Reductive amination using a combination of CaH2 and noble metal†
Carole Guyon‡
,
Eric Da Silva,
Romain Lafon,
Estelle Métay* and
Marc Lemaire*
Equipe Catalyse Synthèse Environnement, Institut de Chimie et Biochimie Moléculaires et Supramoléculaires, UMR-CNRS 5246, Université de Lyon, Université Claude Bernard-Lyon 1, Bâtiment Curien, 43 boulevard du 11 Novembre 1918, F-69622 Villeurbanne Cedex, France. E-mail: estelle.metay@univ-lyon1.fr; marc.lemaire.chimie@univ-lyon1.fr; Fax: +33 (0)4 72 43 14 08; Tel: +33 (0)4 72 44 85 07, +33 (0)4 72 43 14 07
First published on 28th November 2014
Abstract
Amines were prepared by a reductive amination reaction in the presence of calcium hydride and Pt/C. The in situ formation of water seems to be the key to activate CaH2 to reduce the intermediate imine.
Occurring in nature, amines are important building blocks in organic synthesis.1 One way amongst others to prepare these compounds is the reductive amination reaction. This transformation, which supposes the reaction between a carbonyl and an amine then the reduction of the intermediate imine, is already well described.2 Two main approaches are considered for the reductive amination: the first one supposes the formation of the imine before the addition of the reducing agent and in the second one all the reactants are present at the beginning of the reaction.3 To perform this reaction several reducing agents have been employed: the most used are sodium borohydride derivatives4 and hydrogen.5,6 Unfortunately, it is well established now that even if boron and aluminium hydrides are efficient for the reduction of organic functions they should be substituted for security and environmental reasons. Hydrosilanes and hydrosiloxanes7 or formates8 have been associated with a metal complex or with an organocatalyst to realize reductive amination. Several reports are also dealing with Hantzsch esters.9 Recently, amines were prepared via a hydrogen autotransfer in the presence of a metal catalyst.10
In the course to find alternative reducing agents to aluminium and boron hydrides, we have previously developed several methods to reduce organic functions with 1,1,3,3-tetramethylsiloxane (TMDS)11 and hypophosphite derivatives.12 After an analysis of the literature data, we were curious to notice that the calcium hydride was poorly explored for the reduction of organic functions. In fact, the calcium hydride alone is reported to reduce disulfide,13 carbon monoxide14 or tetrafluorosilane at temperatures up to 200 °C.15 With a mechanical activation hexachlorobenzene could be dehalogenated and in the presence of a chelating agent silanes were prepared.16 In an ionic liquid, AlCl3–Et3SBr, benzophenone was reduced.17 The reduction of ketones and imines were performed with Lewis acids such as zinc halides with chlorosilane or Ti(Oi-Pr)4.18 The authors generally mentioned the low reactivity of calcium hydride.19 Probably for this reason, Harder reported the synthesis of an organic calcium hydride complex which is able to reduce several organic functions.20
In our laboratory, we observed that the reaction of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
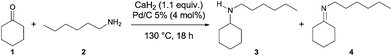
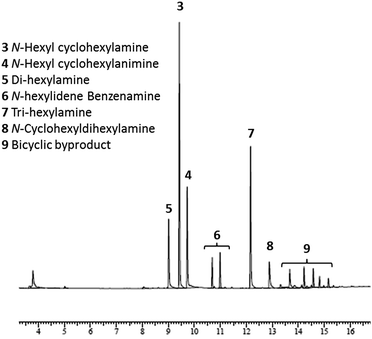
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of cyclohexanone 1 and hexylamine 2 in the presence of 4 mol% of Pd/C and 1.1 equivalent of CaH2 at 130 °C in a sealed tube afforded the N-hexylcyclohexylamine 3 (Scheme 1). More precisely, analysis of the crude showed a complete conversion of the starting material toward the formation of several products (Fig. 1). The two major products were identified by GC-MS as N-hexylcyclohexylamine 3 and N-hexylcyclohexylimine 4. The di- or tri-substituted amine 5, 7 and 8 were formed from hexylamine by condensation reactions. The formation of bicyclic compounds 9 came from aldol reactions. The compounds 6 were identified as products from dehydrogenation reaction. This dehydrogenation reaction was already described in the presence of Pd/C including by our group.21
1 molar ratio of cyclohexanone 1 and hexylamine 2 in the presence of 4 mol% of Pd/C and 1.1 equivalent of CaH2 at 130 °C in a sealed tube afforded the N-hexylcyclohexylamine 3 (Scheme 1). More precisely, analysis of the crude showed a complete conversion of the starting material toward the formation of several products (Fig. 1). The two major products were identified by GC-MS as N-hexylcyclohexylamine 3 and N-hexylcyclohexylimine 4. The di- or tri-substituted amine 5, 7 and 8 were formed from hexylamine by condensation reactions. The formation of bicyclic compounds 9 came from aldol reactions. The compounds 6 were identified as products from dehydrogenation reaction. This dehydrogenation reaction was already described in the presence of Pd/C including by our group.21
In order to increase the selectivity of the reaction, the different parameters were modified. A decrease of the temperature (60 °C) and the load of the catalyst (1 mol% of Pd/C) allowed the isolation of the compound 3 with 68% yield. These conditions were then applied to the formation of amines from benzaldehyde 10 or acetophenone 12 (Scheme 2a). The reaction of benzaldehyde 10 and hexylamine 2 gave the corresponding amine 11 with a 72% of isolated yield. Similar results were obtained with acetophenone 12 as compound 13 was isolated with 74% yield.
