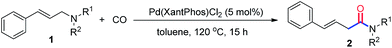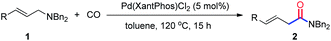Palladium-catalyzed carbonylation of allylamines via C–N bond activation leading to β,γ-unsaturated amides†
Hui Yua,
Guoying Zhangb,
Zong-Jian Liu*a and
Hanmin Huang*ab
aCollege of Chemical Engineering, Zhejiang University of Technology, Hangzhou 310024, China. E-mail: zjliu@zjut.edu.cn
bState Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou 730000, China. E-mail: hmhuang@licp.cas.cn
First published on 19th November 2014
Abstract
Pd(Xantphos)Cl2 has been identified as an efficient catalyst for the direct carbonylation of allylamines via C–N bond activation. The reaction proceeds smoothly and provides β,γ-unsaturated amides in good to excellent yields under relatively mild conditions.
The amide functional group is one of the most important motifs and exists in many natural products, pharmaceutical molecules as well as functional materials. As a result, method development for the efficient synthesis of amides continues to attract much interest from both academia and industry.1 Many synthetic routes, including many named reactions (Beckmann, Wolff, Schmidt, Ritter, and Ugi reaction, etc.),2 aminocarbonylation,3 carbonylation of aryl halides with formamides and their derivatives,4 transamidation reactions,5 and oxidative coupling reactions with amines,6 have been developed to access such kinds of molecules.
As an important class of amides, β,γ-unsaturated amides have gained considerable attention because of their unique synthetic utility.7 Among the many types of synthesis methods access to β,γ-unsaturated amides documented, transition-metal-catalyzed carbonylation of allyl halides or pseudohalides with amines and CO has provided a rapid and straightforward access to such kind of amide skeletons.8 However, the use of organic halides or pseudohalides produced large amounts of wasteful by-products which leads to low atom-economy. To circumvent this problem, the direct carbonylation of allylamines via C–N bond cleavage has been firstly developed by Murahashi and co-workers,9 revealing one of the most atom and step economical process. Despite the significance of the reaction, the harsh reaction conditions were still needed. Therefore, a practical and efficient catalytic protocol for the direct carbonylation of allylamines with CO under relatively mild condition is still urgent. Inspired by the results and in connection with our interests in the carbonylation and C–N bonds activation.10 Herein, we present an efficient palladium catalytic system for the carbonylation of allylamines, which provides a highly atom economical protocol for the synthesis of β,γ-unsaturated amides under mild condition.
Our initial investigation was conducted in the presence of 10 atm of CO by using N,N-dibenzyl-3-phenylprop-2-en-1-amine (1a) as model substrate for optimizing the reaction conditions. On the basis of our experience with carbonylation reactions,10c,10e a series of Pd complexes were firstly investigated. To our delight, the desired product 2a was obtained in 43% yield with PdCl2 as the catalyst and NMP/THF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the solvent (Table 1, entry 1). Several other palladium species, such as Pd(PPh3)2Cl2, Pd(DPPP)Cl2, Pd(Xantphos)Cl2, Pd(BINAP)Cl2 and Pd(DPEPhos)Cl2 were examined, but most of them furnished poor results except Pd(Xantphos)Cl2 (Table 1, entries 2–6). Once a suitable catalyst was identified, optimizations of solvent, CO pressure were conducted. Screening of solvents revealed that the reaction performed in toluene and xylene could afford the desired product with more than 90% yields (Table 1, entries 7–12). The best yield of 2a was obtained when the reaction conducted under 10 atm of CO whereas no appreciable increase in yield was obtained under decreasing pressure of CO. It was noteworthy that the reaction still worked well even at 6 atm of CO and 2a was obtained in 90% yield (Table 1, entries 13 and 14). The catalyst loading was lowered from 5 to 0.1 mol%, giving the corresponding product 2a in 16% yield (TON = 160) (Table 1, entry 15). In the absence of the Pd catalyst, however, no carbonylation reaction occurred under otherwise identical conditions (Table 1, entry 16).
