Synthesis of photoluminescent carbon dots for the detection of cobalt ions†
Chi-Lin Lia,
Chih-Ching Huangbcd,
Arun Prakash Periasamya,
Prathik Roya,
Wei-Cheng Wuef,
Chia-Lun Hsua and
Huan-Tsung Chang*a
aDepartment of Chemistry, National Taiwan University, 1, Section 4, Roosevelt Road, Taipei 10617, Taiwan. E-mail: changht@ntu.edu.tw; Fax: +886-2-33661171; Tel: +886-2-33661171
bDepartment of Bioscience and Biotechnology, National Taiwan Ocean University, 2, Pei-Ning Road, Keelung, 20224, Taiwan
cCenter of Excellence for the Oceans, National Taiwan Ocean University, 2, Pei-Ning Road, Keelung, 20224, Taiwan
dSchool of Pharmacy, College of Pharmacy, Kaohsiung Medical University, Kaohsiung, 80708, Taiwan
eDepartment of Engineering and System Science, National Tsing Hua University, Hsinchu, 30013, Taiwan
fNano Science and Technology Program, Taiwan International Graduate Program, Academia Sinica, Taipei, 11529, Taiwan
First published on 27th November 2014
Abstract
We have developed a simple assay for the sensing of cobalt ions (Co2+), based on the analyte induced photoluminescence (PL) quenching of carbon dots (C-dots). The C-dots (mean diameter 3.6 ± 0.3 nm) prepared from L-cysteine through a simple hydrothermal process at 300 °C for 2 h have a quantum yield of 13.2%. The C-dots have strong blue PL with a maximum PL intensity at 395 nm under an excitation wavelength of 325 nm. Through the reactions of Co2+ ions with cysteine molecules/residues on the surfaces of the C-dots, non-photoluminescent CoxSy nanoparticles are formed. As-formed CoxSy nanoparticles and the C-dots further form aggregates in the solution, leading to PL quenching. The C-dot probe allows detection of Co2+ ions over a concentration range from 10 nM to 100 μM (R2 = 0.992). This reliable, rapid, sensitive, and selective C-dot probe has been utilized for the determination of the concentrations of Co2+ ions in vitamin B12 and natural water samples.
1 Introduction
Carbon dots (C-dots) are a fascinating class of newly synthesized nanomaterials for sensing, catalysis, fuel cells, and imaging.1–6 Low-cost C-dots with sizes <10 nm are promising photoluminescent nanomaterials because of their interesting characteristics, including high dispersibility in aqueous solution, high photoluminescence (PL) quantum yield (ϕf), tunable PL excitation and emission wavelengths, low photobleaching, and excellent biocompatibility.1–6The detection of cobalt (Co) has received considerable attention due to its significant roles in chemical, biological, and environmental fields.7–9 In spite of the fact that Co is an essential element required in minute amounts as a component of vitamin B12 for people (the recommended daily intake of vitamin B12 is 6 μg), excessive Co uptake can lead to diseases (e.g., allergic dermatitis, rhinitis, asthma, cardiomyopathy) and death.8 Exposure of rats to short-term high levels of cobalt in the drinking water or food results in effects on the blood, liver, kidneys, and heart. The oral LD50 (lethal dose, 50%) value of soluble Co salts in rats is about 150 to 500 mg kg−1.10 Co is also considered as a possible carcinogen for humans and a toxic element to aquatic organisms.8,9,11 Mean Co concentrations in rainwater are 0.3–1.7 μg mL−1, while that in soil vary widely from 1 to 40 mg mL−1.12 Co concentrations in drinking water are usually less than 1–2 μg mL−1.12 The maximum level of Co in drinking water permitted should be lower than 40 μg L−1.12 Hence, it is important to develop sensitive, selective, and reliable analytical techniques for the determination of the concentrations of Co2+ in natural water samples and supplements (e.g. vitamin B12).
Many spectrophotometric techniques have been developed for the detection of Co2+ ions, with advantages of high selectivity, sensitivity, and fast response.13–24 However, some of them are commercially unavailable, expensive, and hydrophobic. Herein, we report a label-free C-dot probe for the detection of Co2+ ions in water samples, based on the analyte induced PL quenching (Scheme 1). The C-dots were prepared from cysteine through a hydrothermal process. Through specific interactions between Co2+ and cysteine/residue on the surfaces of C-dots, CoxSy nanoparticles were formed. The as-formed CoxSy nanoparticles and C-dots further formed aggregates in the solution. Practicality of this sensitive and selective C-dot probe was demonstrated by determination of the concentrations of Co2+ ions in stream and lake water and in vitamin B12 supplement samples.