When chloride derivative 14 reacted with hexylamine the desired product was not formed. Only dehalogenated compounds 12, 13 and 15 were obtained as shown in Scheme 2b. As a consequence, the parameters of the reaction were investigated. The reductive amination of benzaldehyde 10 and hexylamine 2 in the presence of calcium hydride (1.2 equiv.), Pd/C (1 mol%), 60 °C, 16 h led to the incomplete reduction of the formed imine 16 (8% GC yield), the formation of the amine 11 (72% GC yield) and toluene 17 (9% GC yield). The reduction of CaH2 equivalent from 1.5 to 0.6 did not affect the conversion into the amine 11 and reduced the formed quantity of imine (5% GC yield) and toluene (5% GC yield).
In order to reduce the amount of toluene 17, metal catalysts were screened with calcium hydride (60 mol%), metal (1 mol%) at 60 °C for 16 h (Table 1). With Ru/C the conversion of benzaldehyde 10 into imine 16 was complete however reduction of the in situ formed imine 16 yielded only 5% of amine 11 (Table 1, entry 1). The reductive amination in the presence of palladium or platinum catalysts led to the formation of the amine 11 with 71–79% GC yields (Table 1, entries 2–6). The platinum catalysts had the advantage to afford less toluene 17 than palladium catalysts respectively 2% against 5–10% GC yields. The conditions using Pt/SiO2 as catalyst were applied to the reductive amination of acetophenone 12 and hexylamine 2. After 16 h, only 45% GC yield of the corresponding amine 13 was observed (Table 1, entry 9). Screening of the catalysts showed a strong effect of the support in this case. Palladium and platinum on carbon led to the complete conversion of acetophenone 12 and 76–77% GC yields into the amine 13 against 37–45% with palladium and platinum on silica (Table 1, entries 7–10). In addition, with palladium on silica incomplete conversion of acetophenone 12 was observed while with platinum on silica 20% GC yield of unreduced imine 15 was detected with 21% GC yield of 1-phenylethanol 20 (Table 1, entries 8 and 9).
| Entry | Metal | Conversion of benzaldehyde 10 | GC yieldsa | |||
|---|---|---|---|---|---|---|
| Amine 11 | Imine 16 | Toluene 17 | Benzylalcohol 18 | |||
| a GC yields were determined by GC using dodecane as internal standard; N.D. = not determined. | ||||||
| 1 | Ru/C | 100 | 5 | 80 | 0 | 2 |
| 2 | Pd/Al2O3 | 100 | 76 | 6 | 5 | 0 |
| 3 | Pd/C | 100 | 71 | 5 | 5 | 0 |
| 4 | Pd/SiO2 | 100 | 71 | 5 | 10 | 0 |
| 5 | Pt/SiO2 | 100 | 79 | 3 | 2 | 1 |
| 6 | Pt/C | 100 | 73 | 4 | 2 | 0 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Conversion of acetophenone 12 | GC yieldsa | |||||
| Amine 13 | Imine 15 | Ethylbenzene 19 | 1-Phenylethanol 20 | |||
| 7 | Pd/C | 100 | 76 | 0.5 | N.D. | 0 |
| 8 | Pd/SiO2 | 66 | 37 | 4 | N.D. | 12 |
| 9 | Pt/SiO2 | 96 | 45 | 20 | N.D. | 21 |
| 10 | Pt/C | 100 | 77 | 4 | N.D. | 7 |
The following conditions were retained for the study of the scope and limitations of the reaction: platinum on carbon (1 mol%), CaH2 (60 mol%), 60 °C for 16 h (Table 2).
