Undergraduate students' conceptions of enthalpy, enthalpy change and related concepts
Tor
Nilsson
* and
Hans
Niedderer
School of Education, Culture and Communication, Mälardalen University, PO Box 325, SE-63105, Eskilstuna, Sweden. E-mail: tor.nilsson@mdh.se; Tel: +46 (0)16-153419
First published on 2nd October 2013
Abstract
Research shows that students have problems understanding thermodynamic concepts and that a gap exists at the tertiary level related to more specific chemistry concepts such as enthalpy. Therefore, the aim of this study is to construct undergraduate students' conceptions of enthalpy, its change and related concepts. Three explorative small-scale studies were conducted at two Swedish universities. Questionnaires, exam questions, hand-ins and interviews covered a range of issues from chemical thermodynamics in general to specific questions about enthalpy and its change, internal energy and its change, heat and work. Data were analysed iteratively and qualitative categories were constructed (F1–F2, F4–F9). The underlying conceptions indicate that constant pressure is explicitly expressed but disregarded as the answer is given (F1), that work is described as mechanical work (F2), that enthalpy is used as a form of energy (F4), and that enthalpy is used for enthalpy change and vice versa (F5). The logical conceptions indicate that molar enthalpy determines the heat given off by a reaction and not the path taken (F6), that constant pressure/constant volume and the definition of enthalpy change are problematic (F7), that students argue for the case when ΔH = ΔU instead of ΔH = q (F8), and that there are different ways to interpret the given tasks (F9). This study offers insight into the way students use enthalpy and its change when arguing and solving qualitative tasks. How the categories may be used as well as other implications for teaching and research are addressed in this paper.
Introduction and aim
Science teaching in general – and specifically the teaching of thermodynamic concepts – is based on algorithms, and teachers view qualitative understanding as a consequence of performing calculations. However, the opposite is often shown in research (e.g.Mazur, 1997; Carson and Watson, 1999, 2002; Carson, 2001; Sözbilir, 2001; Kim and Pak, 2002; Gabel, 2003; Greenbowe and Meltzer, 2003). One common suggestion is to introduce qualitative descriptions before calculations in order to overcome the problem.Research has shown that chemical thermodynamics is problematic for students (Tsaparlis, 2008). Upper secondary students taking chemistry and physics base their understanding of thermodynamics on the forms of the energy framework from physics (Goedhart and Kaper, 2002). In this framework the internal energy is the sum of all forms (Kaper and Goedhart, 2002), but there are crossovers with thermodynamics because fundamental concepts such as energy, work and heat are part of both frameworks.
However, the teaching of thermodynamics changes from upper secondary school to higher education, in the sense that the meanings of concepts change as do the entire frameworks in which they are included (Goedhart and Kaper, 2002). Often, students' prior understanding of the fundamental concepts is assumed by teachers (Carson and Watson, 1999). Undergraduate students have already used concepts such as enthalpy changes, exothermic and endothermic, heat and work during upper secondary education in order to describe the energetics of chemical reactions. In other words, there is a relationship between physics and chemistry (Goedhart and Kaper, 2002) and there is a gap between secondary- and tertiary-level education (de Jong, 2000). In order to address undergraduate students' difficulties with concepts used in chemistry, applications and situations must be taken from chemistry (e.g.Carson, 2001; Sözbilir, 2001; Goedhart and Kaper, 2002). Since only a few studies have focused on higher education and students' understanding of enthalpy, its change and relationships with internal energy, heat and work, (e.g.Carson and Watson, 1999; Carson, 2001; Sözbilir, 2001; Greenbowe and Meltzer, 2003; Nilsson and Niedderer, 2012), the aim of this study is to construct students' conceptions of enthalpy, its change and related concepts as qualitative/conceptual tasks. The research question is:
• What conceptions of enthalpy, its change and related concepts can be constructed in order to describe students' difficulties with specific concepts?
Theoretical background
This section includes the scientific background and a description of conceptions, understandings and algorithmic/conceptual questions, and ends with previous research findings.Scientific background
Enthalpy H and internal energy U and their changes are related, but the similarities and differences are complex. They are state functions, and the changes include heat and work. The unit is Joule (J) and internal energy is the sum of different forms (Kaper and Goedhart, 2002), but neither enthalpy nor enthalpy change can be transformed into a form of energy. Both ΔU and ΔH can be used to describe what happens during a chemical reaction, for instance bond energy or bond enthalpy, but in upper secondary school and at the undergraduate level enthalpy and its change are often used instead of internal energy and its change. A change in enthalpy is defined as dH = dU + d(pV) or ΔH = H2 − H1. Since d(pV) is a change of a product, mathematics states that d(pV) = p![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dV + V
dV + V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dp. The overall change of H does not depend on the path taken from H1 to H2, but dU is path-dependent and ΔU = q + w is valid for a closed system (Laidler et al., 2003; Atkins and de Paula, 2005; IUPAC, 2007). One reason to introduce enthalpy and its change is the possibility to disregard the expansion work, since dH = dU + d(pV) or dH = đq + đw + đw′+ p
dp. The overall change of H does not depend on the path taken from H1 to H2, but dU is path-dependent and ΔU = q + w is valid for a closed system (Laidler et al., 2003; Atkins and de Paula, 2005; IUPAC, 2007). One reason to introduce enthalpy and its change is the possibility to disregard the expansion work, since dH = dU + d(pV) or dH = đq + đw + đw′+ p![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dV + V
dV + V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dp where đ indicates a path-dependent variable and w′, for instance, is electrical work. The above equation probably contains mathematical notions that undergraduate chemistry students are unaware of, and a more simple – although not entirely correct – way is to express the definition as ΔH = q + w + pΔV + VΔp.
dp where đ indicates a path-dependent variable and w′, for instance, is electrical work. The above equation probably contains mathematical notions that undergraduate chemistry students are unaware of, and a more simple – although not entirely correct – way is to express the definition as ΔH = q + w + pΔV + VΔp.
If expansion work is defined as đw = −pex![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dV where pex is the external pressure (i.e. the pressure the system does work against in order to expand), hence pex = p, the equation becomes dH = đq + đw′+ V
dV where pex is the external pressure (i.e. the pressure the system does work against in order to expand), hence pex = p, the equation becomes dH = đq + đw′+ V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dp, which is integrated into ΔH = q + w′ + VΔp. If the pressure is constant, like in many chemical reactions, Δp = 0. If no other work is allowed, w′ = 0, the enthalpy change is heat at constant pressure ΔH = qp. However, enthalpy changes may also be determined as dH = đq + đw + đw′+ p
dp, which is integrated into ΔH = q + w′ + VΔp. If the pressure is constant, like in many chemical reactions, Δp = 0. If no other work is allowed, w′ = 0, the enthalpy change is heat at constant pressure ΔH = qp. However, enthalpy changes may also be determined as dH = đq + đw + đw′+ p![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dV + V
dV + V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dp, which, at constant volume dV = 0 and when no other work is allowed, becomes dH = đq + V
dp, which, at constant volume dV = 0 and when no other work is allowed, becomes dH = đq + V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dp or integrated ΔH = q + VΔp, where VΔp is technical work. In many undergraduate textbooks the constant pressure and only expansion work case is emphasized (e.g.Chang, 2007; Zumdahl and Zumdahl, 2007), but sometimes this definition is not valid (e.g. in a fuel cell where w′ ≠ 0).
dp or integrated ΔH = q + VΔp, where VΔp is technical work. In many undergraduate textbooks the constant pressure and only expansion work case is emphasized (e.g.Chang, 2007; Zumdahl and Zumdahl, 2007), but sometimes this definition is not valid (e.g. in a fuel cell where w′ ≠ 0).
Conceptions
Research on conceptions may be “concerned with the qualitative differences among the conceptions that students use to explain scientific phenomena and examine students' topic-related understanding of scientific concepts” (Duit and Treagust, 1998, p. 6). Conceptions are often viewed as mental models (Petri and Niedderer, 1998; Hartmann and Niedderer, 2005; Treagust and Duit, 2008a, 2008b). Single individuals (Harrison et al., 1999) and groups of students (Thomas and Schwenz, 1998) have been followed, and students' conceptions of single concepts (e.g.Carson and Watson, 1999), single tasks (e.g.Sözbilir, 2001) or a phenomenon (e.g.Andersson and Wallin, 2000) have been researched. Different methods and techniques have been used, and the tradition is mostly cognitive or constructivist whereby individuals have parallel conceptions (Petri and Niedderer, 1998; Hartmann and Niedderer, 2005), a conceptual profile (Mortimer, 1995), misconceptions and preconceptions (Driver and Easley, 1978) or intermediate conceptions (Driver, 1989; Niedderer et al., 2007).The scientific concepts serve as a frame when analysing students' responses. Then, descriptions of how students perceive the theory differently may be constructed. Alternative conceptions are different from the scientific descriptions (Carson and Watson, 1999) and may arise due to the use of similar words in, for instance, everyday life or formal and informal education, which may lead to robust frameworks such as the physical framework forms of energy, which often describe how students understand chemical thermodynamics (Carson, 2001; Goedhart and Kaper, 2002). However, conceptions are never nonsense (Vosniadou, 2008a), can exist in parallel (Petri and Niedderer, 1998; Hartmann and Niedderer, 2005), and are more likely to occur in some situations (Mortimer, 1995). Therefore, one aim of science teaching is to let students develop a more valid scientific conception, and the conceptual change may be done in many ways (Vosniadou, 2008b). On the path towards the scientific conception, different intermediate conceptions may be identified (Petri and Niedderer, 1998).
Understanding and algorithmic/conceptual questions
Conceptual understanding is a complex phenomenon (Nieswandt, 2007). Often, the question is “Does he/she understand X?” and the answer is yes or no (White and Gunstone, 1992), thus not offering any relevant information. How someone understands X is a more valid question. Darmofal et al. (2002) summarize conceptual understanding as the ability to apply knowledge to a range of examples and circumstances that are not necessarily known beforehand. Using, adapting and changing the knowledge is also necessary, but teaching is often focused on promoting a fragmented acquisition of discrete facts and algorithms instead of creating a knowledge structure (Claesgens et al., 2002). In research, both tasks/problems or the answer given may be quantitative or qualitative. Also, questions can be solved algorithmically or conceptually (Bowen and Bunce, 1997). For instance:• Quantitative questions with algorithmic or conceptual answers.
What is the enthalpy change when MgO is formed if 3.4 g Mg is burnt in air? (algorithmic)
If 3.4 g Mg is burnt in air is the enthalpy change more, less or equal to −601.7 kJ? (conceptual)
• Qualitative questions with algorithmic or conceptual answers.