 | ||
| Scheme 1 Schematic representation of hydrothermal preparation of carbon dots (C-dots) from cysteine for the detection of Co2+ ions. | ||
2 Experimental
2.1 Chemicals
Cobalt sulfate, L-cysteine, ethylenediaminetetraacetate (EDTA), glycine, hydrochloric acid, nitric acid, quinine sulphate, and all of the metal salts used in this study were purchased from Sigma-Aldrich (Milwaukee, WI, USA). Monobasic and dibasic sodium phosphates obtained from J. T. Baker (Phillipsburg, NJ, USA) were used to prepare a phosphate buffer (50 mM, pH 9.0). Ultrapure water (18.2 MΩ cm) from a Milli-Q ultrapure water system (Millipore, Billerica, MA, USA) was used throughout the experiments.2.2 Synthesis of C-dots
Cysteine solution (1 M, 15 mL) prepared in ultrapure water was heated hydrothermally in a stainless steel autoclave at 300 °C for 2 h. The resulting brownish yellow solution was cooled to ambient temperature (25 °C) and subsequently centrifuged at a relative centrifugal force (RCF) of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 min to remove large or agglomerated particles. The supernatant containing C-dots was then filtered through a 0.2 μm polyethersulfone membrane to remove large particles. The as-purified solution contained C-dots at a concentration of 3.3 mg mL−1. Aliquots (14.6 mL) of the as-prepared solution containing C-dots were freeze-dried in a lyophilizer for 24 h and stored at −20 °C in dark. The purified C-dots were diluted to required concentrations with sodium phosphate buffer (10 mM, pH 9.0). The ϕf value of C-dots was calculated by comparing their integrated PL intensity (excited at 365 nm) and absorbance at 365 nm with those of quinine sulphate. Quinine sulphate (ϕf = 0.54) was dissolved in 0.1 M H2SO4 (refractive index, 1.33) and C-dots were dispersed in water (refractive index, 1.33). The absorbance values of the two solutions in 1 cm (optical path length) cuvettes were kept under 0.1 at their excitation wavelengths to minimize the re-absorption effects. Excitation and emission slit widths were both set at 5.0 nm.
000g for 10 min to remove large or agglomerated particles. The supernatant containing C-dots was then filtered through a 0.2 μm polyethersulfone membrane to remove large particles. The as-purified solution contained C-dots at a concentration of 3.3 mg mL−1. Aliquots (14.6 mL) of the as-prepared solution containing C-dots were freeze-dried in a lyophilizer for 24 h and stored at −20 °C in dark. The purified C-dots were diluted to required concentrations with sodium phosphate buffer (10 mM, pH 9.0). The ϕf value of C-dots was calculated by comparing their integrated PL intensity (excited at 365 nm) and absorbance at 365 nm with those of quinine sulphate. Quinine sulphate (ϕf = 0.54) was dissolved in 0.1 M H2SO4 (refractive index, 1.33) and C-dots were dispersed in water (refractive index, 1.33). The absorbance values of the two solutions in 1 cm (optical path length) cuvettes were kept under 0.1 at their excitation wavelengths to minimize the re-absorption effects. Excitation and emission slit widths were both set at 5.0 nm.
2.3 Characterization of C-dots
Transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) images of C-dots were recorded using JEOL JSM-1230 (Hitachi, Tokyo, Japan) and FEI Tecnai-G2-F20 (GCEMarket, NJ, USA) systems operating at 200 kV, respectively. Prior to conducting TEM measurements, the as-prepared C-dots (3.3 mg mL−1) were diluted 10-fold with ultrapure water. The as-diluted C-dots were carefully deposited on 400-mesh carbon-coated Cu grids and excess solvents were evaporated at ambient temperature and pressure. A double-beam UV-Vis spectrophotometer (Cintra 10e, Dandenong, VIC, Australia) was used to measure the absorption spectra of the C-dots in ultrapure water. The PL spectra of the as-prepared C-dots were recorded using a Cary Eclipse PL spectrophotometer (Varian, Palo Alto, CA, USA) that was operated at excitation wavelengths in the range 325–405 nm. A Raman microscopic system with a 50× objective (Dongwoo Optron, Kyunggi-do, Korea) was used to analyze air-dried C-dots on a silicon wafer coupled with a diode laser at an excitation wavelength of 532 nm.A Varian 640 FT-IR spectrophotometer was employed to analyze possible functional groups present in the C-dots. Prior to X-ray photoelectron spectroscopy (XPS; PHI 5000 VersaProbe XPS, Ulvac Technologies, Methuen, MA, USA) analysis, C-dot solution (3.3 mg mL−1, 10 μL) was placed on a Si substrate and then dried at ambient temperature. The XPS spectrum of C-dots was recorded in a constant analyzer energy mode with a pass energy of 28 eV and Al Kα (1486.6 eV) radiation as the excitation source. A zetasizer (Nano-HT, Malvern, UK) was employed to record the dynamic light scattering (DLS) histogram and zeta potential of C-dots (0.33 mg mL−1). X-Ray diffraction (XRD) sample was prepared by depositing the C-dot solution (3.3 mg mL−1, 20 μL) on a silicon wafer. XRD measurement was performed by using a X-ray diffractometer (D/MAX 2200 VPC, Rigaku, Sendagaya, Shibuya-Ku, Tokyo, Japan) with the Cu Kα1 line (λ = 1.54 Å, energy = 8.8 keV).