| Entry | Carbonyl derivative | Amine | Product | GC yielda (%) amine product | Isolated yield (%) | |||
|---|---|---|---|---|---|---|---|---|
| a GC yields were determined by GC using dodecane as internal standard.b Toluene ([S] = 2 M).c Ethyl acetate ([S] = 0.5 M).d CaH2 (1.2 equiv.).e 2.2 equiv. of CaH2.f N.D. = not determined; general conditions: 1 equiv. of carbonyl, 1 equiv. of amine, Pt/C (1 mol%), CaH2 (60 mol%), 60 °C, sealed tube, 16 h, stirring 700 rpm. | ||||||||
| 1 |  |
10 | 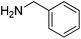 |
21 |  |
22 | 94 | 87 |
| 2b |  |
23 |  |
24 | 96 | 82 | ||
| 3b |  |
25 | 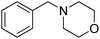 |
26 | 87 | 75 | ||
| 4b | 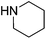 |
27 |  |
28 | 48 | 40 | ||
| 5b |  |
29 |  |
30 | 58 | 58 | ||
| 6c |  |
31 |  |
32 | 0 | 0 | ||
| 7b,d,e |  |
33 |  |
34 | N.D.f | 71 | ||
| 8b,d,e | NH4OAc | 35 | 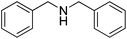 |
22 | N.D.f | 67 | ||
| 9 |  |
2 |  |
11 | 89 | 88 | ||
| 10b |  |
36 |  |
37 | N.D.f | 67 | ||
| 11 |  |
12 |  |
13 | 77 | 80 | ||
| 12 | 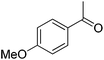 |
38 |  |
39 | 94 | 81 | ||
| 13 |  |
40 |  |
41 | N.D.f | 62 | ||
| 14 |  |
1 |  |
3 | 63 | 62 | ||
| 15 |  |
42 | 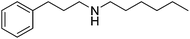 |
43 | 62 | 58 | ||
Reductive amination of benzaldehyde 10 with benzylamine 21 proceeded with an excellent isolated yield of 87% in dibenzylamine 22 (Table 2, entry 1). The reaction with secondary amines such as dibutylamine 23, morpholine 25 and piperidine 27 afforded the corresponding tertiary amines 24, 26 and 28 with moderate to good isolated yields of 40–82% (Table 2, entries 2–4). The formation of benzyl alcohol 18 (12–17% GC yields), as co-product, was observed when morpholine 25 and piperidine 27 were used (Table 2, entries 3 and 4). The reaction of benzaldehyde 10 with aniline 29, a less nucleophilic amine than aliphatic ones, allowed the formation of 30 with moderate 58% yield (Table 2, entry 5). The benzamide 31, poor nucleophile and poorly soluble, did not react. Benzaldehyde 10 was detected in 84% GC yield after reaction with the formation of 10% GC yield of benzyl alcohol 18 (Table 2, entry 6). The reaction with phenylalanine ester hydrochloride salt 33 led to the amine 34 with 71% isolated yield (Table 2, entry 7). The reaction with ammonium acetate led to the formation of dibenzylamine 22 with 67% isolated yield (Table 2, entry 8). The reactions starting from benzaldehyde 10 and acetophenone 12 as carbonyl compounds gave good isolated yields of respectively 88% and 80% into amine 11 and 13 (Table 2, entries 9 and 11).
p-Chlorobenzaldehyde 36 led efficiently to the corresponding product of reductive amination 37 in 67% isolated yield without formation of dehalogenated products. The GC analysis of the crude showed a proportion between amine and imine of 78/22 explaining the moderate yield. The reaction of p-nitrobenzaldehyde with hexylamine led to complete conversion of the starting materials and a mixture of imines: imine from p-nitrobenzaldehyde with hexylamine and imine from p-formylaniline. No product of reductive amination has been observed. After column chromatography, 58% of the p-nitrobenzaldehyde was recovered. p-Formylbenzaldehyde and p-nitrosobenzaldehyde were isolated respectively with 8% and 18% yield. Electro donating group on acetophenone such as para methoxy did not impair the reactivity with 81% isolated yield of 39 compared with acetophenone where 13 was obtained with 80% yield (Table 2, entries 11 and 12). The reaction in the presence of α-tetralone 40 led to the amine 41 with an average yield of 62% (Table 2, entry 13). The crude of the reaction contained an important quantity of unreduced imine (GC proportion: amine/imine = 81/19). However, no other side-product was observed. The reductive amination of hexylamine 2 with aliphatic carbonyl compounds such as cyclohexanone 1 and 3-phenylpropionaldehyde 42 proceeded well leading to the corresponding amines 3 and 43 in moderate yields of 62 and 58% (Table 2, entries 14 and 15).
The reductive amination of p-cyanobenzaldehyde 44 with hexylamine 2 in toluene under the optimized conditions did not lead to the expected product of reductive amination but led to the diamine 45 resulting from the pinacol reaction of the intermediate imine (Scheme 3). The diamine 45 was isolated with 73% yield as a mixture of rac/meso mixture in a 50/50 ratio. This family of diamines have already been observed in the radical reduction of imines of p-cyanobenzaldehyde 44 by NaTeH22 or by sodium metal.23 More generally, the pinacol reaction of imine can take place in the presence of Zn/TMSCl.24 This method has been applied to the synthesis of ligand (R,R)- and (S,S)-N,N′-dimethyl-1,2-diphenylethylene-1,2-diamine on 10 g scale.25 Recently, this method has been selected for the synthesis on 100 g.26
From 2,6-dimethylcyclohexanone 46 (83/17 cis/trans mixture) two diastereoisomers 47A and 47C were isolated respectively in 23% and 25% yield (Scheme 4). Three different products could have been obtained: two diastereoisomers (47A and 47B) and one pair of enantiomers (47C). 47A and 47C had been assigned thanks to carbon and NOESY RMN27 and supported by the literature.28
The monomethylation of amines is a challenge in spite of the number of pathways.29 The alkylation reaction remains difficult as the monomethylated product is more reactive than the starting materials.30 Reductive amination reactions issue from Eschweiler–Clarke reaction often affords a mixture of products.31 In this case, under the optimized conditions in toluene (1 M) in the presence of Pt/C and CaH2, the reaction of paraformaldehyde 48 (1 equiv.) and hexylamine (1.06 equiv.) gave a mixture of hexylamine 2 (11%), N-methylhexylamine 49 (67%) and N,N-dimethylhexylamine 50 (22%) with a global yield of 95% (Scheme 5). The products have not been separated. The distillation is difficult due to close boiling points: hexylamine 2 (130 °C), N-methylhexylamine 49 (135 °C), N,N-dimethylhexylamine 50 (145 °C). Even if the major product is the monomethylated and this result is similar to the literature, the selectivity should be improved.