When is the enthalpy change the heat? (algorithmic)
In which set of apparatuses does the measured heat equal the enthalpy change? (conceptual)
In this description, questions with numerical values are quantitative and the answer is either algorithmic (calculation) or conceptual (motivation). Mazur (1997) uses conceptual (theoretical)–conventional (calculation) questions instead. In this study, the terms qualitative and conceptual are used synonymously in order to describe students' understanding or the characteristics of a question. In Mazur's study about 52% of the students succeed equally well with both kinds of questions, 9% are less fortunate on the conventional questions and 39% are less fortunate on the conceptual questions. But Mazur's results were not unique.
Bodner and Herron (2002) write that “Problem-solving research has provided strong evidence that our students, successful as well as unsuccessful problem solvers, don't grasp the concepts very well. Indeed, one of the most important contributions of research in this area has been the revelation of just how poorly students may understand the ideas underlying problems they have answered correctly. This revelation is despite the fact that teachers frequently justify their emphasis on numerical problem-solving by arguing that it enhances conceptual understanding” (p. 259). Related to Bodner and Herron's statement is how exams are designed (White and Gunstone, 1992), since many teachers believe that numerical problem-solving will enhance conceptual understanding and therefore primarily include calculations in exams. However, algorithmic problem-solving exercises do not promote conceptual understanding and students will, most likely, never get that far (Gabel, 2003). Empirical evidence suggests that a real problem exists since science teaching does not prepare students for conceptual problem-solving (e.g.Kim and Pak, 2002; Gabel, 2003). Students are most likely to succeed in doing chemical thermodynamics calculations, but lack qualitative understanding (e.g.Goldring and Osborne, 1994; Carson and Watson, 1999, 2002; Sözbilir, 2001). The course content must be reduced and qualitative aspects must be addressed prior to algorithmic problem-solving. Carson (2001), Gabel (2003) and Johnstone (2010) argue in a similar manner. In this study, qualitative or conceptual understanding of enthalpy and enthalpy change is an amalgam of the following statements, which all the written tasks and interviews addressed in some way:
• The student shows that he/she is aware of when and whether enthalpy and enthalpy change are applicable to a certain problem. This corresponds to the purpose of introducing the concepts in the scientific theory.
• The student uses enthalpy and enthalpy change to explain a phenomenon (e.g. reaction or experiment).
• The student uses enthalpy and enthalpy change to predict a phenomenon.
• The student explains the difference between enthalpy, enthalpy change and related concepts.
• The student uses enthalpy and enthalpy change in more than one context.
• The student interprets and analyses a phenomenon with the aid of figures and diagrams.
Previous research findings
In chemistry education researchers have mainly focused on bonds, bond energies and enthalpy change (e.g.Granville, 1985; Boo, 1998; Barker and Millar, 1999, 2000; Boo and Watson, 2001; Galley, 2004). Both Carson (2001) and Sözbilir (2001) argue that there are too few empirical studies investigating undergraduate students' understanding of chemical thermodynamics. The results presented below are obtained from both physics and chemistry education research, and different ages are covered. In the latter part of this section the focus is on results regarding enthalpy, its change, heat and expansion work.Table 1 shows conceptions of heat and work that are relevant to this study as approximately the same ages are covered. As seen in Table 1, the first law of thermodynamics (ΔU = q + w) is problematic for students: heat and work as states instead of processes and parts of the energy conversion between different forms (e.g.Johnstone et al., 1977; Driver and Warrington, 1985; van Roon et al., 1994; Sözbilir, 2003).
| Results and conceptions | Age | Source | Relevant for conception(s) |
|---|---|---|---|
| Heat is a form of energy | 6–13 | Sözbilir (2003, p. 28) | F4 |
| Adolescents and adults | Lewis and Linn (1994) | ||
| Heat is not an extensive quantity but an intensive one | 15–16 | Sözbilir (2003, p. 29) | F6 |
| Two bodies with the same temperature have the same energy or heat | 15–16 | Sözbilir (2003, p. 29) | F6 |
| Heat is considered a state quantity instead of a process quantity | Students | van Roon et al. (1994) | F5 |
| q, heat, is energy and the unit is Kelvin (q = T or q = ΔT is used) | Students | Greenbowe and Meltzer (2003) | F4 |
| The amount of heat liberated does not depend on the work done | ∼16–18 | Johnstone et al., (1977) | F6, F7 |
| Energy and work are not separated | 13, 16, 18 | Driver and Warrington (1985) | F5 |
| Work is always involved when energy is transferred | 13, 16, 18 | Driver and Warrington (1985) | F2 |
| Instead of a thermodynamic concept, work is generally considered a mechanical concept | Students | van Roon et al. (1994) | F2 |
| Work is regarded as a convertible form of energy instead of a form of energy conservation | Students | van Roon et al. (1994) | F2 |
| Work is not genuinely seen as a process | Students | van Roon et al. (1994) | F2 |
| The gas leaving the system brings energy with it and this energy is the work | Students | Carson (2001) | F2 |
In chemistry, heat and work are energy inputs or outputs and not forms. They stem from the fundamental process known as a chemical reaction, hence bond breaking and bond formation. The relationships between bonding and thermodynamics are difficult for upper secondary students to understand and, in fact, students make no links between the two areas (Barker and Millar, 2000).
In Table 2 the main results with respect to enthalpy and its change, (internal) energy and its change, heat and work are summarized. Note that Carson's (2001) results on enthalpy and expansion work can also be read in Carson and Watson (1999). According to Carson (2001), work is sensitized post teaching, even if the meaning is still unclear. Post teaching, four of 22 students explain expansion work acceptably, while eight have a range of alternative conceptions. The most common one, held by three students, is that gas only does work when it pushes a piston. Also, work raises weights and is hence considered a mechanical concept, as previously stated by van Roon et al. (1994). According to Carson (2001), one problem is that university teachers assume prior knowledge regarding some concepts (e.g. heat and work), and she suggests that thermodynamic entities are defined qualitatively before they are defined quantitatively; for instance, in Carson's study empirical evidence suggests that students have a limited understanding of the expression ΔH = ΔU + pΔV; they read it but do not know the meaning of the symbols.
| Results and conceptions | Age | Source | Relevant for conception(s) |
|---|---|---|---|
| Endothermic and exothermic are solely based on the sign of ΔH | Students | Granville (1985) | F1, F5 |
| Imprecise terminology regarding enthalpy and enthalpy change | Students | Beall (1994) | F5 |
| Prior to teaching, work is not associated with chemical reactions | Students | Carson (2001) | F2 |
| pV work is unknown prior to teaching | Students | Carson (2001) | F2 |
| Post teaching, work is sensitized but the meaning remained unclear | Students | Carson (2001) | F2 |
| Post teaching, a moving piston or raising of a weight is needed to recognize work | Students | Carson (2001) | F2 |
| Students frequently forget one or several of heat, constant pressure or pV work as they define enthalpy change, making the definition incomplete | Students | Carson (2001) | F1, F6, F7, F8 |
| Enthalpy is a form of energy | Students | Carson (2001) | F4 |
| Enthalpy change is heat at constant volume | Students | Carson (2001) and Sözbilir (2001) | F1, F6, F7, F8 |
| Enthalpy change is heat since no work is done | Students | Carson (2001) and Sözbilir (2001) | F7 |
| Students can calculate the reaction of enthalpy but are not aware that it equals the heat | Students | Sözbilir (2001) and Greenbowe and Meltzer (2003) | F1, F5 |
| The same amount of heat is given out to the surroundings at both constant p and V | Students | Sözbilir (2001) | F6 |
| Bond enthalpies are used in Hess' law instead of formation enthalpies | Students | Teichert and Stacy (2002) | F4 |
| Students separate ΔG from ΔH but they do not know the difference | Students | Ribeiro et al. (1990) | F5 |
| If work is described, the most common way of doing this is without reference to volume and pressure | Students | Nilsson and Niedderer (2012) | F1, F2 |
| When work is described, specific descriptions of volume and its change are more common than specific descriptions of pressure | Students | Nilsson and Niedderer (2012) | F1, F6, F7, F8 |
| Expansion work is not described correctly in actual situations | Students | Nilsson and Niedderer (2012) | F1, F6, F7, F8 |
| Technical work is not often described | Students | Nilsson and Niedderer (2012) | F6, F7, F8 |
One issue regarding enthalpy changes is the relationship between enthalpy and reaction heat, since a majority of students do not distinguish between heat and reaction enthalpy (Sözbilir, 2001). A similar result is presented by Greenbowe and Meltzer (2003) regarding calorimetry, system/surroundings and reaction heat. In the calorimeter the measured heat is the enthalpy change due to constant pressure and only expansion work, and the authors state that “It is notable that none of the students in this study acknowledged the fact that since the reactions occurred under conditions of constant pressure, the heat of reaction (qrxn) is equal to the enthalpy change of the reaction (ΔHrxn)” (p. 786). The results expressed by both Sözbilir (2001) and Greenbowe and Meltzer (2003) are comparable since heat and reaction heat, enthalpy change and reaction enthalpy are, in the described situations, identical. Two authors have previously criticized the imprecise terminology of chemists and textbooks (Zemansky, 1974; Beall, 1994). The use of terminology leads to a situation in which enthalpy and enthalpy changes are expressed synonymously, for instance enthalpy of vaporization instead of enthalpy change due to vaporization (Zemansky, 1974). The result is that students do not realize that Δ indicates a change in enthalpy or free energy. Following Beall's (1994) line of argument, there is a problem in the relationship between the concepts. Enthalpy is related to the process, but Beall does not mention the condition of constant pressure. However, constant pressure is also problematic since students describe enthalpy change as heat at constant volume (Carson, 2001; Sözbilir, 2001). Accordingly, students make connections between enthalpy change and expansion work, arguing that enthalpy change is heat when no work is done (ΔV = 0). Five of 22 students in Carson's (2001) interview study explicitly state constant volume, and Sözbilir's (2001) analysis of questionnaire responses groups this description as one of four that together form one misunderstanding. Recently, Nilsson and Niedderer (2012) have analysed how students use pressure and volume when describing work, expansion work and technical work. Their contextual analysis shows that about 86% and 69% of the descriptions offered by two groups of students, respectively, are either incomplete or incorrect. However, they point out that students more easily relate volume to work than pressure.
Furthermore, Carson (2001) describes that students perceive enthalpy as a form of energy and the framework forms of energy, which guides their understanding of enthalpy, entropy and Gibbs energy (Carson and Watson, 1999, 2002; Carson, 2001). Also, students often describe heat and work as forms of energy (van Roon et al., 1994), which is labelled the forms of energy context and serves as a framework for how students understand thermodynamics (cf.Goedhart and Kaper, 2002; Kaper and Goedhart, 2002). In the forms of energy context, energy is transformed between different energy forms and the internal energy is the sum of the different forms. In chemistry education research, it is shown that the different forms are not transferable to a chemistry context (Martins and Cachapuz, 1990).