2.4 Detection of Co2+ ions
Stock solutions of inorganic metal ions (0.1 M) were prepared in 0.1 M HNO3, which were then diluted to required concentrations (0.1–1000 μM) in sodium phosphate buffer (10 mM, pH 9.0). Aliquots (100 μL) of metal ion solutions were added separately to sodium phosphate buffer (10 mM, pH 9.0) containing C-dots (17 μg mL−1) to obtain final volumes of 1 mL. After equilibrating at ambient temperature for 1 h, the mixtures were transferred separately into 96-well microtiter plates and then their PL spectra were recorded using a Synergy 4 Multi-Mode monochromatic microplate spectrophotometer (Biotek Instruments, Winooski, VT, USA).2.5 Analysis of Co2+ ions in real samples
Water samples collected from stream and lake near the campus of National Taiwan Ocean University were filtered through a 0.2 μm membrane. For the detection of Co2+ ions, aliquots of the water samples (100 μL) were spiked with standard Co2+ ion solutions (100 μL, 0.1–5 μM). The spiked samples were then diluted to 1 mL with sodium phosphate buffer (10 mM, pH 9.0) containing C-dots (17 μg mL−1). The final concentrations of Co2+ ions in the mixtures were over the range 10–500 nM. Vitamin B12 tablets were obtained from General Nutrition Centers (GNC, Pittsburgh, PA, USA). The tablets were weighed and ground to fine powder. Vitamin B12 (1 mg) powder was dissolved in 50 μL HCl solution (12 M) through ultrasonication for 2 h.25 The resulting solution was further diluted with 950 μL of ultrapure water. Aliquots of the diluted sample (100 μL) were spiked with standard Co2+ ions (0–200 μM; 100 μL) in sodium phosphate buffer (10 mM, pH 9.0). The spiked samples were then diluted to 1 mL with sodium phosphate buffer (10 mM, pH 9.0) containing C-dots (17 μg mL−1). After incubation for 1 h, the mixtures were subjected to PL measurements with excitation and emission wavelengths of 325 and 395 nm, respectively. All the spiked natural water samples were also analyzed by inductively coupled plasma mass spectrometry (ICP-MS).3 Results and discussion
3.1 Characterization of C-dots
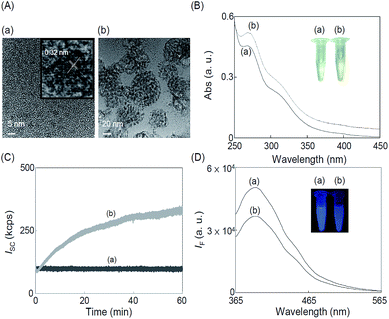
The TEM image of as-prepared C-dots displayed in Fig. 1A(a) shows that they were uniform and monodispersed spheres with a mean diameter of 3.6 ± 0.3 nm (from 100 counts). This autoclave-assisted hydrothermal carbonization method did not require any surface passivation agents or inorganic additives; the sole reagent was cysteine. The cysteine molecules were converted to C-dots via four steps, including dehydration, pyrolysis, carbonization, and passivation.26,27 During the carbonization step, a short single burst of nucleation occurred in the solution as soon as a critical supersaturation level was reached. The as-formed nuclei underwent isotropic growth through the diffusion of solutes toward the particles surfaces and then converted to C-dots.28–31 The lattice structure (d-spacing, 0.32 nm) is discernible in the HRTEM image (the inset to Fig. 1A(a)), which is in good agreement with the XRD pattern (002 plane, at 2θ = 23.5°) as displayed in Fig. 2A.32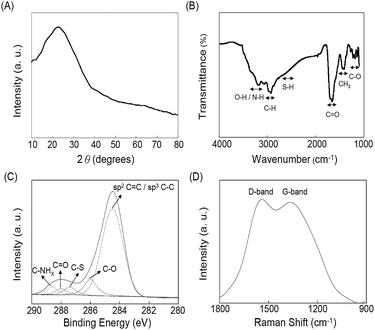 | ||
| Fig. 2 (A) XRD spectrum, (B) FTIR spectrum, (C) C1s XPS spectrum, and (D) Raman spectrum of as-prepared C-dots. Other conditions were the same as those described in Fig. 1. | ||
The functional groups in the C-dots were determined by FTIR (Fig. 2B). The peaks at the wavenumbers around 3300, 3000, 1700 and 1100 cm−1 are assigned separately for the stretching vibrations of O–H/N–H, C–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and C–O bonds, respectively. The peak at the wavenumber about 1400 cm−1 is due to the CH3 band of bending vibration. The S–H stretching vibration mode at the wavenumber close to 2600 cm−1 indicates that the C-dots have the thiol functional group on its surfaces. The presence of hydroxyl and carboxylate groups on the surface of C-dots was further confirmed with its negative zeta potential value (−32.6 ± 3.7 mV; n = 5). The detailed C1s XPS spectrum of C-dots (Fig. 2C) shows five bands at the binding energies of 284.5, 286.1, 287.4, 288.0 and 288.5 eV, which are attributed to C
O, and C–O bonds, respectively. The peak at the wavenumber about 1400 cm−1 is due to the CH3 band of bending vibration. The S–H stretching vibration mode at the wavenumber close to 2600 cm−1 indicates that the C-dots have the thiol functional group on its surfaces. The presence of hydroxyl and carboxylate groups on the surface of C-dots was further confirmed with its negative zeta potential value (−32.6 ± 3.7 mV; n = 5). The detailed C1s XPS spectrum of C-dots (Fig. 2C) shows five bands at the binding energies of 284.5, 286.1, 287.4, 288.0 and 288.5 eV, which are attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/C–C, C–O, C–S, C
C/C–C, C–O, C–S, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and C–NHX bonds, respectively. The Raman spectrum of the C-dots (Fig. 2D) displays D band and G band at 1361 and 1542 cm−1, respectively. The D band is associated with vibrations of carbon atoms with dangling bonds in the terminal plane of disordered carbon, while the G band is related to the vibration of sp2-hybridized carbon atoms in a two-dimensional hexagonal lattice.33 The two bands confirm the graphitic domains present in the network of carbon atoms.
