Synthesis of ethyl (R)-4-cyano-3-hydroxybutyrate in high concentration using a novel halohydrin dehalogenase HHDH-PL from Parvibaculum lavamentivorans DS-1†
Nan-Wei Wanab,
Zhi-Qiang Liuab,
Kai Huangab,
Zhen-Yang Shenab,
Feng Xueab,
Yu-Guo Zheng*ab and
Yin-Chu Shenab
aInstitute of Bioengineering, Zhejiang University of Technology, Hangzhou, Zhejiang 310014, People's Republic of China. E-mail: zhengyg@zjut.edu.cn; Fax: +86-571-88320630; Tel: +86-571-88320614
bEngineering Research Center of Bioconversion and Biopurification of the Ministry of Education, Hangzhou, Zhejiang 310014, China
First published on 19th November 2014
Abstract
We identified and characterized a novel halohydrin dehalogenase HHDH-PL from Parvibaculum lavamentivorans DS-1. Study of substrate specificity indicated that HHDH-PL possessed a high activity toward ethyl (S)-4-chloro-3-hydroxybutanoate ((S)-CHBE). After optimizations of the pH and temperature, whole cell catalysis of HHDH-PL was applied to the synthesis of ethyl (R)-4-cyano-3-hydroxybutyrate (HN) at 200 g L−1 of (S)-CHBE, which gave 95% conversion and 85% yield in 14 h.
Atorvastatin calcium (Lipitor) is an important member of the statin family and widely used as an inhibitor of HMG-CoA reductase for the treatment of hypocholesterolemia and atherosclerosis. HN is the key precursor of the side chains of atorvastatin with an annual demand of more than 100 tons. Many disadvantages have emerged in the chemical routes for HN, including formation of extensive by-products and the usage of toxic catalysts. Recently, two green chemo-enzymatic processes for manufacture of HN have been established to reduce the wastes, hazards and cost of the chemical routes. One of the processes was developed by DeSantis et al., which used a highly enantioselective nitrilase to catalyze asymmetric hydrolysis of 3-hydroxyglutaryl nitrile.1 The other route to prepare HN was via biotransformation of (S)-CHBE using a halohydrin dehalogenase (HHDH), which achieved a substrate concentration of 140 g L−1 and gave 93% yield in 18 h.2 This commercial HHDH was engineered by Richard. J. Fox et al. based on the HHDH (HheC) from Agrobacterium radiobacter AD1. They employed the ProSAR-driven evolution method with 2500-fold improvement in the volumetric productivity for HN.3,4
HHDHs (EC 4.5.1.X) with the promiscuity activity can not only convert the vicinal halohydrins to corresponding epoxides, but also produce β-substituted alcohols undergo ring opening of epoxides in the presence of nucleophiles (NO2−, CN−, N3−, SCN−, HCOO−, OCN− and X−).3,5 The best studied HheC exhibited highly enantioselectiviy and regioselectivity to many halohydrins and epoxides, and was applied in the formation of optically active chloroalcohols, epoxides and β-functionalized alcohols.6–9 So far only a limited number of HHDHs have been cloned and characterized biochemically. These HHDHs showed low activities toward (S)-CHBE which limited the application for preparation of HN. Herein, we reported a new HHDH called HHDH-PL from P. lavamentivorans DS-1 which could tolerate a high concentration of (S)-CHBE. Sequence alignment and substrate specificity studies indicated that HHDH-PL came from a new group of HHDH family. After process optimization, biotransformation of (S)-CHBE at 200 g L−1 with 40 g L−1 (dry cell weight) of HHDH-PL was performed and generated 95% conversion and 85% yield in 14 h (Scheme 1).
Biochemical and structural investigations have shown HHDHs were structurally and mechanistically related to short-chain dehydrogenases/reductases (SDR) enzyme superfamily.3 In order to discover new HHDH genes, we used three kinds of HHDH amino acid sequences (HheAAD2, HheBGP1 and HheC) as queries for PSI-BLAST search against the nr database of NCBI. More than 1200 amino acid sequences were collected for multiple sequence alignment with the three HHDH sequences by ClustalW2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 software in tens. We distinguished SDR sequences with the following standards: the number of amino acids was 220–260; the conserved catalytic triad was Ser-Tyr-Arg (Lys). There were six SDR enzymes sequences from Bacillus cereus ATCC 10876 and P. lavamentivorans DS-1 were collected after 120 times iterations alignment. These SDR genes were cloned and expressed in Escherichia coli BL21 (DE3) for HHDH activity assay (Table 1). The SDR6 gene (HHDH-PL) expressed in E. coli BL21 (DE3) using pET32a (+) as the expression plasmids was identified having HHDH activity. However, no HHDH activities were obtained when it expressed in the plasmids of pET28a (+) or pET20b (+). The other SDRs were tested having no HHDH activity, even after changing the catalytic triad from Ser-Tyr-Lys (SDR catalytic triad) to Ser-Tyr-Arg (HHDH catalytic triad) by site-directed mutagenesis (data not shown).
