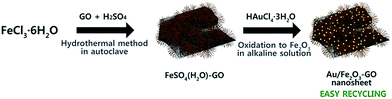Au nanoparticles supported on magnetically separable Fe2O3–graphene oxide hybrid nanosheets for the catalytic reduction of 4-nitrophenol†
Hyunje Wooa,
Ji Woong Kimb,
Miran Kima,
Sungkyun Parkb and
Kang Hyun Park*a
aDepartment of Chemistry and Chemistry Institute for Functional Materials, Pusan National University, Busan 609-735, Korea. E-mail: chemistry@pusan.ac.kr; Fax: +82-51-980-5200; Tel: +82-51-510-2238
bDepartment of Physics, Pusan National University, Busan 609-735, Korea
First published on 22nd December 2014
Abstract
A one-pot hydrothermal synthesis approach was developed to prepare FeSO4·(H2O)–graphene oxide (GO) nanosheets. Au nanoparticles were immobilized onto this support, giving Au/Fe2O3–GO nanocomposites. Fe2O3–GO supports showed high surface area and thermal stability. The Au/Fe2O3–GO and synthesized Au/Fe3O4 nanocomposites exhibited high catalytic activity and recyclability for the reduction of 4-nitrophenol.
4-Nitrophenol is considered to be one of the most abundant refractory pollutants in wastewaters generated by agricultural and industrial sources.1 Until now, there have been many reports about applications of Au nanoparticles (NPs) on various supports as catalysts for the reduction of 4-nitrophenol, since the reduction product, 4-aminophenol, is an important intermediate for the manufacture of many analgesic and antipyretic drugs.2,3 Among these studies, those using water as a solvent under mild conditions have attracted much attention from the environmental viewpoint.
In recent years, various studies have been conducted into hybrid nanostructures in order to fabricate and synthesize multicomponent structures that can combine the physical and chemical properties of the individual components.4,5 This enhanced functionality makes these hybrid nanostructures useful in many research fields such as magnetics, plasmonics, and semiconductors.6 Among various hybrid nanostructures, noble metal NPs with metal oxide supports and graphene oxide (GO) have been applied to organic reactions as catalysts.7–9 For instance, Zhao and coworkers8 developed multi-functional mesoporous composite microspheres with well-designed nanostructures for an integrated catalyst system. Mülhaupt et al.9 reported Pd NPs on graphite oxide and their functionalized graphene derivatives with the aim of creating a hybrid catalytic system. These hybrid nanocatalysts have the advantages of higher catalytic activity and better recyclability than are offered by single metal nanoparticles, owing to electron transfer across the interface as well as their heterogeneous properties.10,11 In a number of supports, iron oxide and GO are important since iron oxide can be separated by an external magnet and GO has high electrical conductivity. Even though iron oxide supports show high recyclability, they are unstable in air and easily aggregate.12 Moreover, taking advantage of surface functional groups as well as the large surface area of GO nanosheets,13 they cannot be easily recycled by an external magnet; therefore, it is essential to combine advantages of iron oxide and GO.
Our ongoing interest in the iron oxide–GO support combining both advantages of iron oxide and GO motivated us to investigate iron oxide–GO supports by a facile approach. In this study, we synthesized FeSO4·(H2O)–GO nanosheets by a one-pot method and then Au NPs were immobilized onto this support. During the immobilization of the Au NPs in situ, FeSO4·(H2O) transformed into Fe2O3, resulting in superparamagnetic Au/Fe2O3–GO nanosheets. To the best of our knowledge, this is the first example of a versatile synthesis of Au/Fe2O3–GO nanosheets. Au/Fe2O3–GO nanosheets were then used as a catalyst for the reduction of 4-nitrophenol in water, and high catalytic activity and recyclability were achieved.
The Au/Fe2O3–GO nanosheets were synthesized via two steps from FeCl3·6H2O (Scheme 1). FeSO4·(H2O)–GO nanosheets were prepared by an in situ hydrothermal method.14 A gold precursor was subsequently injected to yield Au/Fe2O3–GO nanosheets. GO nanosheets were used as the precursor to prepare FeSO4·(H2O)–GO nanocomposites, which were synthesized from natural graphite powders by a modified Hummer's method.15 For comparison, Au/Fe3O4 microspheres were also synthesized.