Research design and methods
Here the design and sampling as well as the different data collections are described.Design and sampling
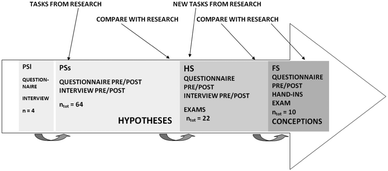
The research design was flexible, explorative and small-scale. A flexible design can be characterized as open to input during the research process (Robson, 2002). The design, data collection methods and data analysis affected each other. An explorative design tries to determine what is happening, seeks new insights and searches for possible results that can be further investigated. It generates hypotheses rather than confirmations, and as a consequence the results presented here can be further elaborated. In Fig. 1, a chronological description of the empirical studies is presented. Some general connection to previous research, data collection and the results in the different studies are also shown. The pilot studies are not described in detail here, but both were designed to increase the researchers' awareness of students' preconceptions regarding different contents generally taught in an undergraduate chemistry course. Also, the pilot studies provided details for the development of the main study, for instance a focus on students' understanding of enthalpy, enthalpy change and related concepts as qualitative/conceptual questions were included (cf.Bowen and Bunce, 1997; Mazur, 1997; Claesgens et al., 2002; Kim and Pak, 2002; Niaz, 2002; Gabel, 2003).During ongoing empirical studies, all chemistry programs at the university were cancelled and therefore the design of the main study became small-scale. Instead of n ≈ 60, a total number of 22 students participated. As a consequence another small-scale (n = 10) follow-up study was conducted at a different university; see more below.
The sampling was always based on voluntary participation and followed the non-randomized principles of “convenience sampling” (Bryman, 2008, p. 183) and “purposive sampling” (Bryman, 2008, p. 458). The difference between these types is that the former is based on available informants and factors the researcher cannot control, while the latter is based on a specific purpose that guides the sampling, and only people relevant to the research questions are included. How participants are chosen affects generalizability and, as volunteering is important, the entire sampling process is to some extent already biased.
With respect to the focused aim from the pilot studies of the main study, categories describing students' answers regarding different contents were created. Here, only categories regarding enthalpy, enthalpy change and related concepts were developed, and in order to saturate them a follow-up study was designed. A comparable group was purposely sought at another university, but the teacher found the tasks too difficult and suggested a group taking a course in chemical thermodynamics at the same university instead. This course was at a more advanced level than the undergraduate course and had fewer students, whom were asked to participate. Table 3 summarizes the data collection instruments and number of participating students in all studies.
| Study | Instrument | Number of students |
|---|---|---|
| PSs | Questionnaire pre teaching | 64 |
| Interview pre teaching | 7 | |
| Video lab open calorimetry and Hess' law | 3 | |
| Video lab electrochemistry | 2 | |
| Questionnaire post teaching | 30 | |
| Video Ellingham diagram | 8 | |
| Interview at the end of semester | 5 | |
| HS | Questionnaire pre teaching (HSE1) | 12 |
| Interview pre teaching (HSI1) | 5 | |
| Exam 1a (HST1a) | 17 | |
| Exam 1b (HST1b) | 16 | |
| Exam 2a (HST2a) | 17 | |
| Exam 2b (HST2b) | 14 | |
| Questionnaire four weeks post teaching (HSE2) | 6 | |
| Interview four weeks post teaching (HSI2) | 4 | |
| FS | Questionnaire pre teaching (FSE1) | 9 |
| Hand-in 2 (FSInl2) | 9 | |
| Hand-in 3 (FSInl3) | 7 | |
| Exam (FST) | 9 | |
| Questionnaire two weeks post teaching (FSE2) | 6 | |
Data collection
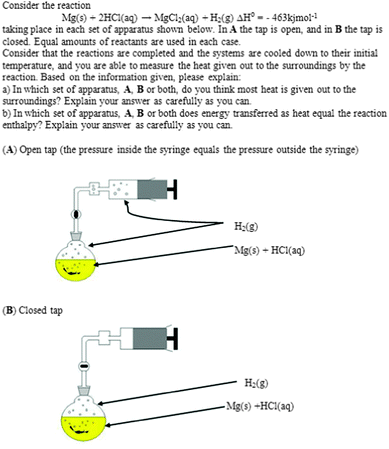
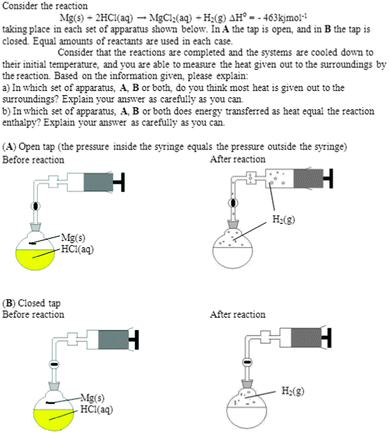
Here, the data collections in the studies are described, with focus on the main and follow-up studies (see Table 3). In Appendix 1 the most important questions in the study are summarized. During the research process, the ethical guidelines developed by the Swedish Research Council (Vetenskapsrådet, 2002) have been followed.In order to fit the setting, most questions were either new or redesigned from previous research (cf.Thomas and Schwenz, 1998; Carson, 2001; Sözbilir, 2001; Galley, 2004). In some cases, for instance one multiple choice question, the distractors were the results from the pilot study: Question (a) was Have you previously heard the concept (word) enthalpy? with the possible answers Yes, No, and Don't know. Follow-up Question (b) was Which of the following alternatives do you consider to be the best explanation of enthalpy? with the possible answers (1) It describes the energy change for a reaction; (2) It describes the heat transfer during a reaction; (3) It describes a possible state for a system; (4) It describes bonds being broken and formed; and (5) None of the above, since… As can be noted, alternatives 1, 2 and 4 are related to enthalpy change instead of enthalpy. Another task was one developed from Sözbilir's (2001) original. It was a key task in the data collections, and the reaction between magnesium metal and hydrochloric acid in a closed apparatus at either constant pressure A or constant volume B was visualized. The questions were (a) In which set of apparatus, A, B or both, do you think most heat is given out to the surroundings? and (b) In which set of apparatus, A, B or both, does energy transferred as heat equal the reaction enthalpy?
This task, together with a figure, was used during the following data collections: HST1a as Question 5ab and HSE2 as Question 7ab.
Since some questions/tasks were used in exams the course teacher, L3, had the final say on which ones to include and how they were included. In the interviews, the students explained three or more of the following reactions taking place in front of them: the endothermic dissolution of ammonium chloride, the exothermic dissolution of sodium hydroxide, the decomposition of sodium hydrogen carbonate and the reaction between magnesium and hydrochloric acid. These reactions have been used before in chemistry education research (e.g.de Vos and Verdonk, 1986; Boo, 1998; Carson, 2001). In the main study, categories describing the content-specific understanding were constructed. In total 969 written responses were analysed, resulting in 115 pages of transcripts.
Qualitative analysis
Niedderer's (2001) description of iterative analysis and the analytical methodology of this study are described here.Qualitative analysis by Niedderer
Fig. 2 shows an applied version of Niedderer's (2001) description of iterative analysis. Iterative means that the analytical process includes both inductive and deductive elements, hence abduction. The aim of the analytical process is to generate theoretical descriptions, conceptions, about students' understanding of some content or phenomenon. The analysis is affected by the researcher's frame of reference and data collected during the research process. Examples of the first are the use of previous research, the scientific content and the wish to offer implications for teaching. These factors influence the researcher to interpret data in a certain way, although Niedderer emphasizes theoretical frames more than is described in Fig. 2. Depending on how the theoretical frames are applied, the researcher can approach the data either deductively or inductively, for instance if hypotheses from previous research form the basis of the analysis or not. If an inductive step is taken, it is not in its most absolute form since there is a frame of reference.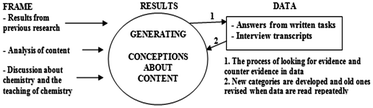 | ||
| Fig. 2 The iterative process for generating conceptions about content (cf.Niedderer, 2001). | ||
In Niedderer's description, iterative also means that the process is systematic and non-linear. It means that data analysis has a determined purpose but the analytical path cannot be pre-determined. The data and the frame of reference create a process which cannot be predicted but instead has to be described afterwards.
The relationship between this description and the actual analysis is described below, and at the same time the example will clarify the dynamic nature described in Fig. 2. The method of analysis is complex, and in order to make implicit issues explicit the steps of data analysis are summarized and examples from the authentic analytical process are given. But doing so also means that our description of the analytical process cannot only be traced back to Niedderer's description of iterative analysis; other descriptions of performing qualitative analysis are also of importance (e.g.Glaser and Strauss, 1967; Erickson, 1998; Carson, 2001; Sözbilir, 2001; Bryman, 2008).
The analytical methodology
The general and specific guidelines used during the entire data analysis process are described below. Since the analytical process led to a gradually focused aim, which in turn led to a changed analytical process, this section is characterized by the description of a changed and increasingly focused analytical process. The guidelines are intertwined and should best be viewed as related principles rather than unique ones:• Compile data
In order to prepare the coding, all data were compiled or transcribed immediately after collection and, if possible, frequencies were calculated (e.g. which answer was chosen).
• Explain wrong answers
Wrong answers to certain tasks were described in a process following Niedderer's (2001) description and the view of an alternative conception; hence, the process explicitly included content in the data-analytical frame of reference. This part of the analysis primarily resulted in conceptions with logical patterns.
• Describe patterns
A greater proportion of data was analysed especially during the main study, and it was not only wrong answers that were analysed. Since the chemical department was shut down, thus lowering the number of informants, both general patterns and specific patterns of certain tasks were sought. During this process, conceptions were constructed to describe both correct and incorrect answers given by students; hence data were analysed without prejudice, which is common in qualitative analysis (e.g.Glaser and Strauss, 1967; Erickson, 1998; Bryman, 2008). Even if an answer is correct it could include incorrect descriptions with respect to scientific theory, but these conceptions are not alternative and logical in the sense that they explain wrong answers. Instead, they describe how students tend to use the concepts (here denoted as underlying).
In the Results section, patterns that create the conception are described. Using empirical evidence to support a pattern is like painting a picture for the reader. Sometimes it is almost impossible to give one quote that clearly states yes, this is the case…, but through a systematic reading of the data and interpretation of the students' expressions, a picture emerged. Thus, it is important to keep in mind that one student's expression is not evidence of a student conception; instead, it was evidence contributing to the interpretation and the pattern. Interpreting meant twisting and turning the data, a process which was easier with interview transcripts since students had to elaborate on their answers during the interviews. Evidence from questionnaires, hand-ins and exams was also used in order to deepen and widen the picture. Evidence was included to support the interpretation or sometimes to show that different interpretations were possible.