1) as the solvent (Table 1, entry 1). Several other palladium species, such as Pd(PPh3)2Cl2, Pd(DPPP)Cl2, Pd(Xantphos)Cl2, Pd(BINAP)Cl2 and Pd(DPEPhos)Cl2 were examined, but most of them furnished poor results except Pd(Xantphos)Cl2 (Table 1, entries 2–6). Once a suitable catalyst was identified, optimizations of solvent, CO pressure were conducted. Screening of solvents revealed that the reaction performed in toluene and xylene could afford the desired product with more than 90% yields (Table 1, entries 7–12). The best yield of 2a was obtained when the reaction conducted under 10 atm of CO whereas no appreciable increase in yield was obtained under decreasing pressure of CO. It was noteworthy that the reaction still worked well even at 6 atm of CO and 2a was obtained in 90% yield (Table 1, entries 13 and 14). The catalyst loading was lowered from 5 to 0.1 mol%, giving the corresponding product 2a in 16% yield (TON = 160) (Table 1, entry 15). In the absence of the Pd catalyst, however, no carbonylation reaction occurred under otherwise identical conditions (Table 1, entry 16).
| Entry | [Pd] (5 mol%) | CO (atm) | Solvent | Yield (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.5 mmol), CO (10 atm), [Pd] (0.025 mmol) solvent (2.0 mL), 120 °C, 15 h, yields determined by GC using n-hexadecane as an internal standard.b Isolated yields.c Pd(Xantphos)Cl2 (0.1 mol%), 48 h. | ||||
| 1 | PdCl2 | 10 | NMP/THF (1/1) | 43 |
| 2 | Pd(PPh3)2Cl2 | 10 | NMP/THF (1/1) | 38 |
| 3 | Pd(DPPP)Cl2 | 10 | NMP/THF (1/1) | 0 |
| 4 | Pd(Xantphos)Cl2 | 10 | NMP/THF (1/1) | 82 |
| 5 | Pd(BINAP)Cl2 | 10 | NMP/THF (1/1) | 0 |
| 6 | Pd(DPEPhos)Cl2 | 10 | NMP/THF (1/1) | 29 |
| 7 | Pd(Xantphos)Cl2 | 10 | NMP | 77 |
| 8 | Pd(Xantphos)Cl2 | 10 | THF | 87(81)b |
| 9 | Pd(Xantphos)Cl2 | 10 | CH3CN | 0 |
| 10 | Pd(Xantphos)Cl2 | 10 | Toluene | 99(91)b |
| 11 | Pd(Xantphos)Cl2 | 10 | Xylene | 99 |
| 12 | Pd(Xantphos)Cl2 | 10 | Mesitylene | 22 |
| 13 | Pd(Xantphos)Cl2 | 6 | Toluene | 90 |
| 14 | Pd(Xantphos)Cl2 | 2 | Toluene | 44 |
| 15 | Pd(Xantphos)Cl2 | 10 | Toluene | 16b,c |
| 16 | — | 10 | Toluene | 0 |
With the optimized conditions in hand, we next investigated the substrate scope of allylamines. As summarized in Table 2, many cinnamyl amines derived from dialkyl amines were well-tolerated in this carbonylation process, furnishing the corresponding products 2a–2f in good to excellent yields. It appeared that the steric hindrance of the substituents on the amine moiety of the allylamines has no remarkable influence on the reactivity (Table 2, entries 2–6). Moreover, cinnamyl amines derived from cyclic amines could also be used as carbonylation partners, giving the corresponding β,γ-unsaturated amides 2g–2i in 51%, 66% and 83% yields, respectively. Besides, N-benzyl-3-phenyl-N-propylprop-2-en-1-amine 1j was transformed into the corresponding amide 2j in 63% yield.