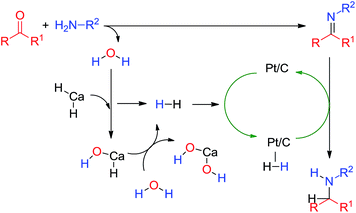
A mechanism is proposed to explain these results (Scheme 6). The amine reacts with the carbonyl group to form the corresponding imine after elimination of water. The water would then react with calcium hydride to release one molecule of hydrogen. The hydrogen could be adsorbed on the metal catalyst and hydrogenate the in situ formed imine into amine. A control experiment was carried out on the reductive amination of benzaldehyde and hexylamine 2 under the optimized conditions in the absence of Pt/C: CaH2 (60 mol%), 60 °C. After 16 h, only the imine 16 was observed showing the importance of the catalyst. The reaction of CaH2 (60 mol%) and Pt/C (1 mol%) on the dry imine 16 at 60 °C for 16 h led to the formation of 44% of the amine 11. It has been attributed to the presence of water in the Pt/C.25 It has been observed with different batch of Pt/C.
Conclusion
We have showed that calcium hydride can be used directly in the reductive amination of carbonyl compounds. The calcium hydride seems to react with the formed water and then liberate hydrogen. CaH2 could be considered as a hydrogen reservoir. This hydrogen adsorbed on the metal catalyst could reduce the formed imine. The reaction leads to good yield in amines, however, it is sensitive to the nucleophilicity of the amine and the steric hindrance of the substrates.This method allows an easy to handle reductive amination without the need of dedicated equipment and selectivity issue due to excess of hydrogen.
Notes and references
- S. Gomez, J. A. Peters and T. Maschmeyer, Adv. Synth. Catal., 2002, 344, 1037 CrossRef CAS.
-
(a) A. Tarasevich and N. G. Kozlov, Russ. Chem. Rev., 1999, 68, 55 CrossRef PubMed;
(b) A. F. Abdel-Magid and S. J. Mehrman, Org. Process Res. Dev., 2006, 10, 971 CrossRef CAS;
(c) R. O. Hutchins, Reduction of C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N to CHNH by Metal Hydrides, in Comprehensive Organic Synthesis, ed. B. N. Trost and I. Fleming, Pergamon Press, New York, 1991, vol. 8, p. 47 Search PubMed;
(d) K. S. Hayes, Appl. Catal., A, 2001, 221, 187 CrossRef CAS;
(e) S. G. Ouellet, A. M. Walji and D. W. C. Macmillan, Acc. Chem. Res., 2007, 40, 1327 CrossRef CAS PubMed;
(f) V. I. Tararov and A. Börner, Synlett, 2005, 203 CrossRef CAS PubMed;
(g) T. C. Nugent and M. El-Shazly, Adv. Synth. Catal., 2010, 352, 753 CrossRef CAS;
(h) M. Klussmann, Angew. Chem., Int. Ed., 2009, 48, 7124 CrossRef CAS PubMed.
N to CHNH by Metal Hydrides, in Comprehensive Organic Synthesis, ed. B. N. Trost and I. Fleming, Pergamon Press, New York, 1991, vol. 8, p. 47 Search PubMed;
(d) K. S. Hayes, Appl. Catal., A, 2001, 221, 187 CrossRef CAS;
(e) S. G. Ouellet, A. M. Walji and D. W. C. Macmillan, Acc. Chem. Res., 2007, 40, 1327 CrossRef CAS PubMed;
(f) V. I. Tararov and A. Börner, Synlett, 2005, 203 CrossRef CAS PubMed;
(g) T. C. Nugent and M. El-Shazly, Adv. Synth. Catal., 2010, 352, 753 CrossRef CAS;
(h) M. Klussmann, Angew. Chem., Int. Ed., 2009, 48, 7124 CrossRef CAS PubMed. - R. P. Tripathi, S. S. Verma, J. Pandey and V. K. Tiwari, Curr. Org. Chem., 2008, 12, 1093 CrossRef CAS.