• Coding and saturation
Data were coded to construct the conceptions. The first step was to become familiar with the data, to start asking questions of the data and then code it in an unprejudiced manner. Words, rows, sentences, passages and the text as a whole were coded, thus labelling different evidence with the same code. The constructed codes changed during the coding process. If similar codes and patterns emerged more general codes and categories were defined, and as the comparison continued the codes and categories became more general and abstract. When necessary additional codes and categories were constructed, some related to old ones and some completely new. Finally, conceptions were constructed, and when no empirical evidence changed the definition or description of a conception as it was applied on the data, each conception was considered to be saturated (cf.Glaser and Strauss, 1967).
• Increased generalizability, abstraction, data reduction
The use of more data during the analytical process increased the generalizability, as the constructed conception became more abstract and complex. The conception was used to analyse more data, and during this process generalizability was related to applicability rather than previous research. As codes were merged to form a conception, the analytical process also led to data reduction.
• A gradually changing and increasingly focused aim
The analytical process was driven by the revised aim in the empirical studies. To test the descriptive categories, or conceptions, that had been constructed in the main study, a new empirical study was designed. The aim was therefore gradually revised from chemical thermodynamics in general to enthalpy, enthalpy change and related concepts. To gradually deepen and focus the aim of a study, data collection and data analysis are important in the qualitative research process since data analysis aids in the formulation of interesting questions.
• Content
During the data analysis, reference to the subject content was a principle. Due to the focused research process the researchers' understanding of the content changed, especially during the follow-up study. As students' explanations and descriptions were compared to the definition of enthalpy and enthalpy change, the authors posed questions that forced a revision of their understanding of the content. This process was necessary in order to understand the students' responses. This means that even though the content was in principle the same, the researchers' frame regarding this content changed as the analysis progressed.
• Using literature
Hypotheses, categories and conceptions were formulated as results in the different empirical studies and research literature was included more and more as a comparison and frame. Tasks from the literature were also included throughout the research process.
• Interrater agreement
To increase trustworthiness an interrater reliability test, testing how well two independent researchers categorize the answers, was performed during the pilot study with satisfactory results. During the main and follow-up studies the second author repeatedly categorized answers to the most relevant tasks. The categorization was done in one of two ways: either questions were categorized using all conceptions, or a conception was chosen and then applied to the data. Whenever answers were categorized differently, the conceptions and definitions were discussed and revised. Due to continuous reviews, almost complete agreement was reached.
(1) When the amount of substance is identical the enthalpy change as heat is identical regardless of the apparatus in which the reaction takes place. The amount of substance is the key to understanding why students tend to describe the heat as equal in Apparatuses A and B. As the analysis continued the conception changed, and at one stage during the follow-up study it was described with the following sub-conceptions:
Conception 2. If it is the same reaction it is always the same enthalpy change and same heat
F2.1 If same amount of substance then same heat
F2.2 If same reactants/products then same heat
F2.3 Since C p = CVq is the same
F2.4 If same state function then same heat
F2.5 Closed valve gives alternative explanations based on F2.1–F2.4
F2.6 Different power but same q
In the Results section some features of those described here can still be seen in F6, The same reaction gives the same enthalpy change and heat, but for instance F2.5 is now described in Conception F9 instead.
Results
The conceptions constructed are presented in Table 4 and the presentation is elaborated below, where the salient aspects of the conceptions are given. Conception F3 is excluded in this presentation since it was based on another analytical method (authors, in press).| Conceptions | Sub-conception (F) or pattern (M) | Frequencies HS | Frequencies FS |
|---|---|---|---|
| F1. The mantra of enthalpy change as heat at constant pressure |
F1.1. The required demand for constant pressure is explicitly expressed but disregarded as the answer is given.
F1.2. The required constant pressure is not mentioned as the enthalpy change as heat is explained. |
F1.1 24% (12/51)
F1.2 27% (21/79) |
F1.2 11% (1/9)
F1.2 29% (12/42) |
| F2. If a visual change in the position of an artefact cannot be observed, work during a chemical reaction is difficult for students to identify. |
M1. When a chemical reaction occurs, the work is difficult to identify since it is abstract.
M2. When an artefact moves, for instance a piston, work is easier for the student to identify. |
17% (14/82) | 36% (25/69) |
| F4. Enthalpy is a form of energy. |
M1. Enthalpy and enthalpy change are implicitly and explicitly described synonymously with energy–energy change.
M2. Enthalpy and enthalpy change follow the first law of thermodynamics, ΔH = q + w. M3. Enthalpy and enthalpy change are described as forms of energy, for instance as taking place in energy transformations. |
26% (23/90) | 27% (13/49) |
| F5. Enthalpy and enthalpy change describe similar aspects of a chemical reaction. | — | 12% (6/50) | 16% (7/43) |
| F6. The same reaction gives the same enthalpy change and heat. |
M1. Same R ⇒ same q
M2. Same R ⇒ same ΔH ⇒ same q M3. Same R ⇒ same q ⇒ same ΔH |
Question A 29% (5/17)
Question B 12% (2/17) |
— |
| F7. Enthalpy change is heat when no work is done. |
M1. Enthalpy change is heat in Apparatus B since ΔH = ΔU + pΔV and ΔV = 0, hence ΔH = q as the work is 0 (incorrect choice/incorrect argument).
M2. Enthalpy change is heat in Apparatus A since ΔH = q + VΔp and Δp = 0, hence ΔH = q as the work is 0 (correct choice/incorrect argument). |
26% (6/23) | 39% (13/33) |
| F8. Enthalpy change is heat since the energy transferred as heat is ΔU | — | Question B 35% (6/17) | 13% (4/30) |
| F9. An alternative interpretation of the apparatuses provides an alternative logic |
M1. Efficiency/flow
M2. Static figures M3. An open or closed system |
21% (7/34) | 47% (7/15) |
Conception F1. The mantra of enthalpy change as heat at constant pressure
Students have learned to express parts of the definition by heart and the conception includes two subcategories: first F1.1, that the required demand for constant pressure is explicitly expressed but disregarded as the answer is given, whereby students explicitly state that enthalpy change is heat at constant pressure but argue for a case in which the pressure is not constant. For instance, S11 (HST1a, Question 5a), Both give off the same amount of heat because the Enthalpy does not change if the same products and reactants are involved ΔH = ΔHprod − ΔHreac (which is only valid at constant pressure). S11 explicitly states that the equation is only valid at constant pressure, but argues that the heat is the same in both Apparatuses A (constant p) and B (constant V).The second subcategory is F1.2, where the requirement constant pressure is not mentioned as the enthalpy change as heat is explained, whereby students do not use the definition of enthalpy change as heat at constant pressure (when only expansion work is allowed) when answering questions explicitly addressing the definition. For instance, S7 (HST1a, Question 5b), It must be B too, since the volume is constant, thus no work is done and all energy is heat. Here, enthalpy change is related to constant volume instead of constant pressure. The definition of enthalpy change is not explicitly mentioned, which indicates sub-Conception F1.2.
Conception F2. If a visual change in the position of an artefact cannot be observed, work during a chemical reaction is difficult for students to identify
The conception indicates that a common case in chemistry, expansion work against the surroundings (atmosphere), is not regarded as work by students. Also, the abstract boundary between a system and its surroundings, where the actual expansion work is taking place, is easier to understand when the movement of a randomly chosen artefact, for instance a piston, can be identified by the naked eye. The two patterns that have emerged are M1, When a chemical reaction occurs, the work is difficult to identify since it is abstract, and M2, When an artefact moves, for instance a piston, work is easier for the student to identify. One example of M2 is the answer given by SK3 (FSInl2, Question 9a): It ought to be System B that is giving away the most heat. In System A, energy will be used to push the piston, hence energy will be spent on that and not as much can be transferred as heat. Here, the student tells in which set of apparatuses, A, B or both, the most energy is given as heat. The correct alternative is chosen, and in the explanation of work an artefact (piston) is affected (M2). In the following transcript it can be noted that S7 identifies that no work is being done. However, work is described as the energy transformation between different forms in terms of transformation of potential energy. At the same time, something should do the transformation. This something may push something if the pressure is increased; hence something may be interpreted as an artefact, especially if it makes a turbine run (M2).S7 (HSI2, parts of excerpt from t ∼ 5.20–7.20, ammonium chloride dissolving in water, I = interviewer, S = student):I: […] is any work done[?]
S: no, I don't think so
I: no, how come[?]
S: because [silence 2 sec] no work is done [silence 2 sec] in some way there's no [silence 2 sec] transformation of potential energy or something like that, so no, I don't think any is done
[…]
I: but if you were to explain the concept of work, what do you mean by saying work [?]
S: work, then [silence 2 sec] something should do a transformation of energy [silence 7 sec] you do so something gets a higher energy [silence 4 sec], for instance if the pressure increases and you push something so it moves, then…
I: ok
S: …I think work is done [.] You make a turbine run
I: but here we have a chemical reaction
S: hmm
I: but isn't it…
S: but no work
It is noted that Conception F2 is primarily found for tasks investigating the artefact's importance for work. In the interview, S7 identifies that no work is done because nothing interacts. In fact, in both pre and post interviews, even though gas is produced the students describe an absence of work since no artefacts are included.
Conception F4. Enthalpy is a form of energy
The conception indicates that enthalpy, energy, heat and work are related to each other and that students describe, apply and explain the concepts as if they are forms of energy. This means that students tend to understand enthalpy as related to physics and the forms of energy framework in which different forms are transformed into each other. Three patterns have emerged: M1, M2 and M3. In M1, enthalpy and enthalpy change are implicitly and explicitly described synonymously with energy/energy change. The following transcript shows one of a series of statements made by S1. Returning to a previous statement regarding enthalpy and its change, S1 stated that it is measured in joules per mol, which is true, but as joule is energy it is possible to use both energy and enthalpy and since Joule is the unit, enthalpy must be energy. S1 (HSI2, short excerpt from t ∼ 11.50–12.50, ammonium chloride dissolving in water, explain using energy and enthalpy, I = interviewer, S = student):I: […] because now in your explanation you began with enthalpy and endothermic and then you went on using energy
S: [laughs]
I: what [silence 2 sec] how is it possible that we can talk of both energy and enthalpy for one and the same reaction [?]
S: [silence 2 sec] yeah, I just think they're almost the same but I don't know […] it's measured in joules per mol or something like that and […] joule is energy [laughs]
The other two patterns are M2, enthalpy and enthalpy change follow the first law of thermodynamics, ΔH = q + w, and M3, enthalpy and enthalpy change are described as forms of energy, for instance as taking place in energy transformations. The answer from SK8 serves as an example of both patterns: SK8 (FSInl2, Question 9b): In B since the reaction is taking place in a closed vessel, then no mass transfer will take place and the enthalpy change will be transformed into heat. SK8 explains that enthalpy change is transformed into heat (M3). But, Apparatus B is actually the wrong answer and the heat does not correspond to the enthalpy change as heat. In B no work is done; hence pattern (M2) is exemplified.