Transmission electron microscopy (TEM) images show the morphology of Au/Fe3O4 microspheres, FeSO4·(H2O)–GO and Au/Fe2O3–GO nanosheets (Fig. 1). Low-resolution TEM images of FeSO4·(H2O)–GO and Au/Fe2O3–GO nanosheets are shown in Fig. S1a and b (ESI†). Prior to the immobilization of the Au NPs onto the Fe3O4 microspheres, pretreatment procedures such as coating polymers or SiO2 onto Fe3O4 were not required. In fact, the Au NPs were easily immobilized onto the surface of Fe3O4 in situ because of the interaction of the carboxyl groups of trisodium citrate with Au (Fig. 1a and d).16 Size distribution graphs of the Au NPs are shown in Fig. S1c (ESI†), indicating the average size of the Au NPs was 8.0 nm. The X-ray diffraction (XRD) pattern of the Au/Fe3O4 microspheres matched the cubic spinel structure of Fe3O4 (JCPDS no. 19-0629) and face-centered cubic structure of Au (JCPDS no. 04-0784) (Fig. 2a).
 | ||
| Fig. 1 TEM images of Au/Fe3O4 microspheres (a and d), FeSO4·(H2O)–GO (b and e) and Au/Fe2O3–GO nanosheets (c and f). | ||
The TEM images in Fig. 1b and e show FeSO4·(H2O)–GO nanocomposites. GO and FeSO4·(H2O)–GO were also imaged by non-tapping mode dynamic force microscope (DFM). Fig. S2 (ESI†) shows the DFM images of GO and FeSO4·(H2O)–GO, showing topology of nanocomposites. As shown in Fig. 2a, the XRD pattern shows anorthic FeSO4·(H2O) reflections (JCPDS no. 81-0019). Fourier-transform infrared (FT-IR) spectra were also measured to confirm the structure of GO and FeSO4·(H2O)–GO (Fig. 3). In the case of GO, the bands at ca. 1200 cm−1, 1070 cm−1, and 1720 cm−1 are attributed to the C–O epoxy stretching, C–O alkoxy stretching, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O carbonyl stretching vibrations, respectively; the absorption band at ca. 3450 cm−1 corresponds to O–H stretching.17 Additionally, FeSO4·(H2O)–GO shows strong bands corresponding to FeSO4·(H2O). The asymmetric bending mode of SO42− is presented as triplet at 606, 625, and 667 cm−1. The asymmetric bending mode of OH− is located at 555 and 823 cm−1. The symmetrical stretching mode of SO42− is registered at 1020 cm−1. The asymmetrical stretching mode of SO42− is split into two peaks, due to lower symmetry: 1080 and 1130 cm−1. The symmetric bending modes of the hydroxyl group and crystal water are registered at 1500 and 1650 cm−1, respectively. The broad band of OH− in the range 3000–3500 cm−1 is associated with strong hydrogen bonds.18 Elemental analysis of FeSO4·(H2O)–GO was confirmed by using X-ray photoelectron spectroscopy (XPS). The Fe2+ 2p3/2 peak of FeSO4·(H2O) is 710.9 eV; the binding energies of the doublet for S6+ 2p1/2 (168.1 eV) and S6+ 2p3/2 (168.6 eV) are shown in Fig. S3 (ESI†) and are consistent with the previously reported FeSO4·(H2O) data (Fig. S3a and c, ESI†).19 In the case of GO, curve fitting of the C 1s and O 1s spectra was conducted using a Gaussian–Lorentzian function following Shirley background correction. The binding energy of the C–C is assigned at 284.3 eV and shifts of +1.6 and +3.3 eV are typically assigned to the C–OH and C
O carbonyl stretching vibrations, respectively; the absorption band at ca. 3450 cm−1 corresponds to O–H stretching.17 Additionally, FeSO4·(H2O)–GO shows strong bands corresponding to FeSO4·(H2O). The asymmetric bending mode of SO42− is presented as triplet at 606, 625, and 667 cm−1. The asymmetric bending mode of OH− is located at 555 and 823 cm−1. The symmetrical stretching mode of SO42− is registered at 1020 cm−1. The asymmetrical stretching mode of SO42− is split into two peaks, due to lower symmetry: 1080 and 1130 cm−1. The symmetric bending modes of the hydroxyl group and crystal water are registered at 1500 and 1650 cm−1, respectively. The broad band of OH− in the range 3000–3500 cm−1 is associated with strong hydrogen bonds.18 Elemental analysis of FeSO4·(H2O)–GO was confirmed by using X-ray photoelectron spectroscopy (XPS). The Fe2+ 2p3/2 peak of FeSO4·(H2O) is 710.9 eV; the binding energies of the doublet for S6+ 2p1/2 (168.1 eV) and S6+ 2p3/2 (168.6 eV) are shown in Fig. S3 (ESI†) and are consistent with the previously reported FeSO4·(H2O) data (Fig. S3a and c, ESI†).19 In the case of GO, curve fitting of the C 1s and O 1s spectra was conducted using a Gaussian–Lorentzian function following Shirley background correction. The binding energy of the C–C is assigned at 284.3 eV and shifts of +1.6 and +3.3 eV are typically assigned to the C–OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functional groups, respectively (Fig. S3b, ESI†).20
O functional groups, respectively (Fig. S3b, ESI†).20
In order to immobilize the Au NPs onto the FeSO4·(H2O)–GO support, a method of exchanging sodium cations with noble metallic ions was applied.16 In alkaline aqueous solution, iron salts precipitate,21 leading to the oxidation of FeSO4·(H2O) to Fe2O3 during immobilization of the Au NPs, resulting in Au/Fe2O3–GO nanosheets. TEM images show highly monodisperse Au NPs immobilized onto Fe2O3–GO nanosheets (Fig. 1c and f). Size distribution graphs of the Au NPs are displayed in Fig. S1d (ESI†), showing the average size of the Au NPs was 7.6 nm. Since it was difficult to assign the crystal structure of Fe2O3 due to the low intensity in the XRD pattern (Fig. 2a), we confirmed the Fe2O3 structure by using the XPS technique. In the case of high-spin iron, the Fe 2p peak is always split into two due to spin–orbit coupling (Fe 2p1/2, Fe 2p3/2). In the oxidized state (Fig. S3d, ESI†), an additional satellite peak appears in between the Fe 2p1/2 and Fe 2p3/2 components: for the case of Fe2O3, the satellite occurs 8 eV above the Fe 2p3/2 component.22 The binding energy of the C–C is also assigned at 284.3 eV and shifts of +1.5 and +3.8 eV are typically assigned for the C–OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functional groups, respectively (Fig. S3e, ESI†).20 When the Au/Fe2O3–GO nanosheets were synthesized, GO was further oxidized. The binding energies of the doublet for Au 4f7/2 (83.1 eV) and Au 4f5/2 (87.0 eV) shown in Fig. S3f (ESI†) are characteristic of Au0.23
O functional groups, respectively (Fig. S3e, ESI†).20 When the Au/Fe2O3–GO nanosheets were synthesized, GO was further oxidized. The binding energies of the doublet for Au 4f7/2 (83.1 eV) and Au 4f5/2 (87.0 eV) shown in Fig. S3f (ESI†) are characteristic of Au0.23
As a control experiment, Fe2O3–GO nanosheets were also synthesized without addition of HAuCl4·3H2O. As shown in the N2 adsorption/desorption isotherms (Fig. 2b), Fe2O3–GO and Au/Fe2O3–GO nanosheets showed typical IV isotherms, indicating the presence of mesopores (4.4 nm) and high surface area (292 m2 g−1). The textural characteristics of the corresponding samples are summarized in Table S1 (ESI†). Surprisingly, the surface area of FeSO4·(H2O)–GO was less than 20 m2 g−1 (Table S1, ESI†). Thermogravimetric analysis (TGA) was then used to characterize the thermal stability of the three samples (Fig. 2c). TGA data also showed a different tendency of weight loss (%) between FeSO4·(H2O)–GO and Fe2O3–GO nanocomposites. It is considered that the physical and chemical properties of FeSO4·(H2O)–GO were drastically changed in alkaline solution during the Au NP immobilization. The elemental compositions of the respective Au/Fe3O4, FeSO4·(H2O), Fe2O3–GO, and Au/Fe2O3–GO nanocomposites were obtained using energy-dispersive X-ray spectroscopy (EDS) (Fig. S4, ESI†). The superconducting quantum interference device (SQUID) data showed the magnetic curves as a function of the applied field at 300 K (Fig. S5, ESI†). The saturation magnetization value of Au/Fe2O3–GO nanocomposites was 8.92 emu g−1, which was similar to that of the Fe2O3–GO (9.85 emu g−1). Moreover, both the remanence (Mr) and coercivity (Hc) of nanocomposites were close to zero, indicating superparamagnetism.