- (a) G. W. Gribble, P. D. Lord, J. Skotnicki, S. E. Dietz, J. T. Eaton and J. L. Johnson, J. Am. Chem. Soc., 1974, 96, 7812 CrossRef CAS; (b) A. F. Abdel-Magid, C. A. Mayanoff and K. G. Carson, Tetrahedron Lett., 1990, 31, 5595 CrossRef CAS; (c) A. F. Abdel-Magid, K. G. Carson, B. D. Harris, C. A. Maryanoff and R. D. Shah, J. Org. Chem., 1996, 61, 3849 CrossRef CAS PubMed; (d) E. R. Burkhardt and K. Matos, Chem. Rev., 2006, 106, 2617 CrossRef CAS PubMed; (e) M. D. Bomann, I. C. Guch and M. DiMare, J. Org. Chem., 1996, 60, 5995 CrossRef; (f) S. Sato, T. Sakamoto, E. Miyazawa and Y. Kikugawa, Tetrahedron, 2004, 60, 7899 CrossRef CAS PubMed; (g) S. Yoshida, J. Hayashida, Y. Morinaga, S. Mizobata, A. Okada, K. Kawai, S. Tanoue, T. Nakata, M. Kitayama, A. Ohigashi, M. Matsuura, T. Takahashi, S. Ieda and M. Okada, Org. Process Res. Dev., 2014, 18, 725 CrossRef CAS; (h) R. F. Borch, M. D. Bernstein and H. D. Durst, J. Am. Chem. Soc., 1971, 93, 2897 CrossRef CAS.
- (a) M. O. Frederick, S. A. Frank, J. T. Vicenzi, M. E. LeTourneau, K. Derek Berglund, A. W. Edward and C. A. Alt, Org. Process Res. Dev., 2014, 18, 546 CrossRef CAS; (b) F. Fache, L. Jacquot and M. Lemaire, Tetrahedron Lett., 1994, 35, 3313 CrossRef CAS; (c) T. Mohy El Dine, S. Chapron, M.-C. Duclos, N. Duguet, F. Popowycz and M. Lemaire, Eur. J. Org. Chem., 2013, 5445 CrossRef CAS; (d) G. S. Vanier, Synlett, 2007, 131 CrossRef CAS PubMed; (e) N. Levi and R. Neumann, ACS Catal., 2013, 3, 1915 CrossRef CAS; (f) H.-U. Blaser, C. Malan, B. Pugin, F. Spindler, H. Steiner and M. Studer, Adv. Synth. Catal., 2003, 345, 103 CrossRef CAS; (g) T. Ikenaga, K. Matsushita, J. Shinozawa, S. Yada and Y. Takagi, Tetrahedron, 2005, 61, 2105 CrossRef CAS PubMed; (h) M. Freifelder, J. Org. Chem., 1966, 31, 3875 CrossRef CAS; (i) L. Hu, X. Cao, D. Ge, H. Hong, Z. Guo, L. Chen, X. Sun, J. Tang, J. Zheng, J. Lu and H. Gu, Chem.–Eur. J., 2011, 17, 14283 CrossRef CAS PubMed; (j) T. Ikawa, Y. Fujita, T. Mizusaki, S. Betsuin, H. Takamatsu, T. Maegawa, Y. Monguchi and H. Sajiki, Org. Biomol. Chem., 2012, 10, 293 CAS; (k) S. K. Sharma, J. Lynch, A. M. Sobolewska, P. Plucinski, R. J. Watson and J. M. J. Williams, Catal. Sci. Technol., 2013, 3, 85 RSC.
- (a) W. S. Emerson and H. W. Mohrman, J. Am. Chem. Soc., 1940, 62, 69 CrossRef CAS; (b) W. S. Emerson and C. A. Uraneck, J. Am. Chem. Soc., 1941, 63, 749 CrossRef CAS; (c) Y. Yamane, X. Liu, A. Hamasaki, T. Ishida, M. Haruta, T. Yokoyama and M. Tokunaga, Org. Lett., 2009, 11, 5162 CrossRef CAS PubMed; (d) V. I. Tararov, R. Kadyrov, T. H. Riermeier and A. Börner, Chem. Commun., 2000, 1867 RSC; (e) T. Gross, A. M. Seayad, M. Ahmad and M. Beller, Org. Lett., 2002, 4, 2055 CrossRef CAS PubMed; (f) C. Li, B. Villa-Marcos and J. Xiao, J. Am. Chem. Soc., 2009, 131, 6967 CrossRef CAS PubMed; (g) P. Mattei, G. Moine, K. Püntener and R. Schmid, Org. Process Res. Dev., 2011, 15, 353 CrossRef CAS; (h) S. Werkmeister, K. Junge and M. Beller, Green Chem., 2012, 14, 2371 RSC; (i) M. D. Bhor, M. J. Bhanushali, N. S. Nandurkar and B. M. Bhanage, Tetrahedron Lett., 2008, 49, 965 CrossRef CAS PubMed; (j) S. Fleischer, S. Zhou, K. Junge and M. Beller, Chem.–Asian J., 2011, 6, 2240 CrossRef CAS PubMed; (k) A. Pagnoux-Ozherelyeva, N. Pannetier, M. Diagne Mbaye, S. Gaillard and J.-L. Renaud, Angew. Chem., Int. Ed., 2012, 51, 4976 CrossRef CAS PubMed; (l) S. Moulin, H. Dentel, A. Pagnoux-Ozherelyeva, S. Gaillard, A. Poater, L. Cavallo, J.-F. Lohier and J.-L. Renaud, Chem.–Eur. J., 2013, 19, 17881 CrossRef CAS PubMed.