Conception F5. Enthalpy and enthalpy change describe similar aspects of a chemical reaction
This conception is based on all collected data and describes both correct and incorrect answers as well as how students use the concepts in discussions. The picture emerging in the analysis is as follows. Enthalpy has the same meaning as enthalpy change and is sometimes used as an abbreviation for, for instance, reaction enthalpy. This means that students use enthalpy to describe the process or actual reaction instead of the state before and after a reaction. In the following example the student argues that the most heat is given off in Apparatus B, which is correct, but uses the state function enthalpy H = E + PV to describe an enthalpy change: S8 (HST1a, Question 5a): B gives the most heat to the surroundings since in Case (A) energy is used for work. H = E + PV. The same tendencies hold for other concepts as well, for instance in the following example where enthalpy (H) and Gibbs free energy (G) are expressed as ΔH and ΔG. Negative enthalpy means ΔH < 0, not H < 0.S1 (HST2b, Question 3): Enthalpy ΔH, Gibbs free energy ΔG and entropy S […] Gibbs free energy ΔG can be: […] the reaction with negative enthalpy (ΔH)[…] The reaction with positive enthalpy must have had ΔH < TΔS…Conception F6. The same reaction gives the same enthalpy change and heat
This conception is related to the tasks based on Sözbilir's (2001) original. The common denominator is the conclusion that the same reaction (amount of substance and/or reactants and products) will always give the same heat regardless of whether the process is carried out at a constant pressure or volume. Hence, students draw the wrong conclusions about heat, change in internal energy, enthalpy change and the meaning of a state. Three patterns have emerged: M1, Same R† ⇒ same q; M2, Same R ⇒ same ΔH ⇒ same q; and M3, Same R ⇒ same q ⇒ same ΔH.In the following example, molar enthalpy seems to be a valid description of how the students describe the same heat in Apparatuses A and B (M2). The argument is that the path taken is unimportant, which is the opposite of the scientific view.
S12 (HST1a, Question 5a): At constant pressure the amount of heat, change in enthalpy, will be equal to the energy of the system. Same reaction, same amount of substance, same reactants. When all reactants have reacted, the sum of the same – that is the path the reaction takes – is unimportant. All reactants have been used in both cases. The heat to the surroundings should be the same.
Conception F7. Enthalpy change is heat when no work is done
This conception offers insight into some answers to the task designed from Sözbilir's (2001) original. Here, students describe that enthalpy change is heat when no work is done. Two related patterns have emerged. The first is M1, enthalpy change is heat in Apparatus B since ΔH = ΔU + pΔV and ΔV = 0, hence ΔH = q as the work is 0 (incorrect choice/incorrect argument) – for instance in the answer by S7 (HST1a, Question 5b), it must be B too, because the volume is constant and therefore no work is done all energy is transformed into heat. Here, constant volume is explicitly addressed and the student chooses the wrong Apparatus, B. However, it is true that all energy is transferred as heat in Apparatus B due to constant volume. Here we argue that the student bases the argument on ΔH = ΔU + pΔV, which is only valid at constant pressure, and since ΔV = 0 and pΔV = 0 the logical answer seems to be enthalpy change as heat when no work is done. The other pattern is M2, enthalpy change is heat in Apparatus A since ΔH = q + VΔp and Δp = 0, hence ΔH = q as the work is 0 (correct choice/incorrect argument) – for instance given by SK6 (FSInl2, Question 9b), Apparatus A, since dH = dq + V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dp where dp = 0. Here the student chooses the correct apparatus but uses a formula for constant volume when volume increases.
dp where dp = 0. Here the student chooses the correct apparatus but uses a formula for constant volume when volume increases.
Conception F8. Enthalpy change is heat since the energy transferred as heat is ΔU
This conception, a deepening of Conception F7 in which enthalpy change is heat when no work is done is the foundation, describes that ΔH = ΔU = q instead of ΔH = q and the fact that energy transferred as heat is denoted ΔU (or ΔE). S1 (HST1a, Question 5b): in Case (B) the volume is constant → ΔH = ΔE. In Case (A) the volume varies and the expansion requires energy otherwise given to enthalpy ΔH ≠ ΔE.The student shows ΔH = ΔU (ΔE) instead of ΔH = q, implicitly indicating that the energy transferred as heat is ΔU (ΔE). The student also identifies constant volume in B and varying volume in A. Since the student expresses that volume is constant → ΔH = ΔE it is reasonable to assume that the student bases the explanation on the definition of enthalpy change at constant pressure ΔH = ΔU + pΔV.
Conception F9. An alternative interpretation of the apparatuses provides an alternative logic
Students have interpreted Apparatuses A and B in a different way than anticipated. The analysis of the interpretations gave the three following patterns: M1, Efficiency/Flow, in which answers regarding the greatest efficiency or flow instead of heat are grouped; M2, Static figures, is best described as a picture. Here, both apparatuses have a specific constant pressure and volume, hence students describe them as states instead of as ongoing processes; in M3, An open or closed system, answers in which students describe an open valve as an open system are included, as described in the following example: S19 (HST1a, Question 5b), B energy transfer as heat should be the same as enthalpy change in Case (B) with a closed valve but not with the configuration. The explanation is based on ΔH° = −464 kJ/mol−1, which multiplied by mol/amount of substance for the products gives the heat transfer in kilo-Joule/kJ. Since the connection between the two is based on molar amounts, they can only be the same if no substances are leaking out, something which will surely happen if the valve is open, since hydrogen gas/H2(g) would leak out and change the molar amounts compared to a closed valve keeping the hydrogen there.In this response there is a passage to an open valve, or tap, indicating that the amount of substance changes (M3). Again, molar enthalpy seems to be a valid description and an open valve is an open system and not a closed system at constant pressure. At the same time, a closed valve means a closed system instead of a closed system and constant volume.
Discussion and concluding remarks
In this section the contribution to the knowledge base, different methodological considerations and implications are addressed.The constructed conceptions – contribution to the knowledge base
The conceptions constructed during the analytical process describe qualitative differences (cf.Duit and Treagust, 1998) and follow Carson's and Watson's (1999) description that no matter how small the differences are from the scientific conceptions, the label is an alternative conception. The scientific frame is described in order to make the analysis possible; thus, how students perceive the theory relates to the comparison between the answer and the theory. The frequencies are based on the number of answers categorized as the conception/total number categorized answers to all tasks in which the conception is present. Thus the frequencies may seem higher or lower than they actually are, but according to research they are probably more common than expressed here (Mortimer, 1995).There are different ways to contribute to the knowledge base. New conceptions can be constructed; other conceptions have been constructed before but new data provide new insights; and finally, previous conceptions can be generalized as they are.
Conception F1, The mantra of enthalpy change as heat at constant pressure, has not been stated before. It firstly describes how students express constant pressure but argue for a case in which constant pressure is not valid, and secondly it includes the absence of constant pressure when it is explicitly asked for. Both expressed patterns give a clearer picture of how students apply the definition of enthalpy change and constant pressure. The entire conception is of the underlying type, according to the description of a mantra (Nationalencyklopedin, 2012), which means that F1 does not govern how students argue; instead, it is a description of how they include/exclude different statements in their answers as they argue another case. As noted, the mantra differs from the scientific definition since only expansion work is left out. Although it has not been described previously, some similarities with previous research exist; for instance, Greenbowe and Meltzer (2003) note that none of the students in their study equate reaction heat with enthalpy change as processes are carried out at constant pressure. Carson (2001) expresses a similar finding based on the fact that only two of 22 students describe enthalpy change completely. Carson states “The special importance of constant pressure and the limitation to pV work appeared not to have impinged on students” (p. 114). Nilsson and Niedderer (2012) show results similar to those found by Carson (2001), since expansion work is not explained correctly in actual situations and since students relate volume to work more easily than pressure. Also, Carson (2001) and Sözbilir (2001) report that students frequently state that enthalpy change is heat at constant volume, indicating that there is a general problem with constant pressure and only expansion work. Earlier, Granville (1985) reported that students tend to focus only on the sign ΔH when determining whether a reaction is endothermic or exothermic, and miss the fact that the definition is only valid if the only work allowed is expansion work.
Learning to express things by heart is an example of algorithmic learning, and the mantra is no different. Researchers have suggested that the problem is how the theory is applied rather than how the calculations are done (e.g.Bowen and Bunce, 1997; Mazur, 1997; Claesgens et al., 2002; Niaz, 2002; Gabel, 2003).
Conception F8, Enthalpy change is heat since the energy transferred as heat is ΔU, also provides new insight into how students argue when enthalpy change is the heat given off in an apparatus. As described above, Conception F8 is an elaboration of F7. As it seems, students actually argue for when ΔU is the energy transferred as heat. This means that students end up choosing an incorrect answer. Thus far, no evidence of this conception has been found, and Sözbilir (2001) only describes results similar to Conception F7, as does Carson (2001). However, when analysed with Nilsson and Niedderer's (2012) matrix, the examples given would yield both incomplete and incorrect descriptions of the expansion work. Therefore, it is possible that this conception deepens previous results regarding why students argue that enthalpy change is heat at constant volume. Logic shows that the student argues when ΔH = ΔU instead of ΔH = q, implicitly stating that ΔU = q.
Another way to contribute to research is to describe conceptions that are similar or identical to previous research results. Conception F6, The same reaction gives the same enthalpy change and heat, has been stated before but this study has provided new insights. Students argue that the heat and enthalpy change is equal in both Apparatuses A and B (constant p vs. constant V). It has previously been identified that students frequently forget one or more of the factors heat, constant pressure and expansion work as they define enthalpy change (Carson, 2001). This study supports this finding, since students tend not to focus on constant pressure and expansion work as they argue why the heat must be the same. Nilsson and Niedderer's (2012) matrix shows how students use pressure and volume when explaining work, expansion work and technical work, and stress the importance of using the general definition of enthalpy change since it is difficult for students to argue for a constant volume/varying pressure case if they have only been introduced to ΔH = ΔU + pΔV. This finding is in accordance with Carson's (2001) and Sözbilir's (2001) results that enthalpy change is heat at constant volume. Johnstone et al. (1977) also showed a result among younger students that the amount of heat does not depend on the work done.
In Sözbilir's (2003) review heat is an intensive quantity, but this study contradicts this since molar enthalpy can be used to explain students' answers; hence, heat is an extensive quantity. Also, Sözbilir (2001) reports that students confuse total energy with heat and for Question A in the post test a frequency of 5% is reported. Even though a limited number of students participated in this study, empirical evidence suggests that answers to Question b can be included in the categorization, at least in the main study.