| Entry | R1 | R2 | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: allylamine 1 (0.5 mmol), Pd(Xantphos)Cl2 (0.025 mmol) in toluene (2.0 mL) under CO (10 atm) at 120 °C for 15 h.b Isolated yields.c Ratios of Z/E determined by 1H NMR are given within parentheses. Major isomers are shown. | |||
| 1 | Bn | Bn | 2a, 91 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)c 1)c |
| 2 | Et | Et | 2b, 93 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)c 1)c |
| 3 | n-Pr | n-Pr | 2c, 82 (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)c 1)c |
| 4 | i-Pr | i-Pr | 2d, 78 (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)c 1)c |
| 5 | n-Bu | n-Bu | 2e, 75 (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)c 1)c |
| 6 | 1-Octane | 1-Octane | 2f, 72 (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)c 1)c |
| 7 | –(CH2)4– | 2g, 51 (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)c 1)c |
|
| 8 | –(CH2)5– | 2h, 66 (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)c 1)c |
|
| 9 | –(CH2CH2OCH2CH2)– | 2i, 83 (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)c 1)c |
|
| 10 | Bn | n-Pr | 2j, 63 (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)c 1)c |
Allylamines with different substituents attached in aromatic rings were also investigated in this carbonylation reaction (Table 3). The carbonylation of cinnamyl amines bearing an electron-withdrawing or electron-donating group at the meta or para position of the phenyl ring proceeded smoothly to provide corresponding adducts 2k–2r in 73–86% yields. It is noteworthy that the tolerance of halide substituents in this transformation offers an opportunity for subsequent cross-coupling reactions, which facilitates expedient synthesis of functional amides. N,N-dibenzyl-3-(naphthalen-2-yl)prop-2-en-1-amine 1s was a suitable substrate, giving the desired amide 2s in 84% yield under the optimized conditions. Besides cinnamyl amines, the simple alkyl group substituted allylamine 1t was subjected to the reaction and transformed into the corresponding product 2t in 67% yields, but with lower stereoselectivity.
| Entry | R | Yieldb (%) |
|---|---|---|
| a Reaction conditions: allylamine 1 (0.5 mmol), Pd(Xantphos)Cl2 (0.025 mmol) in toluene (2.0 mL) under CO (10 atm) at 120 °C for 15 h.b Isolated yields.c Ratios of Z/E determined by 1H NMR are given within parentheses. Major isomers are shown. | ||
| 1 | 4-CH3C6H4 | 2k, 75 (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)c 1)c |
| 2 | 3-CH3C6H4 | 2l, 73 (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)c 1)c |
| 3 | 4-t-BuC6H4 | 2m, 76 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)c 1)c |
| 4 | 4-MeOC6H4 | 2n, 74 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)c 1)c |
| 5 | 4-EtOC6H4 | 2o, 74 (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)c 1)c |
| 6 | 4-FC6H4 | 2p, 86 (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)c 1)c |
| 7 | 4-ClC6H4 | 2q, 77 (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)c 1)c |
| 8 | 4-AcOC6H4 | 2r, 83 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)c 1)c |
| 9 | Naphthyl-2-yl | 2s, 84 (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)c 1)c |
| 10 | CH3 | 2t, 67 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)c 1)c |
On the basis of our experimental results and previous work, a plausible reaction pathway was outlined in Fig. 1. Initially, Pd(0) species A derived from the reduction of Pd(II) serve as an effective catalytic species to react with allylamine 1a to give rise to the intermediate B through oxidative addition. Then, the intermediate C would be generated via CO insertion. Finally, reductive elimination of C afforded the desired product 2a and along with regeneration of the active catalyst species A for the next catalytic cycle.
In summary, we have identified Pd(Xantphos)Cl2 can act as an efficient catalyst for the direct carbonylation of simple allylamines via C–N activation under mild reaction conditions. With 5 mol% of Pd(Xantphos)Cl2 as the catalyst, the carbonylation reaction can proceed smoothly to afford the desired β,γ-unsaturated amides in good to excellent yields. Further studies on exploring new reactions via C–N activation are ongoing in our group.
Acknowledgements
This research was supported by the Chinese Academy of Sciences and the National Natural Science Foundation of China (21222203, 21372231 and 21133011).Notes and references
- (a) J. M. Humphrey and A. R. Chamberlin, Chem. Rev., 1997, 97, 2243 CrossRef CAS PubMed; (b) M. A. Mintzer and E. E. Simanek, Chem. Rev., 2009, 109, 259 CrossRef CAS PubMed; (c) V. R. Pattabiraman and J. W. Bode, Nature, 2011, 480, 471 CrossRef CAS PubMed; (d) C. L. Allen and J. M. J. Williams, Chem. Soc. Rev., 2011, 40, 3405 RSC; (e) D. W. Zhang, X. Zhao, J. L. Hou and Z. T. Li, Chem. Rev., 2012, 112, 5271 CrossRef CAS PubMed.