10 software in tens. We distinguished SDR sequences with the following standards: the number of amino acids was 220–260; the conserved catalytic triad was Ser-Tyr-Arg (Lys). There were six SDR enzymes sequences from Bacillus cereus ATCC 10876 and P. lavamentivorans DS-1 were collected after 120 times iterations alignment. These SDR genes were cloned and expressed in Escherichia coli BL21 (DE3) for HHDH activity assay (Table 1). The SDR6 gene (HHDH-PL) expressed in E. coli BL21 (DE3) using pET32a (+) as the expression plasmids was identified having HHDH activity. However, no HHDH activities were obtained when it expressed in the plasmids of pET28a (+) or pET20b (+). The other SDRs were tested having no HHDH activity, even after changing the catalytic triad from Ser-Tyr-Lys (SDR catalytic triad) to Ser-Tyr-Arg (HHDH catalytic triad) by site-directed mutagenesis (data not shown).
| SDR | Plasmids enzyme site | Genebank accession | Activity assay |
|---|---|---|---|
| a Expressed successfully and tested having no HHDH activity.b Expressed unsuccessfully and tested having no HHDH activity.c Barely expressed in soluble and tested having no HHDH activity.d Expressed successfully and tested having HHDH activity. | |||
| SDR1 | pET20b(+) NdeI, XhoI | ABS62758.1 | −a |
| SDR2 | pET20b(+) NdeI, XhoI | ABS62418.1 | −a |
| SDR3 | pET28a(+) NcoI, XhoI | ABS61994.1 | −a |
| SDR4 | pET20b(+) NcoI, XhoI | AAS42725.1 | −a |
| SDR5 | pET28a(+) NcoI, XhoI | ACJ78562.1 | −a |
| SDR6 | pET28a(+) NcoI, XhoI | ABS64560.1 | −b |
| SDR6 | pET20b(+) NcoI, XhoI | ABS64560.1 | −c |
| SDR6 | pET32a(+) NcoI, XhoI | ABS64560.1 | +d |
Sequence blasting study indicated that HHDH-PL had the conserved catalytic triad Ser-Tyr-Arg and halogen binding site of HHDHs family. The identities of HHDH-PL with the other three groups of HHDHs were calculated by clustalW2 software and listed in Table 2. The results showed that HHDH-PL shared 32.8%, 28.1% and 34.8% identity with HheAAD2, HheBGP1 and HheC, respectively, which revealed that HHDH-PL was a novel HHDH.
| Group | HHDHs | Organisms | Identity (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HheAAD2 | HheA | HheAAm | HheBGP1 | HheB | HheC | HalB | HHDH-PL | |||
| A | HheAAD2 | Arthrobacter sp. strain AD2 | 100 | 97.0 | 97.13 | 18.3 | 18.3 | 33.2 | 30.0 | 32.8 |
| HheA | Corynebacterium sp. strain N-1074 | 100 | 97.13 | 18.7 | 18.7 | 33.2 | 30.0 | 32.8 | ||
| HheAAm11 | Agromyces mediolanus strain ZJB120203 | 100 | 18.3 | 18.3 | 33.2 | 30.0 | 33.6 | |||
| B | HheBGP1 | Mycobacterium sp. strain GP1 | 100 | 98.3 | 23.0 | 17.8 | 28.1 | |||
| HheB | Corynebacterium sp. strain N-1074 | 100 | 22.6 | 17.4 | 28.5 | |||||
| C | HheC | A. radiobacter AD1 | 100 | 91.3 | 34.8 | |||||
| HalB | A. tumefaciens | 100 | 31.3 | |||||||
| This work | HHD-PL | P. lavamentivorans DS-1 | 100 | |||||||
To investigate the substrate specificity of HHDH-PL, the steady-state kinetic parameters to a range of aliphatic and aromatic halohydrins were determined (Table 3). The results indicated that HHDH-PL could efficiently convert aliphatic and aromatic halohydrins to their corresponding epoxides. HHDH-PL showed a high activity toward 1,3-dichloro-2-propanol (1) with kcat and Km of 4651 ± 8.2 s−1 and 30.5 ± 1.2 mM, respectively. In comparison with HheC, HHDH-PL had a lower activity to 2,3-dichloro-1-propanol (11). HHDH-PL exhibited a preference toward the bromo-substituted haloalcohol substrates which was similar to the other characterized HHDHs.12 The kcat and Km of (S)-CHBE (5) were 2935 ± 9.6 s−1 and 9.9 ± 1.4 mM respectively. These results demonstrated that HHDH-PL possessed high activities on both short carbon-chain (C2–C3) and long carbon-chain (C4–C6) halohydrins. Surprisingly, HHDH-PL showed significantly higher activity in conversion of (S)-CHBE to HN as compared to HheAAD2, HheBGP1 and HheC (Fig. S5†).