Like F6, Conception F9 – An alternative interpretation of the apparatuses provides an alternative logic – provides similar but not identical insight to previous research. The conception indicates that students interpret the questions and figures in alternative ways, and as a result the conception is fruitful from both a methodological and conceptual point of view. The fact that the figures have been interpreted as static in the main study led to a change in the follow-up study: Conception F9 was still present, but the pattern indicating static figures did not appear. However, patterns showing students' descriptions of efficiency and/or flow were identified in both studies as well as in one of Sözbilir's (2001) misunderstandings. A key issue regarding the patterns seems to be how systems are described in textbooks. In some common textbooks open, closed and isolated systems are presented according to the test-tube principle (Atkins and de Paula, 2005; Chang, 2007) and visual differences between system/surroundings/universe (Laidler et al., 2003; Atkins and de Paula, 2005; Chang, 2007; Zumdahl and Zumdahl, 2007), but tasks to prepare students for differentiating between these fundamental aspects of a chemical system are seldom included. Differentiating between properties of the system and the surroundings is important, but Carson (2001) shows that students have difficulty separating a system from its surroundings, although results suggest that post teaching the situation has improved. However, Greenbowe and Meltzer (2003) show the opposite result. In their study, 22% (of n = 207) correctly identify the chemical reaction as the system while only 6% correctly identify the surroundings. Although research shows some opposing tendencies, the common ground is to differentiate a system from its surroundings in order to identify the properties of the reacting systems.
The last four conceptions generalize previous research findings. Since scientific knowledge is based on different authors showing similar results, this result is important since the different studies concern different countries, educational levels and methodologies. Below, the last four conceptions are related to previous research findings.
Conception F2 – If a visual change in the position of an artefact cannot be observed, work during a chemical reaction is difficult for students to identify – shows the importance of an artefact when identifying expansion work since work against the surroundings (atmosphere) is disregarded. We do not argue against the obvious fact that work is done, but merely describe that without the artefact no work is done. Work, as described in chemistry, has been found to be difficult for students to identify. Carson (2001) describes that “students had difficulty in associating work with chemical reactions” (p. 118), and presents findings such as work equals bond breaking, the presence of a piston or raising weights. Nilsson and Niedderer (2012) show that in their two groups 59% and 37% of the answers regarding work are left blank. This could be interpreted as students having difficulty seeing the work actually being done. Empirical evidence suggests that work is done when gas and an arbitrarily chosen artefact interact. The formation of gas that expands is not regarded as work. The results here, together with Carson's results, indicate that the description of work by van Roon et al. (1994) as a mechanical concept still seems to be the best and, sometimes, the only valid description of how students perceive work in chemistry.
Conception F4, Enthalpy is a form of energy, describes when enthalpy and its change are described and applied in a similar way to (internal) energy and its change and hence no differentiation is made between the concepts. Neither enthalpy nor enthalpy change is a form of energy by definition (Kaper and Goedhart, 2002). Also, neither enthalpy nor enthalpy change follows the first law of thermodynamics. However, it is true that the first law of thermodynamics is a fundamental part of enthalpy change. In this study students commonly express enthalpy, Gibbs energy and entropy as forms of energy and, in the explanation of the framework forms of energy, transformation is a key concept (Carson, 2001). In a review it is shown that students base their perceptions of different concepts on transformations, and the framework is robust (Goedhart and Kaper, 2002). Therefore, university students accept new thermodynamic concepts as part of the framework (Carson, 2001). The following interpretation of students' answers sheds some light on why it might be easy to include enthalpy as part of the framework: if heat is a form of energy and enthalpy change is heat then, surely, enthalpy change must be a form of energy.
In Conception F5, Enthalpy and enthalpy change describe similar aspects of a chemical reaction, both H = U + pV and ΔH = ΔU + Δ(pV) are used, for instance, to describe changes, and when students use enthalpy it is related to changes. Beall (1994) reports similar findings for enthalpy and Gibbs energy, and students forget to indicate the change for internal energy (van Huis and van den Berg, 1993). More recently, Sözbilir (2001) reports that students use reaction heat, enthalpy change and enthalpy similar to Beall's (1994) findings, and like Sözbilir (2001), Greenbowe and Meltzer (2003) show that students have difficulty relating reaction heat to the enthalpy change. Long ago, Driver and Warrington (1985) showed similar patterns for work and energy since students do not tend to separate them. However, the present study differs from most previous ones since both correct and incorrect answers are analysed. A feature of the conception is how students use the concepts in actual settings, and even though the students choose/give a correct answer the concepts may be used incorrectly. Improper use does not necessarily result in an incorrect answer.
In Conception F7, Enthalpy change is heat when no work is done, both patterns indicate that students pick an alternative that does not correspond to the definition of enthalpy change they themselves are using. This in turn leads to contextually correct descriptions of volume but incorrect descriptions of pressure (e.g. if ΔV = 0 then pΔV = 0 even if the context is Δp ≠ 0 and ΔV = 0). Nilsson and Niedderer (2012) show that students often correctly relate volume to work, but pressure is not mentioned often. Also, Carson (2001) and Sözbilir (2001) describe results similar to those presented here. In the latter study a misunderstanding is present in 47% and 36% of the answers on the pre and post questionnaires, respectively, where one sub-conception of four is similar to F7. In Carson's (2001) interview study, five of 22 students explicitly state that enthalpy change is heat when the volume is constant since no work is done. Although related to the previous results, the different educational levels in this study provide further insight into students' understanding of the relationship between work and enthalpy change.
Based on the results presented and the contribution to the knowledge base, it is possible to conclude that enthalpy and its change do not necessarily offer students new ways of solving, anticipating and explaining problems in chemistry.
Relationships between the conceptions
During the data analysis, the labels underlying and logical were used to group the conceptions; see Table 5. More general tendencies were denoted as underlying, whereas alternative logics to the scientific conception were denoted as logical. Grouping the conceptions also means that they are each placed on one side of a continuum, but in reality they should be regarded as more/less underlying/logical. When arguing for the different conceptions, it became obvious that the division is rather rough. However, the main idea was to show how students used the concepts (i.e. underlying) or argued for a certain incorrect answer when there was a predefined correct answer (i.e. logical). Also, the underlying conception is more general and unprejudiced than a logical conception, more fluid on the continuum and goes beyond the correct/incorrect answer to a certain task. However, it is still an alternative conception since it deviates from the scientific frame. Since underlying and logical conceptions are constructed in similar ways, as described in the data analysis, they may seem more alike than they actually are since the idea of a conception is to describe a characteristic, which means that statements based on the definition of the conception are either included or excluded. The grouping may also be traced back to the methods, tasks and questions used (e.g.Carson, 2001; Sözbilir, 2001). Carson (2001) formulates conceptions based on interview data and a validated list of statements. Sözbilir (2001) formulates conceptions of different tasks based on a coding scheme. One reason for the need to divide the conceptions into underlying and logical is the use of different tasks and data collection methods. The data reduction led to some general patterns, the underlying conceptions and some task specific conceptions, the logical ones. In retrospect, the data analysis carried out can perhaps best be described as a synthesis of Carson's (2001) and Sözbilir's (2001) analyses.| Type of conception | Conception |
|---|---|
| Underlying | F1. The mantra of enthalpy change as heat at constant pressure |
| F2. If a visual change in the position of an artefact cannot be observed, work during a chemical reaction is difficult for students to identify | |
| F4. Enthalpy is a form of energy | |
| F5. Enthalpy and enthalpy change describe similar aspects of a chemical reaction | |
| Logical | F6. The same reaction gives the same enthalpy change and heat |
| F7. Enthalpy change is heat when no work is done | |
| F8. Enthalpy change is heat since the energy transferred as heat is ΔU | |
| F9. An alternative interpretation of the apparatuses provides an alternative logic | |
The conceptions are related to each other in different ways, but each describes unique properties of the students' answers. Three relationships between the conceptions are described below.
• Relationship R1: evidence of two conceptions in one answer
One answer may act as evidence of two different conceptions, since an answer can contain different theoretical aspects. An example of this is Conceptions F4 and F5, which can be used in parallel. Theoretically they are mutually exclusive, but they can be used to describe the same answer, as described here:
S1 (HST1a, Question 5b): In Case (B) the volume is constant → ΔH = ΔE. In Case (A) the volume varies and the expansion requires energy otherwise given to enthalpy ΔH ≠ ΔE.
Conception F4 is applied, since the expansion requires energy otherwise given to enthalpy. Conception F5 is applied since enthalpy is expressed but related to ΔH ≠ ΔE.
• Relationship R2: conceptions that are logically alike
If conceptions are logically alike it means that one conception leads to another, since they share some logic. An example of this is Conceptions F7 and F8:
S1 (HST1a, Question 5b): In Case (B) the volume is constant → ΔH = ΔE. In Case (A) the volume varies and the expansion requires energy otherwise given to enthalpy ΔH ≠ ΔE. Here F7 is activated, since volume is constant → ΔH = ΔE which implicitly indicates that pΔV = 0. Conception F8 gives a deeper understanding of the answer, since the student argues for the case when ΔH = ΔU (ΔE) instead of ΔH = q. As a consequence, volume is constant and no work is done (F7). However, not all answers categorized as F7 can be categorized as F8.
• Relationship R3: conceptions facilitate each other
These conceptions are mutually exclusive but facilitate each other. An example of this is Conceptions F6 and F1 (F1.1), since F6 facilitates F1:
S11 (HST1a, Question 5a): both give off the same amount of heat because the Enthalpy does not change if the same products and reactants are involved ΔH = ΔHprod− ΔHreac(which is only valid at constant pressure). The student explicitly states that the equation or statement is only valid at constant pressure, indicating that constant pressure is known. At the same time, the actual argument is that both apparatuses give off the same heat. The relationship between the conceptions exists, since F6 facilitates F1.
Decisions during the data analysis – a gradually focused aim
This study used local opportunities to develop teaching and construct new conceptions. Since the pilot study with teachers and students was designed to develop a frame for the study (e.g. identify preconceptions and develop relevant tasks), the original idea to follow one course and its teacher was kept even though the chemistry department was shut down just prior to the main study. Also, since only a limited number of studies about undergraduate students' conceptions of enthalpy and its change and related concepts can be found (e.g.Carson and Watson, 1999; Carson, 2001; Sözbilir, 2001; Greenbowe and Meltzer, 2003; Nilsson and Niedderer, 2012), the research design was kept but was adjusted to the decrease in participants. The explorative, flexible design allowed for this, and the aim was gradually narrowed, which is also common in qualitative research (Glaser and Strauss, 1967; Robson, 2002). However, in retrospect some things could have been done differently, for instance the data collection.Since we wanted to keep the study within the developed frame (i.e. L3's aims), we chose not to include data from other universities during the main study. Instead a larger group was sought for the follow-up study, but the new teacher found the tasks too difficult for the students and recommended another, more advanced, course with fewer students instead. Although we find the categories to be saturated, additional data may shed new light on the categories developed.
A different approach would be to use Carson's (2001) or Sözbilir's (2001) research designs, which would offer an opportunity to quantitatively analyse the data, or at least use the authors' developed categories. However, with this study design and the focus of the pilot study with students (i.e. chemical thermodynamics in general), the authors' categories would not have corresponded with most data. The use of pre-designed categories could also have hindered one outcome in the research process, namely the first author's ability, through a systematic re-reading of the data, to assign definitions and categories to the patterns emerging from the data. This ability must be developed during a research process, and this was considered important. However, the tasks developed from Sözbilir's (2001) original should have been used in interviews as well in order to allow for students to fully describe their answers and justifications.