- (a) E. Beckmann, Ber. Dtsch. Chem. Ges., 1886, 89, 988 CrossRef; (b) L. Wolff, Justus Liebigs Ann. Chem., 1912, 384, 25 Search PubMed; (c) R. F. Schmidt, Ber. Dtsch. Chem. Ges., 1924, 57, 704 CrossRef; (d) J. J. Ritter and P. P. Minieri, J. Am. Chem. Soc., 1948, 70, 4045 CrossRef CAS; (e) I. Ugi, Angew. Chem., Int. Ed., 1962, 1, 8 CrossRef.
- (a) A. Schoenberg and R. F. Heck, J. Org. Chem., 1974, 39, 3327 CrossRef CAS; (b) M. Beller, B. Cornils, C. D. Frohning and C. W. Kohlpaintner, J. Mol. Catal. A: Chem., 1995, 104, 17 CrossRef CAS; (c) D. J. Knapton and T. Y. Meyer, Org. Lett., 2004, 6, 687 CrossRef CAS PubMed; (d) S. H. Cho, E. J. Yoo, I. Bae and S. Chang, J. Am. Chem. Soc., 2005, 127, 16046 CrossRef CAS PubMed; (e) J. R. Martinelli, T. P. Clark, D. A. Watson, R. H. Munday and S. L. Buchwald, Angew. Chem., Int. Ed., 2007, 46, 8460 CrossRef CAS PubMed; (f) E. M. Beccalli, G. Broggini, M. Martinelli and S. Sottocornola, Chem. Rev., 2007, 107, 5318 CrossRef CAS PubMed; (g) Y. Yasui and Y. Takemoto, Chem. Rec., 2008, 8, 386 CrossRef CAS PubMed; (h) J. R. Martinelli, D. A. Watson, D. M. M. Freckmann, T. E. Barder and S. L. Buchwald, J. Org. Chem., 2008, 73, 7102 CrossRef CAS PubMed; (i) A. Brennführer, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2009, 48, 4114 CrossRef PubMed; (j) X.-F. Wu, H. Neumann and M. Beller, Chem.–Asian J., 2010, 5, 2168 CrossRef CAS PubMed; (k) M. V. Khedkar, T. Sasaki and B. M. Bhanage, ACS Catal., 2013, 3, 287 CrossRef CAS.
- (a) P. J. Tambade, Y. P. Patil, M. J. Bhanushali and B. M. Bhanage, Tetrahedron Lett., 2008, 49, 2221 CrossRef CAS PubMed; (b) Y. Jo, J. Ju, J. Choe, K. H. Song and S. Lee, J. Org. Chem., 2009, 74, 6358 CrossRef CAS PubMed; (c) D. N. Sawant, Y. W. Wagh, P. J. Tambade, K. D. Bhatte and B. M. Bhanage, Adv. Synth. Catal., 2011, 353, 781 CrossRef CAS; (d) T. He, H. Li, P. Li and L. Wang, Chem. Commun., 2011, 47, 8946 RSC; (e) Y.-X. Xie, R.-J. Song, X.-H. Yang, J.-N. Xiang and J.-H. Li, Eur. J. Org. Chem., 2013, 2013, 5737 CrossRef CAS.
- (a) S. E. Eldred, D. A. Stone, S. H. Gellman and S. S. Stahl, J. Am. Chem. Soc., 2003, 125, 3422 CrossRef CAS PubMed; (b) A. Klapars, S. Parris, K. W. Anderson and S. L. Buchwald, J. Am. Chem. Soc., 2004, 126, 3529 CrossRef CAS PubMed; (c) T. A. Dineen, M. A. Zajac and A. G. Myers, J. Am. Chem. Soc., 2006, 128, 16406 CrossRef CAS PubMed; (d) N. A. Stephenson, J. Zhu, S. H. Gellman and S. S. Stahl, J. Am. Chem. Soc., 2009, 131, 10003 CrossRef CAS PubMed; (e) T. B. Nguyen, J. Sorres, M. Q. Tran, L. Ermolenko and A. Al-Mourabit, Org. Lett., 2012, 14, 3202 CrossRef CAS PubMed; (f) C. L. Allen, B. N. Atkinson and J. M. Williams, Angew. Chem., Int. Ed., 2012, 51, 1383 CrossRef CAS PubMed; (g) M. Zhang, S. Imm, S. Bähn, L. Neubert, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2012, 51, 3905 CrossRef CAS PubMed.