O, and C–NHX bonds, respectively. The Raman spectrum of the C-dots (Fig. 2D) displays D band and G band at 1361 and 1542 cm−1, respectively. The D band is associated with vibrations of carbon atoms with dangling bonds in the terminal plane of disordered carbon, while the G band is related to the vibration of sp2-hybridized carbon atoms in a two-dimensional hexagonal lattice.33 The two bands confirm the graphitic domains present in the network of carbon atoms.
3.2 Sensing of Co2+ ions
Fig. 1A(b) reveals that as-prepared C-dots in the presence of 10 μM Co2+ ions aggregated to form large granular C-dots/cobalt sulfide (C-dots/CoxSy) nanomaterials with 40–80 nm in diameter. XPS results (Fig. S1A, ESI†) reveal that the C-dots/CoxSy nanomaterials contain 56.1%, 20.6%, 9.6%, 8.8%, and 4.9% of C, O, S, N and Co, respectively. The XRD patterns of the CoxSy (Fig. S1B, ESI†) show characteristic diffraction peaks at 2θ values of 29.6° and 61.7°, which correspond to Co8S9 (111) and CoS2 (230), respectively. The representative STEM-EDS (Fig. S2, ESI†) of C-dots/CoxSy nanomaterials reveals that the C-dots and CoxSy coexisted and were homogeneously distributed. Co2+ ions reacted with the cysteine/ligand on the surfaces of C-dots to form CoxSy nanoparticles. The as-formed CoxSy nanoparticles and the unreacted C-dots formed aggregates in the solution.The UV-Vis absorption spectrum (Fig. 1B) of the C-dots exhibited two absorption bands at the wavelengths around 272 and 320 nm corresponding to π → π* (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond) and n → π* (peroxide and/or epoxide groups) transitions, respectively.31,34 In the presence of Co2+ (10 μM), the color of solution changed from light yellow to gray black as a result of aggregation of C-dots with the as-formed CoxSy (the inset in Fig. 1B). Fig. S3 (ESI†) illustrates the PL spectra of the C-dots, showing red shifts of the PL with decreased intensities under excitations at the wavelengths from 325 to 405 nm. These excitation wavelength-dependent PL properties are due to different hybridized compositions, structures, emissive trap sites, and sizes of the C-dots.1–3 The C-dots have strong blue PL as shown in the inset (Fig. 1D), with a maximum PL intensity at 395 nm under an excitation wavelength of 325 nm (Fig. S3, ESI†). The ϕf value (λex = 365 nm) was determined to be 13.2% when using quinine sulfate (ϕf ∼ 54% in 0.1 M H2SO4) as the reference. The obtained ϕf value is comparable with that of C-dots prepared through hydrothermal methods.1–6,35,36 Co2+ induced PL quenching of the C-dots (Fig. 1D) as a result of the formation of non-photoluminescent CoxSy nanoparticles. PL quenching is also possible through electron and energy transfer between the C-dots and CoxSy.1,37,38 The deposition of CoxSy on the surfaces of C-dots may interrupt photoinduced charge separation and/or radiative recombination of C-dots.37
C bond) and n → π* (peroxide and/or epoxide groups) transitions, respectively.31,34 In the presence of Co2+ (10 μM), the color of solution changed from light yellow to gray black as a result of aggregation of C-dots with the as-formed CoxSy (the inset in Fig. 1B). Fig. S3 (ESI†) illustrates the PL spectra of the C-dots, showing red shifts of the PL with decreased intensities under excitations at the wavelengths from 325 to 405 nm. These excitation wavelength-dependent PL properties are due to different hybridized compositions, structures, emissive trap sites, and sizes of the C-dots.1–3 The C-dots have strong blue PL as shown in the inset (Fig. 1D), with a maximum PL intensity at 395 nm under an excitation wavelength of 325 nm (Fig. S3, ESI†). The ϕf value (λex = 365 nm) was determined to be 13.2% when using quinine sulfate (ϕf ∼ 54% in 0.1 M H2SO4) as the reference. The obtained ϕf value is comparable with that of C-dots prepared through hydrothermal methods.1–6,35,36 Co2+ induced PL quenching of the C-dots (Fig. 1D) as a result of the formation of non-photoluminescent CoxSy nanoparticles. PL quenching is also possible through electron and energy transfer between the C-dots and CoxSy.1,37,38 The deposition of CoxSy on the surfaces of C-dots may interrupt photoinduced charge separation and/or radiative recombination of C-dots.37