As shown in Fig. 4a and b, the UV/vis spectrum of the reaction mixture was monitored with time during the catalytic reduction of 4-nitrophenol. Specifically, the absorption of 4-nitrophenol at 400 nm decreased rapidly with a concomitant increase in the peak at 300 nm, that can be attributed to the formation of the reduction product, 4-aminophenol. The reduction was complete in 12 min and 6 min when Au/Fe2O3–GO (5.0 mol%) and Au/Fe3O4 (2.5 mol%) was used as the catalyst, respectively (100 equiv. of NaBH4 per equiv. substrate at 298 K) (Fig. 4a and b). The real experimental absorption capacity of 4-nitrophenol by Au/Fe2O3–GO is 1150 mg g−1 at the concentration of 104 mg L−1, resulting in high adsorption capacity at room temperature. As shown in Fig. 4c, the Au/Fe3O4 catalyst showed a higher reaction constant k (0.284 min−1) than the respective catalyst, Au/Fe2O3–GO (0.212 min−1) nanocomposites. Slightly higher catalytic activity of the Au/Fe3O4 catalyst is attributed to its better dispersion stability in water. Because Fe3O4 has many citrate groups and OH− anions, the dispersion stability of Au/Fe3O4 in water is enhanced. As shown in Fig. 4d, the reaction rate constant k was also compared under different temperatures using 5.0 mol% of Au/Fe2O3–GO and 50 equiv. of NaBH4. As expected, the highest catalytic efficiency (0.252 min−1) was obtained at 35 °C.
Both Au/Fe3O4 and Au/Fe2O3–GO catalysts exhibited superior catalytic activity relative to previously reported Au–Fe3O4 nanocomposites and Au–graphene nanosheets in terms of the reaction rate constant (k) value.24,25 It is believed that the electronic structures of both the Au and the metal oxide components are modified by electron transfer across the interface of the Au–metal oxide hybrid NPs, giving rise to oxygen vacancies on the interfacial metal oxide that act as active sites for oxygen absorption and activation.26,27 Thus, the hybrid NPs are catalytically more active than each individual component for the reduction of 4-nitrophenol.
Remarkably, after the reaction, the Au/Fe2O3–GO catalyst could be recovered from the reaction mixture by an external magnet and reused seven times with only slight loss of catalytic activity (Fig. 5a). Even though the Au/Fe3O4 catalyst showed higher catalytic activity than the Au/Fe2O3–GO catalyst, reusability of the Au/Fe3O4 catalyst was drastically decreased within four uses (Fig. 5b). As shown in Fig. S6 (ESI†), the structure of the Au NPs on the Fe2O3–GO nanosheets was slightly decomposed after the seven recycling reactions, demonstrating the recyclability of the catalyst.28
In conclusion, FeSO4·(H2O)–GO nanosheets were synthesized by a one-pot method and Au NPs were immobilized onto this support, giving an Au/Fe2O3–GO nanocomposite. A Fe2O3–GO support showed higher surface area and thermal stability. Both the Au/Fe2O3–GO and the Au/Fe3O4 nanocomposites exhibited higher catalytic activity and reusability for the reduction of 4-nitrophenol. The present findings may open up a new aspect in the development of high-performance metal/Fe2O3–GO nanocomposites, which will be useful in heterogeneous catalysis.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (no. 2013R1A1A1A05006634) and the Human Resource Training Project for Regional Innovation (no. 2012H1B8A2026225). K.H.P thanks to the TJ Park Junior Faculty Fellowship and LG Yonam Foundation. H.W. thanks to the Dr Yunhyeong Jang (Nextron, Republic of Korea) for AFM measurements.Notes and references
- T. Mori, T. Watanuki and T. Kashiwaguru, Environ. Toxicol., 2007, 22, 58 CrossRef CAS PubMed.
- Y.-C. Tsao, S. Rej, C.-Y. Chiu and M. H. Huang, J. Am. Chem. Soc., 2014, 136, 396 CrossRef CAS PubMed.
- J. Wei, H. Wang, Y. Deng, Z. Sun, L. Shi, B. Tu, M. Luqman and D. Zhao, J. Am. Chem. Soc., 2011, 133, 20369 CrossRef CAS PubMed.
- K. Bramhaiah and N. S. John, RSC Adv., 2013, 3, 7765 RSC.