- (a) Y. Tamaru, Y. Yamada and Z. Yoshida, Synthesis, 1983, 474 CrossRef CAS PubMed; (b) T. Naota and S. Murahashi, Synlett, 1991, 693 CrossRef CAS; (c) A. Tillack, I. Rudloff and M. Beller, Eur. J. Org. Chem., 2001, 523 CrossRef CAS; (d) W.-J. Yoo and C.-J. Li, J. Am. Chem. Soc., 2006, 128, 13064 CrossRef CAS PubMed; (e) C. Gunanathan, Y. Ben-David and D. Milstein, Science, 2007, 317, 790 CrossRef CAS PubMed; (f) J. W. W. Chang and P. W. H. Chan, Angew. Chem., Int. Ed., 2008, 47, 1138 CrossRef CAS PubMed; (g) C. Zhang and N. Jiao, J. Am. Chem. Soc., 2010, 132, 28 CrossRef CAS PubMed; (h) C. Zhang, Z. Xu, L. Zhang and N. Jiao, Angew. Chem., Int. Ed., 2011, 50, 11088 CrossRef CAS PubMed; (i) Y. Wang, D. P. Zhu, L. Tang, S. J. Wang and Z. Y. Wang, Angew. Chem., Int. Ed., 2011, 50, 8917 CrossRef CAS PubMed; (j) Z. Liu, J. Zhang, S. Chen, E. Shi, Y. Xu and X. Wan, Angew. Chem., Int. Ed., 2012, 51, 3231 CrossRef CAS PubMed.
- (a) K.-K. Chan and G. Saucy, J. Org. Chem., 1977, 42, 3828 CrossRef CAS; (b) B. J. L. Royles, Chem. Rev., 1995, 95, 1981 CrossRef CAS; (c) F. Yokokawa and T. Shioiri, J. Org. Chem., 1998, 63, 8638 CrossRef CAS; (d) F.-T. Luo, T.-Y. Lub and C. H. Xue, Tetrahedron Lett., 2003, 44, 7249 CrossRef CAS PubMed.
- (a) P. Doz, G. P. Chiusoli and L. Cassar, Angew. Chem., Int. Ed., 1967, 6, 124 CrossRef; (b) S.-I. Murahashi and Y. Imada, Chem. Lett., 1985, 1477 CrossRef CAS; (c) Y. Imada, O. Shibata and S.-I. Murahashi, J. Organomet. Chem., 1993, 451, 183 CrossRef CAS; (d) T.-a. Mitsudo, N. Suzuki, T. Kondo and Y. Watanabe, J. Org. Chem., 1994, 59, 7759 CrossRef CAS; (e) T.-P. Loh, G.-Q. Cao and Z. Yin, Tetrahedron Lett., 1999, 40, 2649 CrossRef CAS; (f) R. J. van Haaren, H. Oevering, P. C. J. Kamer, K. Goubitz, J. Fraanje, P. W. N. M. van Leeuwen and G. P. F. van Strijdonck, J. Organomet. Chem., 2004, 689, 3800 CrossRef CAS PubMed.
- (a) S.-I. Murahashi, Y. Imada and K. Nishimura, J. Chem. Soc., Chem. Commun., 1988, 1578 RSC; (b) S.-I. Murahashi, Y. Imada and K. Nishimura, Tetrahedron, 1994, 50, 453 CrossRef CAS.
- (a) S. Guo, B. Qian, Y. Xie, C. Xia and H. Huang, Org. Lett., 2011, 13, 522 CrossRef CAS PubMed; (b) Y. Xie, J. Hu, Y. Wang, C. Xia and H. Huang, J. Am. Chem. Soc., 2012, 134, 20613 CrossRef CAS PubMed; (c) P. Xie, Y. Xie, B. Qian, H. Zhou, C. Xia and H. Huang, J. Am. Chem. Soc., 2012, 134, 9902 CrossRef CAS PubMed; (d) Y. Xie, J. Hu, P. Xie, B. Qian and H. Huang, J. Am. Chem. Soc., 2013, 135, 18327 CrossRef CAS PubMed; (e) P. Xie, C. Xia and H. Huang, Org. Lett., 2013, 15, 3370 CrossRef CAS PubMed; (f) J. Hu, Y. Xie and H. Huang, Angew. Chem., Int. Ed., 2014, 53, 7272 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. DOI: 10.1039/c4ra13939a |
| This journal is © The Royal Society of Chemistry 2014 |