Fig. 1C displays that the static scattering light intensities of the C-dots (17 μg mL−1) was much higher than that in the presence of 10 μM Co2+ ions, confirming the as-formed CoxSy nanoparticles further formed aggregates with C-dots. To further understand the Co2+ induced PL quenching of C-dots, lifetimes of the C-dots in the absence and presence of 10 μM Co2+ ions were measured. Fig. S4 (ESI†) shows that the lifetime of C-dots decreased from its original 6.26 to 2.51 ns, indicating that excited-state molecules of C-dots reacted with Co2+ ions. In addition, the lifetime of C-dots decreased gradually upon increasing concentration of Co2+ ions (data not shown). The results revealed that Co2+ induced PL quenching is a dynamic quenching. To further prove that the PL quenching is related to the formation CoxSy nanoparticles and their aggregates with the C-dots, control experiments were done using C-dots that had been purified through dialysis. The PL intensity of the purified C-dots was slightly quenched by Co2+ ions (data not shown). In contrast, PL quenching of the purified C-dots by Co2+ ions was significant by adding cysteine (5 mM) to the solution. The PL of C-dots quenched by Co2+ ions was minimized by adding a strong metal ion-chelating agent such as EDTA to the solution (Fig. S5, ESI†), revealing that the PL quenching associated with CoxSy nanoparticles was minimized. In other words, the Co2+ ions induced PL quenching of C-dots was minimized through a competitive complexation reaction.
3.3 Sensitivity and selectivity
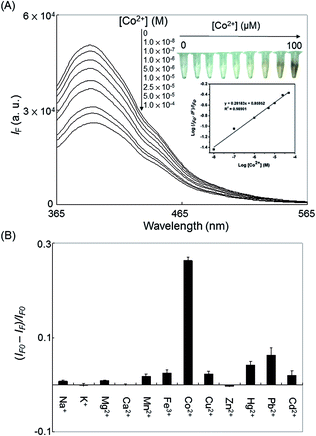
Fig. 3A depicts the PL responses of the C-dots (17 μg mL−1) toward Co2+ ions (0–100 μM) in sodium phosphate buffer (10 mM, pH 9.0). The value of logarithm of (IF0 − IF)/IF0 (IF and IF0 are the PL intensities of the C-dots in the presence and absence of Co2+ ions, respectively) of C-dots solution increased linearly (R2 = 0.992) upon increasing the concentration of Co2+ ions over the range from 10 nM to 100 μM. The C-dot probe allowed detection of Co2+ ions as low as ∼5 nM (∼0.3 μg mL−1), which is much below the maximum level of Co2+ ions permitted (∼40 μg mL−1) for drinking water. Compared with other nanoparticles-based optical sensors,14–24 this approach opens up various avenues toward the detection of Co2+ ions in environmental and biological samples. We noted that only cysteine solution without any hydrothermal treatment (control) allowed detection of Co2+ ions down to 1.0 μM through the absorption measurement at 450 nm (Fig. S6, ESI†). The absorption band at 450 nm is due to the as-formed [Co(Cys)2] complexes. Relative to the cysteine-operated colorimetric approach, the PL approach provides much better sensitivity toward Co2+ ions. To investigate the selectivity of C-dots probe toward Co2+ ions, metal ions such as Na+, K+, Mg2+, Ca2+, Mn2+, Fe3+, Co2+, Cu2+, Zn2+, Hg2+, Pb2+, and Cd2+ ions (10 μM) were added separately into the probe solution. The relative PL quenching [(IF − IF0)/IF0] for other metal ions is presented in Fig. 3B, where, IF and IF0 are the PL intensities at 395 nm of the C-dots in the presence and absence of metal ions, respectively. Fig. 3B reveals that the C-dot probe was specific toward Co2+ ions. The selectivity for Co2+ over the selected metal ions resulted mainly from the specific interactions between Co2+ and cysteine.39,40 C-dots prepared from organic compounds (e.g., glycine, EDTA) did not exhibit any obvious selectivity toward Co2+ ions (data not