptual change approach in learning and instruction, Amsterdam, The Netherlands: Elsevier Science, pp. 1–15.
- Vosniadou S. and Brewer W. F., (1992), Mental models of the earth: a study of conceptual change in childhood, Cognitive Psychol., 24, 535–585.
- Wandersee J. H., Mintzes J. J. and Novak J. D., (1994), Research on alternative conceptions in science, in Gabel D. L. (ed.), Handbook of research on science teaching and learning, NY: Macmillan Publishing Company, pp. 177–210.
- Yalcin F. A., (2012), Pre-service primary science teachers' understandings of the effect of temperature and pressure on solid–liquid phase transition of water, Chem. Educ. Res. Pract., 13, 369–377.
| This journal is © The Royal Society of Chemistry 2014 |