- [Si] (a) O.-Y. Lee, K.-L. Law, C.-Y. Ho and D. Yang, J. Org. Chem., 2008, 73, 8829 CrossRef CAS PubMed; (b) T. Mizuta, S. Sakaguchi and Y. Ishii, J. Org. Chem., 2005, 70, 2195 CrossRef CAS PubMed; (c) F. Lehmann and M. Scobie, Synthesis, 2008, 1679 CAS; (d) D. Menche, F. Arikan, J. Li and S. Rudolph, Org. Lett., 2007, 9, 267 CrossRef CAS PubMed; (e) R. Apodaca and W. Xiao, Org. Lett., 2001, 3, 1745 CrossRef CAS PubMed; (f) J. R. Bernardo, S. C. A. Sousa, P. R. Florindo, M. Wolff, B. Machura and A. C. Fernandes, Tetrahedron, 2013, 69, 9145 CrossRef CAS PubMed; (g) T. Matsumura and M. Nakada, Tetrahedron Lett., 2014, 55, 1829 CrossRef CAS PubMed; (h) T. Mizuta, S. Sakaguchi and Y. Ishii, J. Org. Chem., 2005, 70, 2195 CrossRef CAS PubMed; (i) F.-M. Gautier, S. Jones, X. Li and S. J. Martin, Org. Biomol. Chem., 2011, 9, 7860 RSC; (j) J. P. Patel, A.-H. Li, H. Dong, V. L. Korlipara and M. J. Mulvihill, Tetrahedron Lett., 2009, 50, 5975 CrossRef CAS PubMed; (k) S. Chandrasekhar, Ch. Raji Reddy and M. Ahmed, Synlett, 2000, 1655 CAS; (l) S. Enthaler, Catal. Lett., 2011, 141, 55 CrossRef CAS; (m) H. Jaafar, H. Li, L. C. M. Castro, J. Zheng, T. Roisnel, V. Dorcet, J.-B. Sortais and C. Darcel, Eur. J. Inorg. Chem., 2012, 3546 CrossRef CAS; (n) R. Cano, M. Yus and D. J. Ramón, Tetrahedron, 2011, 67, 8079 CrossRef CAS PubMed; (o) V. Kumar, S. Sharma, U. Sharma, B. Singh and N. Kumar, Green Chem., 2012, 14, 3410 RSC; (p) J. Zheng, T. Roisnel, C. Darcel and J.-B. Sortais, ChemCatChem, 2013, 5, 2861 CrossRef CAS.
- (a) R. Leuckart, Ber. Dtsch. Chem. Ges., 1885, 18, 2341 CrossRef; (b) J. Dalmolen, M. van der Sluis, J. W. Nieuwenhuijzen, A. Meetsma, B. de Lange, B. Kaptein, R. M. Kellogg and Q. B. Broxterman, Eur. J. Org. Chem., 2004, 1544 CrossRef CAS; (c) W. Eschweiler, Ber. Dtsch. Chem. Ges., 1905, 38, 880 CrossRef; (d) H. T. Clarke, H. B. Gillespie and S. Z. Weisshaus, J. Am. Chem. Soc., 1933, 55, 4571 CrossRef CAS; (e) B. Basu, S. Jha, Md. M. H. Bhuiyan and P. Das, Synlett, 2003, 555 CrossRef CAS PubMed; (f) E. E. Drinkel, R. R. Campedelli, A. M. Manfredi, H. D. Fiedler and F. Nome, J. Org. Chem., 2014, 79, 2574 CrossRef CAS PubMed; (g) M. Allegretti, V. Berdini, M. Candida Cesta, R. Curti, L. Nicolini and A. Topai, Tetrahedron Lett., 2001, 42, 4257 CrossRef CAS; (h) V. Berdini, M. C. Cesta, R. Curti, G. D'Anniballe, N. Di Bello, G. Nano, L. Nicolini, A. Topai and M. Allegretti, Tetrahedron, 2002, 58, 5669 CrossRef CAS; (i) E. Byun, B. Hong, K. A. De Castro, M. Lim and H. Rhee, J. Org. Chem., 2007, 72, 9815 CrossRef CAS PubMed.