shown). In comparison with some representative nanoparticles based optical methods (Table 1), our label-free PL-based assay for Co2+ ions is relatively simple, cost-effective, selective and sensitive. In addition, other techniques mostly require covalent conjugation of ligands to nanoparticles.14–24 As shown in Table 1, other kinds of QDs have been used for the detection of Co2+ ions. However, our label-free PL-based assay provides much better sensitivity than that of the reported ones, showing C-dots are the best candidate for the detection of Co2+ ions. Traditional toxic metal-based semiconductor QDs have raised serious health and environmental concerns. In addition, the composite and size of the QDs (i.e., the bandgap of QDs) may influence the selectivity and quenching efficiency of QDs toward heavy metal ions.41,42 Using toxic metal precursors and organic solvents for the preparation of QDs are also problematic. Relative to semiconductor QDs, the preparation of C-dots is simple and straightforward. Avoiding use of toxic precursors and organic solvents are advantageous. Recently, positioning QDs on top of TiO2 layer has been shown to be effective for improving their PL.43 In our future work, C-dots will be coupled with different metal oxide nanostructures including TiO2, ZnO or MnO2 for improving their PL and sensitivity of the detection of Co2+ ions.| Method | Probe unit | LOD (nM) | Real samples | Ref. |
|---|---|---|---|---|
a Thioglycolic acid (TGA) functionalized-hexadecyltrimethylammonium bromide (CTAB) modified gold nanoparticles (Au NPs).b Peptide (CALNNDHHHHHH) modified Au NPs.c Dopamine dithiocarbamate (DDTC) functionalized Ag NPs.d Carboxyl-functionalized CdS quantum dots (QDs).e Dithiocarbamate anchored terpyridine (TPY-DTC) functionalized Ag NPs.f PEG-passivated carbon dots modulated with CTAB to form the CTAB–PEG microenvironment.g CePO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Tb3+ nanocrystals.h N-Acetyl-L-cysteine-capped Mn-doped ZnS QDs.i Thioglycolic acid (TGA)-capped CdTe QDs.j Thioglycolic acid-capped CuInS2/ZnS QDs. Tb3+ nanocrystals.h N-Acetyl-L-cysteine-capped Mn-doped ZnS QDs.i Thioglycolic acid (TGA)-capped CdTe QDs.j Thioglycolic acid-capped CuInS2/ZnS QDs. |
||||
| Absorbance | TGA functionalized CTAB-Au NPsa | 300 | Not provided | 14 |
| Absorbance | P-Au NPsb | 2000 | Tap/river water | 15 |
| Absorbance | DDTC-Ag NPsc | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Not provided | 16 |
| Absorbance | Triazole-carboxyl Ag NPs | 7000 | Drinking water | 17 |
| Absorbance | COF-CdS QDsd | 390 | Drinking/tap/river/lake water | 18 |
| SERS | TPY-DTC-Ag NPse | 1 | Potable water | 19 |
| Chemiluminescence | CTAB@carbon dotsf | 0.67 | HepG2 cells | 20 |
| Photoluminescence | CePO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Tb3+ NCsg Tb3+ NCsg |
3.5 | Tap/river/lake water | 21 |
| Photoluminescence | Mn-doped ZnS QDsh | 60 | Tap/pond water | 22 |
| Photoluminescence | TGA-CdTe QDsi | 7.3 | Not provided | 23 |
| Photoluminescence | CuInS2/ZnS/TGA QDsj | 160 | Simulated water | 24 |
| Photoluminescence | C-dots | 5 | Lake/stream water and vitamin B12 tablet | This study |
3.4 Detection of Co2+ ions in water and vitamin B12 samples
To test the practicality of our developed approach, a standard addition method was applied to determine the concentration of Co2+ ions in water and vitamin B12 samples. As shown in Fig. 4A and B, the PL intensity ratio of C-dot probe decreased upon increasing the spiked concentration of Co2+ ions (10–500 nM) in the 10-fold diluted lake water and stream water samples, respectively. The limits of detection (LOD; S/N = 3) for Co2+ ions (∼10 nM) in these two natural water samples were slightly higher than that obtained in the standard solutions, revealing small matrix effects. Neither the C-dots based sensing probe nor did an ICP-MS approach