Different techniques were used (e.g. multiple choice, yes/no, explanatory or descriptive) to address the description of qualitative or conceptual understanding. However, it became obvious that some techniques were more fruitful than others; for instance, the multiple choice question in the main study with the distractors taken from the pilot study with students, in which an almost identical open question was answered by the students. Hence, it is possible that all answers appealed to some students but since only one answer could be chosen there is a possibility that the frequencies of other distractors are higher than we can show. The yes/no questions served as a complement to other questions, but in retrospect it was easy for the students to guess correctly. The explanatory or descriptive questions were developed during the research process. As the analysis shows, different conceptions were activated, sometimes more than one in one answer. New possibilities therefore exist; see below.
Generalizability and trustworthiness
As noted above, one indication of the quality of this research is the generalizability of the results. Not surprisingly, similar results have been found• by different authors in different countries,
• at different educational levels, and
• with different data collection methods (triangulation).
The use of previously developed tasks and methods increases generalizability since the results are comparable. As some tasks were used both in the main study and follow-up study, generalization across educational levels is possible. Although important from a methodological perspective, the re-designing of the task based on Sözbilir's (2001) original decreased the generalizability. However, the conceptions have been shown to be applicable to different data and situations (i.e. questionnaires, exams, hand-ins and interviews), which increases generalizability.
Generalizability is the possibility that the results are valid in a larger population, and since this study shows similarities to previous studies in the field we believe the results presented here are valid outside the range of this study, in some cases even in upper secondary education.
In this research project, different methodological and analytical choices have been argued and discussed. The empirical evidence included has always been based on a scientific frame of reference and personal interpretations. The personal frame of reference and the interpretations are clarified in the qualitative analysis, but personal values have not guided the analysis. In constructing a conception, the researcher is always the constructor; therefore, it is obvious that a researcher cannot be completely objective, but the researcher's personal views should not have affected the research process or results. It is thus important to stress that the iterative research process was based on empirical evidence interacting with a frame of reference.
The credibility of research is increased when recommendations are followed and other researchers partake in the research process. The design, methodological concerns, data analysis, theoretical frames and conceptions have been discussed at conferences with supervisors, experienced teachers and other PhD students. Using different methods and data triangulation also increases credibility (Robson, 2002). However, using different methods may lead to difficulties in comparing data due to different units of analysis. In order to increase credibility, evidence from interviews was part of the definition of a conception, but the interviews were not analysed in the same way as the written data. Also, Sözbilir's (2001) way of including data from interviews was followed.
Implications for research and teaching
Research on the teaching and learning of thermodynamics at different educational levels has long been discussed (e.g.Duit and Haeussler, 1994; Lewis and Linn, 1994; Kaper and Goedhart, 2002; Millar, 2005; Engström, 2008; Johnstone, 2010). For chemical thermodynamics and higher education Carson (2001), Sözbilir (2001) and Goedhart and Kaper (2002) give more specific suggestions, and we support the idea that qualitative discussions should precede quantitative manipulations of variables (White and Gunstone, 1992; Carson and Watson, 1999; Carson, 2001; Bodner and Herron, 2002; Gabel, 2003; Johnstone, 2010).According to Carson (2001) (and we agree), one problem is that university teachers assume prior understanding of heat and work. They should do this, of course, since fundamental concepts like (internal) energy, heat and work are included in upper secondary education, but it is possible that students' prior understanding is not in accordance with teachers' beliefs. Discussing these concepts first will most likely illuminate the differences, and calculations should be excluded in the first step (cf.Carson, 2001; Gabel, 2003). Also, as enthalpy and its change are introduced using a frictionless piston in a cylinder together with an algorithmic derivation, empirical evidence suggests that the purpose of introducing enthalpy in chemistry will most likely evade students (cf.Carson, 2001; Sözbilir, 2001). Work, especially expansion work, must be further emphasized and exemplified in teaching since one purpose of introducing enthalpy and its change is related to expansion work.
In theory, it is also possible to exclude the abstract concept of enthalpy from teaching altogether, but in practice it seems more difficult. Some students need enthalpy further along their educational path as well as in future employment. Hence, it is difficult to give clear suggestions concerning whether or not to exclude enthalpy. However, one hypothesis is that most students will understand the more fundamental parts of chemical thermodynamics better if enthalpy is excluded.
In Sweden, enthalpy is introduced at the upper secondary level and in most examples used it is not needed as a theoretical foundation. In fact, most students are already in the forms of energy framework (Goedhart and Kaper, 2002), and chemistry teachers can therefore use energy, heat, work and the first law of thermodynamics to prepare them for the chemical perspective. In order to bridge the gap to physics and find similarities, it is also possible to consider energy quality (and entropy) as parts of the chemistry curriculum (cf.Engström, 2008). However, bridging the gap requires teachers' awareness of how different science subjects use the concepts (e.g. the forms of energy framework and biology) and how they are used in everyday life.
Following Beall's (1994) line of argument about scientific terminology, we believe in a revision of how enthalpy changes are expressed. Long ago, Zemansky (1974) proposed that enthalpy of vaporization should instead be expressed as enthalpy change due to vaporization. One step in this direction has been made, with IUPAC (2007) changing the index of the process from ΔHvap to ΔvapH, thus indicating that the enthalpy of a system changes due to vaporization. However, many standard textbooks still use the old expression.
Although the previous suggestions were intended for policymakers, closing the gap between secondary- and tertiary-level chemistry education is of importance since the same content is covered (de Jong, 2000). Enthalpy is a commonly used concept in both upper secondary- and tertiary-level chemistry, and it is surprising how limited the research is on the actual teaching–learning process. Since this research has been carried out at the tertiary level, the research suggestions offered here are relevant to higher education.
We propose three research suggestions: firstly, use the same tasks in order to collect more data, whereby discussions in classrooms may provide new insight; secondly, develop new tasks in order to refine old results and construct new ones. Both these suggestions can be carried out within the frame of a university chemistry course; thirdly, use theoretical triangulation in order to gain different theoretical insights (Robson, 2002).
We chose to continue the discussion of continuous content-related research as posed by Carson (2001), Sözbilir (2001) and other researchers in chemistry education research. In light of the development during the past decade, one suggestion is to address socio-scientific issues in relation to enthalpy and its change. Our empirical evidence indicates that energy, heat and work can be used by undergraduate students to explain different socio-scientific issues, whereas enthalpy and its change cannot.
Appendix 1. Selected tasks translated into English
The first task was developed during the research process. It also includes a question, C, that was a calculation using Hess' law. The task was used in HST1a as Question 4ab; FST as Question 3ab and FSTE2 as Question 3ab. During HS, ΔE was used to describe the change in internal energy (cf.Zumdahl and Zumdahl, 2007) whereas ΔU was used in FS. Below, only ΔU is used.As a chemical reaction occurs, the enthalpy change ΔH for the reaction sometimes differs from the internal energy change (ΔU) for the reaction.
(a) Give an example of a reaction that fulfils ΔH ≠ ΔU. (2p)
(b) Motivate why the chosen reaction fulfils ΔH ≠ ΔU. (3p)
The second task was based on Sözbilir's (2001) original. It was used in HST1a as Question 5ab; in HSE2 as Question 7ab; in FSE1 as Question 3ab; and in FST as Question 10ab.
The third task was developed during the research process. The situation presented here is analogous to the interview tasks in which exothermic/endothermic reactions took place in front of the students. The task was used in HST2b as Question 3.
Assume two identical beakers filled with the same volume of water. Add 1 mol of solid sodium hydroxide to one beaker and 1 mol of solid potassium iodide to the other beaker. A while later an increase in temperature is observed in the beaker with sodium hydroxide while the temperature decreases in the beaker with potassium iodide. The temperature in the surroundings is constant during the experiment. The beakers cannot affect each other's temperature.
Please explain how both reactions can occur despite the increase in temperature in one beaker and decrease in the other. Base your explanation on the concepts enthalpy, Gibbs free energy, entropy and bonds.
The fourth task was presented as HSE2 Question 9. A similar one with magnesium and hydrochloric acid was presented in HSE1, but in this case the questions concerned temperature, energy and enthalpy. Below, the term spontaneously is used in the question since it is used by both Teacher L3 and Zumdahl and Zumdahl (2007).
Consider the following reaction 2Na(s) + Cl2(g) → 2NaCl(s) which occurs spontaneously at 25 °C.
(a) Is it exothermic or endothermic? Describe how you arrive at your answers and the assumptions you make.
(b) What happens to the bond energy during the described reaction (from reactants to products)? Please explain as thoroughly as possible.
(c) Please explain why the bond energy and bond enthalpy do not have the same numerical value.
The fifth task is based on Sözbilir's (2001) original and the results in the main study of students interpreting the figures differently (see Conception F9). The task was used in FSInl2 as Question 9ab and in FSE2 as Question 4ab.
Acknowledgements
We would like to thank the Swedish National Graduate School in Science, Technology and Mathematics Education Research (FontD) for its financial support.References
- Andersson B. and Wallin A., (2000), Students' Understanding of the Greenhouse Effect, the Societal Consequences of Reducing CO2 Emissions and the Problem of Ozone Layer Depletion, J. Res. Sci. Teach., 37, 1096–1111.
- Andersson S., Sonesson A., Stålhandske B., Tullberg A. and Rydén L., (2000), Gymnasiekemi A [Upper Secondary Chemistry A], Stockholm: Liber AB.
- Atkins P. W. and de Paula J., (2005), Elements of Physical Chemistry, Oxford: Oxford University Press.
- Barker V. and Millar R., (1999), Students' reasoning about chemical reactions: what changes occur during a context-based post-16 chemistry course, Int. J. Sci. Educ., 21, 645–666.
- Barker V. and Millar R., (2000), Students' reasoning about basic chemical thermodynamics and chemical bonding: what changes occur during a context-based post-16 chemistry course? Int. J. Sci. Educ., 22, 1171–1200.
- Beall H., (1994), Probing Student Misconceptions in Thermodynamics with In-Class Writing, J. Chem. Educ., 71, 1056–1057.
- Bodner G. M. and Herron J. D., (2002), Problem-Solving in Chemistry, in Gilbert J. K., de Jong O., Justi R., Treagust D. F. and van Driel J. (ed.), Chemical Education: Towards Research-Based Practice, Dordrecht: Kluwer Academic Publishers, pp. 235–266.
- Boo H.-K., (1998), Students' Understanding of Chemical Bonds and the Energetics of Chemical reactions, J. Res. Sci. Teach., 35, 569–581.
- Boo H.-K. and Watson J. R., (2001), Progression in High School Students' (aged 16–18) Conceptualizations about Chemical Reactions in Solution, Sci. Educ., 85, 568–585.