- (a) G. Li, Y. Liang and J. C. Antilla, J. Am. Chem. Soc., 2007, 129, 5830 CrossRef CAS PubMed; (b) D. Menche and F. Arikan, Synlett, 2006, 841 CrossRef CAS PubMed; (c) D. Menche, J. Hassfeld, J. Li, G. Menche, A. Ritter and S. Rudolph, Org. Lett., 2006, 8, 741 CrossRef CAS PubMed; (d) Q. P. B. Nguyen and T. H. Kim, Synthesis, 2012, 1977 CAS.
- Y. Zhang, C.-S. Lim, D. S. Boon Sim, H.-J. Pan and Y. Zhao, Angew. Chem., Int. Ed., 2014, 53, 1399 CrossRef CAS PubMed.
- [Cu], [Bi], [Al] (a) Y.-J. Zhang, W. Dayoub, G.-R. Chen and M. Lemaire, Green Chem., 2011, 13, 2737 RSC; (b) Y.-J. Zhang, W. Dayoub, G.-R. Chen and M. Lemaire, Eur. J. Org. Chem., 2012, 1960 CrossRef CAS ; [Mo], [V] ; (c) L. Pehlivan, E. Métay, S. Laval, W. Dayoub, D. Delbrayelle, G. Mignani and M. Lemaire, Eur. J. Org. Chem., 2011, 7400 CrossRef CAS ; [Pd] ; (d) Y. Shi, W. Dayoub, G.-R. Chen and M. Lemaire, Tetrahedron Lett., 2011, 52, 1281 CrossRef CAS PubMed; (e) L. Pehlivan, E. Métay, O. Boyron, P. Demonchaux, G. Mignani and M. Lemaire, Eur. J. Org. Chem., 2011, 4687 CrossRef CAS ; [Fe] ; (f) L. Pehlivan, E. Métay, S. Laval, W. Dayoub, P. Demonchaux, G. Mignani and M. Lemaire, Tetrahedron Lett., 2010, 51, 1939 CrossRef CAS PubMed; (g) L. Pehlivan, E. Métay, S. Laval, W. Dayoub, P. Demonchaux, G. Mignani and M. Lemaire, Tetrahedron, 2011, 67, 1971 CrossRef CAS PubMed ; [In] ; (h) L. Pehlivan, E. Métay, D. Delbrayelle, G. Mignani and M. Lemaire, Eur. J. Org. Chem., 2012, 4689 CrossRef CAS; (i) L. Pehlivan, E. Métay, D. Delbrayelle, G. Mignani and M. Lemaire, Tetrahedron, 2012, 68, 3151 CrossRef CAS PubMed ; [Ti] ; (j) S. Laval, W. Dayoub, L. Pehlivan, E. Métay, A. Favre-Réguillon, D. Delbrayelle, G. Mignani and M. Lemaire, Tetrahedron Lett., 2011, 52, 4072 CrossRef CAS PubMed; (k) S. Laval, W. Dayoub, A. Favre Réguillon, P. Demonchaux, G. Mignani and M. Lemaire, Tetrahedron Lett., 2010, 51, 2092 CrossRef CAS PubMed; (l) M. Berthod, A. Favre-Réguillon, J. Mohamad, G. Mignani, G. Docherty and M. Lemaire, Synlett, 2007, 1545 CAS; (m) C. Petit, A. Favre Réguillon, B. Albela, L. Bonneviot, G. Mignani and M. Lemaire, Organometallics, 2009, 28, 6379 CrossRef CAS; (n) C. Petit, E. Poli, A. Favre-Réguillon, L. Khrouz, S. Denis-Quanquin, L. Bonneviot, G. Mignani and M. Lemaire, ACS Catal., 2013, 3, 1431 CrossRef CAS; (o) S. Laval, W. Dayoub, L. Pehlivan, E. Métay, A. Favre-Reguillon, D. Delbrayelle, G. Mignani and M. Lemaire, Tetrahedron, 2014, 70, 975 CrossRef CAS PubMed; (p) S. Laval, W. Dayoub, L. Pehlivan, E. Métay, D. Delbrayelle, G. Mignani and M. Lemaire, Tetrahedron Lett., 2014, 55, 23 CrossRef CAS PubMed.
- (a) C. Guyon, M. Baron, M. Lemaire, F. Popowycz and E. Métay, Tetrahedron, 2014, 70, 2088 CrossRef CAS PubMed; (b) M. Baron, E. Métay, M. Lemaire and F. Popowycz, Green Chem., 2013, 15, 1006 RSC; (c) C. Guyon, E. Métay, N. Duguet and M. Lemaire, Eur. J. Org. Chem., 2013, 5439 CrossRef CAS.
- N. S. Gavande, S. Kundu, N. S. Badgujar, G. Kaur and A. K. Chakraborti, Tetrahedron, 2006, 62, 4201 CrossRef CAS PubMed.
- S. Reich and H. O. Serpek, Helv. Chim. Acta, 1920, 3, 138 CrossRef CAS.