detect the presence of Co2+ ions in the non-spiked water samples. Fig. 4C displays the PL responses of the C-dot probe toward Co2+ ions (0–20 μM) spiked into vitamin B12 samples. The concentration of Co2+ ions in the vitamin B12 tablet was determined to be 4.34 ± 0.08 wt% (n = 4). There was no significant difference (at a 95% confidence level) between the value determined using our approach and the certified one (4.36 wt%). The results reveal that the label-free C-dots probe can be utilized for the determination of the concentrations of Co2+ ions in natural water and supplement samples.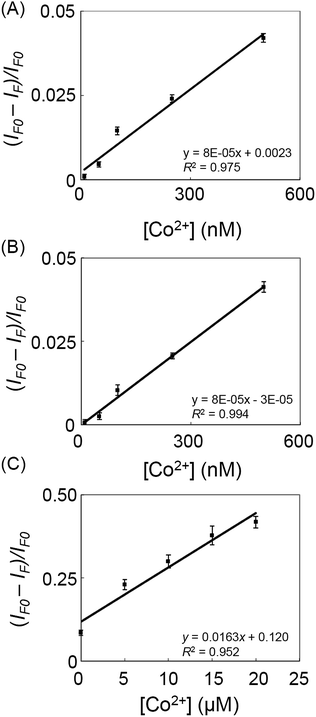 | ||
| Fig. 4 The linear relationship between [(IF − IF0)/IF0] and [Co2+] from 10 to 500 nM in 10-fold diluted (A) lake and (B) stream water samples in sodium phosphate buffer (10 mM, pH 9.0). (C) Analysis of a representative vitamin B12 sample using C-dots probe in sodium phosphate buffer (10 mM, pH 9.0). Aliquots of the diluted vitamin B12 sample (1000 μg mL−1) were spiked with Co2+ ions at concentrations in the range 0–20 μM. Other conditions were the same as those described in Fig. 3. | ||
4 Conclusions
C-dots were successfully synthesized from cysteine through a hydrothermal process. The as-synthesized C-dots were used for the sensitive and selective detection of Co2+ ions in aqueous solution, based on the analyte induced PL quenching. This simple, cost-effective, selective, and sensitive probe allows the detection of Co2+ ions in vitamin B12 and natural water samples. This study revealed that the cysteine played an important role in determining the selectivity. It is thus our belief that C-dots prepared from various precursors are worthy to be tested for different metal ions.Acknowledgements
This study was supported by the Ministry of Science & Technology (MOST) (contract NSC 98-2113M-002-011-MY3), the National Taiwan Health Research Institutes Taiwan (contract NHRI-EX100-10047NI), and the Institute of Nuclear Energy Research (contract 1002001INER082). A.P.P and P.R are grateful to the Department of Chemistry, National Taiwan University and MOST for postdoctoral fellowships under the contract numbers 101-R-4000 and NSC 101-2113-M-002-002-MY3, respectively.Notes and references
- H. Li, Z. Kang, Y. Liu and S.-T. Lee, J. Mater. Chem., 2012, 22, 24230 RSC.
- J. Shen, Y. Zhu, X. Yang and C. Li, Chem. Commun., 2012, 48, 3686 RSC.
- S. N. Baker and G. A. Baker, Angew. Chem., Int. Ed., 2010, 49, 6726 CrossRef CAS PubMed.
- S.-T. Yang, L. Cao, P. G. Luo, F. Lu, X. Wang, H. Wang, M. J. Meziani, Y. Liu, G. Qi and Y.-P. Sun, J. Am. Chem. Soc., 2009, 131, 11308 CrossRef CAS PubMed.
- C. Ding, A. Zhu and Y. Tian, Acc. Chem. Res., 2014, 47, 20 CrossRef CAS PubMed.
- C.-L. Li, C.-M. Ou, C.-C. Huang, W.-C. Wu, Y.-P. Chen, T.-E. Lin, L.-C. Ho, C.-W. Wang, C.-C. Shih, H.-C. Zhou, Y.-C. Lee, W.-F. Tzeng, T.-J. Chiou, S.-T. Chu, J.-S. Cang and H.-T. Chang, J. Mater. Chem. B, 2014, 2, 4564 RSC.
- E. J. Underwood, Nutr. Rev., 1975, 33, 65 CrossRef CAS PubMed.
- D. G. Barceloux, J. Toxicol., Clin. Toxicol., 1999, 37, 201 CrossRef CAS PubMed.
- R. Lauwerys and D. Lison, Sci. Total Environ., 1994, 150, 1 CrossRef CAS.
- G. J. Speijers, E. I. Krajnc, J. M. Berkvens and M. J. van Logten, Food Chem. Toxicol., 1982, 20, 311 CrossRef CAS.
- D. Lison, M. D. Boeck, V. Verougstraete and M. Kirsch-Volders, Occup. Environ. Med., 2001, 58, 619 CrossRef CAS.
- Agency for Toxic Substances and Disease Registry (ATSDR), Toxicological Profile for Cobalt, Public Health Service, U.S. Department of Health and Human Services, Atlanta, GA, 1992.
- C.-Y. Tsai and Y.-W. Lin, Analyst, 2013, 138, 1232 RSC.
- F. Zhang, L. Zeng, Y. Zhang, H. Wang and A. Wu, Nanoscale, 2011, 3, 2150 RSC.
- M. Zhang, Y.-Q. Liu and B.-C. Ye, Analyst, 2012, 137, 601 RSC.
- V. N. Mehta, A. K. Mungara and S. K. Kailasa, Anal. Methods, 2013, 5, 1818 RSC.