- N. Zhang, X. Yu, J. Hu, F. Xuea and E. Ding, RSC Adv., 2013, 3, 13740 RSC.
- Y. Tang, Z. Jiang, Q. Tay, J. Deng, Y. Lai, D. Gong, Z. Dong and Z. Chen, RSC Adv., 2012, 2, 9406 RSC.
- A. M. Karim, V. Prasad, G. Mpourmpakis, W. W. Lonergan, A. I. Frenkel, J. G. Chen and D. G. Vlachos, J. Am. Chem. Soc., 2009, 131, 12230 CrossRef CAS PubMed.
- Y. Deng, Y. Cai, Z. Sun, J. Liu, C. Liu, J. Wei, W. Li, C. Liu, Y. Wang and D. Zhao, J. Am. Chem. Soc., 2010, 132, 8466 CrossRef CAS PubMed.
- G. M. Scheuermann, L. Rumi, P. Steurer, W. Bannwarth and R. Mülhaupt, J. Am. Chem. Soc., 2009, 131, 8262 CrossRef CAS PubMed.
- M. Lamblin, L. N. Hardy, J. C. Hierso, E. Fouquet and F. X. Felpin, Adv. Synth. Catal., 2010, 352, 33 CrossRef CAS.
- S. W. Kim, M. Kim, W. Y. Lee and T. Hyeon, J. Am. Chem. Soc., 2002, 124, 7642 CrossRef CAS PubMed.
- S. Y. Gan and M. Chow, J. Mater. Chem., 2004, 14, 2781 RSC.
- P. V. Kamat, J. Phys. Chem. Lett., 2011, 2, 242 CrossRef CAS.
- D. Su, H.-J. Ahn and G. Wang, Chem. Commun., 2013, 49, 3131 RSC.
- H. Xu, Q. Yang, F. Li, L. Tang, S. Gao, B. Jiang, X. Zhao, L. Wang and C. Fan, Analyst, 2013, 138, 2678 RSC.
- B. Liu, W. Zhang, F. Yang, H. Feng and X. Yang, J. Phys. Chem. C, 2011, 115, 15875 CAS.
- J. I. Paredes, S. Villar-Rodil, A. Martınez-Alonso and J. M. D. Tascon, Langmuir, 2008, 24, 10560 CrossRef CAS PubMed.
- V. Petkova, Y. Pelovski, D. Paneva and I. Mitov, J. Therm. Anal. Calorim., 2011, 105, 793 CrossRef CAS PubMed.
- A. P. Grosvenor, B. A. Kobe, M. C. Biesinger and N. S. McIntyre, Surf. Interface Anal., 2004, 36, 1564 CrossRef CAS.
- D. Yang, A. Velamakanni, G. Bozoklu, S. Park, M. Stoller, R. D. Piner, S. Stankovich, I. Jung, D. A. Field, C. A. Ventrice Jr and R. S. Ruoff, Carbon, 2009, 47, 145 CrossRef CAS PubMed.
- R. Massart, IEEE Trans. Magn., 1981, 17, 1247 CrossRef.
- J.-B. Moussy, J. Phys. D: Appl. Phys., 2013, 46, 143001 CrossRef.
- M. Turner, V. B. Golovko, O. P. H. Vaughan, P. Abdulkin, A. Berenguer-Murcia, M. S. Tikhov, B. F. G. Johnson and R. M. Lambert, Nature, 2008, 454, 981 CrossRef CAS PubMed.
- X. Meng, B. Li, X. Ren, L. Tan, Z. Huang and F. Tang, J. Mater. Chem. A, 2013, 1, 10513 CAS.
- X. Chen, Z. Cai, X. Chen and M. Oyama, J. Mater. Chem. A, 2014, 2, 5668 CAS.
- C. Wang, H. Daimon and S. Sun, Nano Lett., 2009, 9, 1493 CrossRef CAS PubMed.
- Z. Liu, X. Gong, J. Kohanoff, C. Sanchez and P. Hu, Phys. Rev. Lett., 2003, 91, 266102 CrossRef.
- C. W. Corti, R. J. Holliday and D. T. Thompson, Appl. Catal., A, 2005, 291, 253 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures; DFM, XPS, surface areas, pore volumes and pore diameters. See DOI: 10.1039/c4ra13989e |
| This journal is © The Royal Society of Chemistry 2015 |