- (a) A. D. Bulanov, V. V. Balabanov, D. A. Pryakhin and O. Yu. Troshin, Inorg. Mater., 2002, 38, 283 (Neorg. Mater., 2002, 38, 356) CrossRef CAS; (b) G. G. Devyatykh, E. M. Dianov, A. D. Bulanov, O. Yu. Troshin, V. V. Balabanov and D. A. Pryakhin, Dokl. Chem., 2003, 391, 204 (Dokl. Akad. Nauk, 2003, 391, 638) CrossRef CAS; (c) O. Yu. Troshin, A. D. Bulanov, V. S. Mikheev and A. Yu. Lashkov, Russ. J. Appl. Chem., 2010, 83, 984 CrossRef CAS.
- (a) S. Loiselle, M. Branca, G. Mulas and G. Cocco, Environ. Sci. Technol., 1997, 31, 261 CrossRef CAS; (b) G. Mulas, S. Loiselle, L. Schiffini and G. Cocco, J. Solid State Chem., 1997, 129, 263 CrossRef CAS; (c) G. Cao, S. Doppiu, M. Monagheddu, R. Orrù, M. Sannia and G. Cocco, Ind. Eng. Chem. Res., 1999, 38, 3218 CrossRef CAS; (d) G. Cao and R. Orrù, Chem. Eng. J., 2002, 87, 239 CrossRef CAS; (e) I. Pri-Bar and B. R. James, J. Mol. Catal. A: Chem., 2007, 264, 135 CrossRef CAS PubMed; (f) R. Calas and P. Bourgeois, Bull. Soc. Chim. Fr., 1971, 3263 CAS; (g) G. Soula and J.-L. Lepage, FR Patent 2576902, 1985.
- L. Xiao and K. E. Johnson, Can. J. Chem., 2004, 82, 491 CrossRef CAS.
- (a) T. Aida, N. Kuboki, K. Kato, W. Uchikawa, C. Matsuno and S. Okamoto, Tetrahedron Lett., 2005, 46, 1667 CrossRef CAS PubMed; (b) A. Tsuhako, J.-Q. He, M. Mihara, N. Saino and S. Okamoto, Tetrahedron Lett., 2007, 48, 9120 CrossRef CAS PubMed.
- (a) S. Harder, Chem. Commun., 2012, 48, 11165 RSC; (b) S. Harder, Chem. Rev., 2010, 110, 3852 CrossRef CAS PubMed.
- (a) S. Harder and J. Brettar, Angew. Chem., Int. Ed., 2006, 45, 3474 CrossRef CAS PubMed; (b) J. Spielmann and S. Harder, Chem.–Eur. J., 2007, 13, 8928 CrossRef CAS PubMed; (c) J. Spielmann and S. Harder, Eur. J. Inorg. Chem., 2008, 1480 CrossRef CAS.
- M. Sutter, M.-C. Duclos, B. Guicheret, Y. Raoul, E. Métay and M. Lemaire, ACS Sustainable Chem. Eng., 2013, 1, 1463 CrossRef CAS.
- D. H. R. Barton, L. Bohé and X. Lusinchi, Tetrahedron Lett., 1988, 29, 2571 CrossRef CAS.
- J. G. Smith and I. Ho, J. Org. Chem., 1973, 38, 2776 CrossRef CAS.
- A. Alexakis, I. Aujard and P. Mangeney, Synlett, 1998, 873 CrossRef CAS PubMed.
- A. Alexakis, I. Aujard, T. Kanger and P. Mangeney, Org. Synth., 1999, 76, 23 CrossRef CAS.
- S. Karlsson, J. Lindberg and H. Sorensen, Org. Process Res. Dev., 2013, 17, 1552 CrossRef CAS.
- Further information can be found in the ESI.†.
- C. L. Barney, E. W. Huber and J. R. McCarthy, Tetrahedron Lett., 1990, 31, 5547 CrossRef CAS.
- (a) L. Aurelio, R. T. C. Brownlee and A. B. Hughes, Chem. Rev., 2004, 104, 5823 CrossRef CAS PubMed; (b) C. Wu, R. Li, D. Dearborn and Y. Wang, Int. J. Org. Chem., 2012, 2, 202 CrossRef CAS.
- (a) M. Selva and P. Tundo, Tetrahedron Lett., 2003, 44, 8139 CrossRef CAS PubMed; (b) A. Dhakshinamoorthy, M. Alvaro and H. Garcia, Appl. Catal., A, 2010, 378, 19 CrossRef CAS PubMed; (c) T. Lebleu, X. Ma, J. Maddaluno and J. Legros, Chem. Commun., 2014, 50, 1836 RSC.
- R. A. da Silva, I. H. S. Estevamb and L. W. Bieber, Tetrahedron Lett., 2007, 48, 7680 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra14808h |
‡ Carole Guyon held a doctoral fellowship from La Région Rhône-Alpes financed to the amount of 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 116 euros. The authors are grateful for the access to the MS analysis at the Centre Commun de Spectroscopie de Masse and NMR facilities at the Université Lyon 1. 116 euros. The authors are grateful for the access to the MS analysis at the Centre Commun de Spectroscopie de Masse and NMR facilities at the Université Lyon 1. |
| This journal is © The Royal Society of Chemistry 2015 |