- Y. Yao, D. Tian and H. Li, ACS Appl. Mater. Interfaces, 2010, 2, 684 CAS.
- A. H. Gore, D. B. Gunjal, M. R. Kokate, V. Sudarsan, P. V. Anbhule, S. R. Patil and G. B. Kolekar, ACS Appl. Mater. Interfaces, 2012, 4, 5217 CAS.
- D. Tsoutsi, L. Guerrini, J. M. Hermida-Ramon, V. Giannini, L. M. Liz-Marzán, A. Wei and R. A. Alvarez-Puebla, Nanoscale, 2013, 5, 5841 RSC.
- J. Shi, C. Lu, D. Yan and L. Ma, Biosens. Bioelectron., 2013, 45, 58 CrossRef CAS PubMed.
- H. Chen, F. Yuan, J. Xu, Y. Zhang, Y. Wu and L. Wang, Spectrochim. Acta, Part A, 2013, 107, 151 CrossRef CAS PubMed.
- W. Bian, J. Ma, Q. Liu, Y. Wei, Y. Li, C. Dong and S. Shuang, Luminescence, 2014, 29, 151 CrossRef CAS PubMed.
- W. Zhong, J. Liang and J. Yu, Spectrochim. Acta, Part A, 2009, 74, 603 CrossRef PubMed.
- L. Zi, Y. Huang, Z. Yan and S. Liao, J. Lumin., 2014, 148, 359 CrossRef CAS PubMed.
- K. S. Lok, S. Z. B. A. Muttalib, P. P. F. Lee, Y. C. Kwok and N.-T. Nguyen, Lab Chip, 2012, 12, 2353 RSC.
- P.-C. Hsu, Z.-Y. Shih, C.-H. Lee and H.-T. Chang, Green Chem., 2012, 14, 917 RSC.
- P.-C. Hsu and H.-T. Chang, Chem. Commun., 2012, 48, 3984 RSC.
- X. Sun and Y. Li, Angew. Chem., Int. Ed., 2004, 43, 597 CrossRef PubMed.
- C. Liu, P. Zhang, F. Tian, W. Li, F. Li and W. Liu, J. Mater. Chem., 2011, 21, 13163 RSC.
- Y. Li, E. J. Lee, W. Cai, K. Y. Kim and S. O. Cho, ACS Nano, 2008, 2, 1108 CrossRef CAS PubMed.
- Y. Yang, J. Cui, M. Zheng, C. Hu, S. Tan, Y. Xiao, Q. Yang and Y. Liu, Chem. Commun., 2012, 48, 380 RSC.
- Z. Q. Li, C. J. Lu, Z. P. Xia, Y. Zhou and Z. Luo, Carbon, 2007, 45, 1686 CrossRef CAS PubMed.
- A. C. Ferrari and D. M. Basko, Nat. Nanotechnol., 2013, 8, 235 CrossRef CAS PubMed.
- X. Zhang, Y. Zhang, Y. Wang, S. Kalytchuk, S. V. Kershaw, Y. Wang, P. Wang, T. Zhang, Y. Zhao, H. Zhang, T. Cui, Y. Wang, J. Zhao, W. W. Yu and A. L. Rogach, ACS Nano, 2013, 7, 11234 CrossRef CAS PubMed.
- C.-I. Wang, W.-C. Wu, A. P. Periasamy and H.-T. Chang, Green Chem., 2014, 16, 2509 RSC.
- K. Qu, J. Wang, J. Ren and X. Qu, Chem.–Eur. J., 2013, 19, 7243 CrossRef CAS PubMed.
- X. Wang, L. Cao, F. Lu, M. J. Meziani, H. Li, G. Qi, B. Zhou, B. A. Harruff, F. Kermarrec and Y.-P. Sun, Chem. Commun., 2009, 25, 3774 RSC.
- J. Xu, S. Sahu, L. Cao, C. E. Bunker, G. Peng, Y. Liu, K. A. S. Fernando, P. Wang, E. A. Guliants, M. J. Meziani, H. Qian and Y.-P. Sun, Langmuir, 2012, 28, 16141 CrossRef CAS PubMed.
- M. Tada and R. Shino, J. Inorg. Biochem., 1991, 44, 89 CrossRef CAS.
- B. Harman and I. Sóvágó, Inorg. Chim. Acta, 1983, 80, 75 CrossRef CAS.
- Y. S. Xia and C. Q. Zhu, Talanta, 2008, 75, 215 CAS.
- Y. S. Xia, C. Cao and C. Q. Zhu, Chin. J. Chem., 2007, 25, 1836 CrossRef CAS.
- Z.-H. Chen, Y. Wang, Y. Yang, N. Qiao, Y. Wang and Z. Yu, Nanoscale, 2014 10.1039/c4nr03851g.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1–S6. See DOI: 10.1039/c4ra11704b |
| This journal is © The Royal Society of Chemistry 2015 |