- Bowen C. W. and Bunce D. M., (1997), Testing for Conceptual Understanding in General Chemistry, Chem. Educ., 2, 1–17.
- Bryman A., (2008), Social Research Methods, 3rd edn, Oxford: Oxford University Press.
- Carson E., (2001), A Study of Undergraduate Students' Understanding of Selected Concepts in Chemical Thermodynamics, Unpublished PhD, University of London, School of education.
- Carson E. and Watson J. R., (1999), Undergraduate students' understanding of enthalpy change, Univ. Chem. Educ., 3, 46–51.
- Carson E. and Watson J. R., (2002), Undergraduate students' understandings of entropy and Gibbs free energy, Univ. Chem. Educ., 6, 4–12.
- Chang R., (2007), Chemistry, 9th edn, Boston: McGraw-Hill Higher Education.
- Claesgens J., Scalise K., Draney K., Wilson M. and Stacy A., (2002), Perspective of a Chemist: A Framework to Promote Conceptual Understanding of Chemistry, Paper presented at the Annual meeting of the American Educational Research Association.
- Clayden J., Greeves N., Warren S. and Wothers P., (2001), Organic Chemistry, Oxford: Oxford University Press.
- Darmofal D. L., Soderholm D. H. and Brodeur D. R., (2002), Using Concept Maps and Concept Questions to Enhance Conceptual Understanding, Paper presented at the 2002 American Society for Engineering Education Annual Conference & Exposition, Montreal.
- de Jong O., (2000), Crossing the borders: chemical education research and teaching practice, Univ. Chem. Educ., 4, 31–34.
- de Vos W. and Verdonk A. H., (1986), A new road to reactions: part 3. Teaching the heat effects of reactions, J. Chem. Educ., 63, 972–974.
- Driver R., (1989), Students' conceptions and the learning in science, Int. J. Sci. Educ., 11, 481–490.
- Driver R. and Easley J., (1978), Pupils and paradigms: a review of literature related to concept development in adolescent science students, Studies in Science Education, 5, 61–84.
- Driver R. and Warrington L., (1985), Students' use of the principle of energy conservation in problem situations, Phys. Educ., 20, 171–176.
- Duit R. and Haeussler P., (1994), Learning and Teaching Energy, in Fensham P., Gunstone R. and White R. (ed.), The content of science, London: The Falmer Press, pp. 185–200.
- Duit R. and Treagust D. F., (1998), Learning in Science - From Behaviourism Towards Social Constructivism and Beyond, in Fraser B. J. and Tobin K. G. (ed.), International Handbook of Science Education, Dordrecht: Kluwer Academic Publishers, pp. 3–25.
- Engström S., (2008), Fysiken spelar roll!: undervisning om hållbara energisystem, fokus på gymnasiekursen fysik A, Västerås: School of Education, Culture and Communication, Mälardalen University.
- Erickson F., (1998), Qualitative Research Methods for Science Education, in Fraser B. J. and Tobin K. G. (ed.), International Handbook of Science Education Part Two, Dordrecht: Kluwer Academic Publishers, pp. 1155–1173.
- Gabel D., (2003), Enhancing the Conceptual Understanding of Science, Educ. Horiz., 81, 70–76.
- Galley W. C., (2004), Exothermic Bond Breaking: A Persistent Misconception, J. Chem. Educ., 81, 523–526.
- Glaser B. G. and Strauss A. L., (1967), The Discovery of Grounded Theory: Strategies for Qualitative Research, New York: Aldine de Gruyter.
- Goedhart M. J. and Kaper W., (2002), From Chemical Energetics to Chemical Thermodynamics, in Gilbert J. K., de Jong O., Justi R., Treagust D. F. and van Driel J. (ed.), Chemical Education: Towards Research-Based Practice, Dordrecht: Kluwer Academic Publishers, pp. 339–362.
- Goldring H. and Osborne J., (1994), Students' difficulties with energy and related concepts, Phys. Educ., 29, 26–32.
- Granville M. F., (1985), Student Misconceptions in Thermodynamics, J. Chem. Educ., 62, 847–848.
- Greenbowe T. J. and Meltzer D. E., (2003), Student learning of thermochemical concepts in the context of solution calorimetry, Int. J. Sci. Educ., 25, 779–800.
- Harrison A. G., Grayson D. J. and Treagust D. F., (1999), Investigating a Grade 11 Student's Evolving Conceptions of Heat and Temperature, J. Res. Sci. Teach., 36, 55–87.
- Hartmann S. and Niedderer H., (2005), Parallel Conceptions and Learning in the Domain of Force and Motion, in Boersma K., Eijkelhof H., de Jong O. and Goedhart M. J. (ed.), Research and the Quality of Education, Dordrecht: Kluwer Academic Publishers.
- IUPAC, (2007), Quantities, Units and Symbols in Physical Chemistry, 3rd edn, Cambridge: Royal Society of Chemistry.
- Johnstone A. H., (2010), You Can't Get There from Here, J. Chem. Educ., 87, 22–29.
- Johnstone A. H., MacDonald J. J. and Webb G., (1977), Misconceptions in school thermodynamics, Phys. Educ., 12, 248–251.
- Kaper W. H. and Goedhart M. J., (2002), ‘Forms of energy’, an intermediary language on the road to thermodynamics? Part I, Int. J. Sci. Educ., 24, 81–96.
- Kim E. and Pak S.-J., (2002), Students do not overcome conceptual difficulties after solving 1000 traditional problems, Am. J. Phys., 70, 759–765.
- Kvale S., (1996), Interviews: An Introduction to Qualitative Research Interviewing, Thousand Oaks, Calif.: Sage.
- Laidler K. J., Meiser J. H. and Sanctuary B. C., (2003), Physical Chemistry, 4th edn, Boston: Houghton Mifflin.
- Lewis E. L. and Linn M. C., (1994), Heat Energy and Temperature Concepts of Adolescents, Adults, and Experts: Implications for Curricular Improvements, J. Res. Sci. Teach., 31, 657–677.
- Martins I. P. and Cachapuz A. F., (1990), How do pupils perceive the concept of energy in chemical situations? Sch. Sci. Rev., 71, 83–85.
- Mazur E., (1997), Peer Instruction, Upper Saddle River, NJ: Prentice Hall.
- Millar R., (2005), Teaching about energy, York: University of York.
- Mortimer E. F., (1995), Conceptual Change or Conceptual Profile Change, Sci. Educ., 4, 267–285.
- Nationalencyklopedin, (2012), mantra, retrieved 8/10/2010, from http://www.ne.se/lang/mantra.
- Niaz M., (2002), Facilitating conceptual change in students' understanding of electrochemistry, Int. J. Sci. Educ., 24, 425–439.
- Niedderer H., (2001), Analyse von Lehr-Lern-Prozessen beim elektrischen Stromkreis aus Videodaten, in von Aufschneiter S. and Welzel M. (ed.), Nutzung von Videodaten zur Untersuchung von Lern-Lehr-Prozessen - Aktuelle Methoden empirischer pädagogischer Forschung, Münster: Waxmann.
- Niedderer H., Budde M., Givry D., Psillos D. and Tiberghien A., (2007), Learning process studies, in Pintó R. and Couso D. (ed.), Contributions from Science Education Research, Dordrecht: Springer, pp. 159–171.
- Nieswandt M., (2007), Student Affect and Conceptual Understanding in Learning Chemistry, J. Res. Sci. Teach., 44, 908–937.
- Nilsson T. and Niedderer H., (2012), An analytical tool to determine undergraduate students' use of volume and pressure when describing expansion work and technical work, Chem. Educ. Res. Pract., 13, 348–356.
- Petri J. and Niedderer H., (1998), A learning pathway in high-school level quantum atomic physics, Int. J. Sci. Educ., 20, 1075–1088.
- Ribeiro M. G. T. C., Pereira, D. J. V. C., and Maskill R. (1990), Reaction and spontaneity: the influence of meaning from everyday language on fourth year undergraduates' interpretation of some simple chemical phenomena, Int. J. Sci. Educ., 12(4), 391–401.
- Robson C., (2002), Real world research: a resource for social scientists and practitioner-researchers, 2nd edn, Oxford: Blackwell Publishing.
- Sözbilir M., (2001), A Study of Undergraduates' Understandings of Key Chemical Ideas in Thermodynamics, Unpublished PhD, University of York, Department of educational studies.
- Sözbilir M., (2003), A Review of Selected Literature on Students' Misconceptions of Heat and Temperature, Boğaziçi University Journal of Education, 20, 25–40.
- Teichert M., and Stacy A., (2002), Promoting understanding of chemical bonding and spontaneity through student explanation and integration of ideas, J. Res. Sci. Teach., 39(6), 464–496.
- Thomas P. L. and Schwenz R. W., (1998), College Physical Chemistry Students' Conceptions of Equilibrium and Fundamental Thermodynamics, J. Res. Sci. Teach., 35, 1151–1160.
- Treagust D. F. and Duit R., (2008a), Compatibility between cultural studies and conceptual change in science education: there is more to acknowledge than to fight straw men!, Cultural Studies of Science Education, 3, 387–395.
- Treagust D. F. and Duit R., (2008b), Conceptual change: a discussion of theoretical, methodological and practical challenges for science education, Cultural Studies of Science Education, 3, 297–328.
- Tsaparlis G., (2008), Teaching and Learning Physical Chemistry: A review of Educational Research, in Ellison M. D. and Schoolcraft T. A. (ed.), Advances in Teaching Physical Chemistry, Washington, DC: American Chemical Society.
- van Huis C. and van den Berg E., (1993), Teaching energy: a system approach, Phys. Educ., 28, 146–153.
- van Roon P. H., van Sprang H. F. and Verdonk A. H., (1994), ‘Work’ and ‘Heat’: on the road towards thermodynamics, Int. J. Sci. Educ., 16, 131–144.
- Vetenskapsrådet, (2002), Forskningsetiska principer inom humanistisk-samhällsvetenskaplig forskning [research ethics principles in humanistic-social scientific research], Stockholm: Vetenskapsrådet.
- White R. and Gunstone R., (1992), Probing Understanding, London: The Falmer Press.
- Vosniadou S., (2008a), Bridging culture with cognition: a commentary on “culturing conceptions: from first principles”, Cultural Studies of Science Education, 3, 277–282.
- Vosniadou S., (2008b), International Handbook of Research on Conceptual Change, New York: Routledge.
- Zemansky M. W., (1974), A physics teacher looks at chemical thermodynamics, J. Chem. Educ., 51, 572–576.
- Zumdahl S. S. and Zumdahl S. A., (2007), Chemistry, 7th edn, Boston, Mass: Houghton Mifflin Co.
Footnote |
| † Here R is short for state function/reaction/amount of substance/reactants and products. |
| This journal is © The Royal Society of Chemistry 2014 |