| Substrate | Relative activity (%) | kcat (s−1) | Km (mM) | kcat/Km (s−1 mM−1) |
|---|---|---|---|---|
| a The activity to substrate 1 was selected as comparison (100%).b ND: the activities toward the substrates were too low to determine the kinetic parameters. | ||||
| 1 | 100.0a | 4651 ± 8.2 | 30.5 ± 1.2 | 153 ± 6.3 |
| (R,S)-2 | 0.9 ± 0.23 | NDb | ND | ND |
| (R,S)-3 | 5.4 ± 0.71 | ND | ND | ND |
| (S)-4 | 82.4 ± 0.86 | 519 ± 11.2 | 4.1 ± 0.3 | 127 ± 5.9 |
| (S)-5 | 112.9 ± 3.45 | 2935 ± 9.6 | 9.9 ± 1.4 | 296 ± 35.8 |
| (R,S)-6 | 37.6 ± 1.23 | 1534 ± 21.3 | 32.0 ± 1.9 | 47.9 ± 3.7 |
| 7 | 125.7 ± 3.12 | 8015 ± 13.5 | 43.9 ± 1.5 | 182 ± 5.7 |
| (R,S)-8 | 127.2 ± 4.85 | 5243 ± 9.9 | 31.2 ± 2.2 | 168 ± 10.7 |
| (R,S)-9 | 194.1 ± 6.32 | 4070 ± 32.2 | 14.5 ± 0.4 | 280 ± 4.8 |
| (R,S)-10 | 76.7 ± 4.61 | 4255 ± 21.5 | 36.3 ± 0.6 | 117 ± 0.7 |
| (R,S)-11 | 1.5 ± 0.37 | ND | ND | ND |
For further insight into the excellent activity of HHDH-PL on (S)-CHBE based on the structure information, the docking studies were performed by AutoDocK software.13 The generated model structure of HHDH-PL and the crystal structure of HheC (PDB code: 1PWX)14 were docked with (S)-CHBE for comparison. The catalytic triad of HHDHs was composed of Ser-Tyr-Arg, in which Ser was involved in substrate binding and Arg acquired a proton from OH of Tyr to lower its pKa. Tyr then acted as a catalytic base and seized a proton from the OH of (S)-CHBE, subsequently oxygen anion disrupted the carbon-halogen bond to form corresponding epoxide. Docking results in Fig. 1 revealed that HHDH-PL owned a more open substrate-binding pocket than HheC, which was suitable for accepting and transporting of (S)-CHBE. In addition, the distance of the substrate OH between Tyr146 was 3.08 Å for HHDH-PL, while it was 4.35 Å for HheC. And the longer distance made it more difficult for HheC to acquire a proton from the OH of (S)-CHBE than HHDH-PL, which resulted in a lower activity to (S)-CHBE.
 | ||
| Fig. 1 Three-dimensional representation of (S)-CHBE in the active sites of HHDH-PL and HheC by docking. The substrates and catalytic triad residues were described in sticks. | ||
Based on the high efficiency of HHDH-PL for preparation of HN by conversion of (S)-CHBE, we systematically investigated the optimal temperature and pH of this process. Fig. 2 summarized the effects of temperature (ranged from 35 °C to 50 °C) and pH (7.0–8.5) on the formation of HN. The highest yield of 87 g L−1 of HN was found at 40 °C. With the highest conversion of 97%, the yield of HN was just 70 g L−1 at 45 °C. HHDH-PL showed a low activity at 35 °C which gave 85% conversion and 78 g L−1 of HN. When the reaction was performed at 50 °C, the yield of HN decreased to 36 g L−1. From the results it could be concluded that 40 °C was the optimal temperature for production of HN. The lower temperature (35 °C) lowered the catalytic activity of HHDH-PL, and the higher temperature (45 and 50 °C) accelerated the hydrolysis of HN. The optimisation of pH was investigated at 40 °C and showed in Fig. 2B. At pH 8.5, the conversion was 100% in 6 h with 50 g L−1 of HN yield. In this case, the yield was reduced extremely after 2 h which was because the formation rate of HN was lower than the hydrolysis rate. At the neutral pH 7.0, the lowest conversion and yield were obtained. The yield of HN reached 85 g L−1 at pH 8.0 in 8 h and decreased slowly in the following 4 h. Several conclusions could be drawn from the results: pH 8.0 was the optimal pH for synthesis of HN, even if it had a slight effect on the hydrolysis of HN; the high pH of 8.5 improved the activity of HHDH-PL but accelerated the hydrolysis of HN; the pH of 7.0 and 7.5 were unfavourable for the conversion of (S)-CHBN.
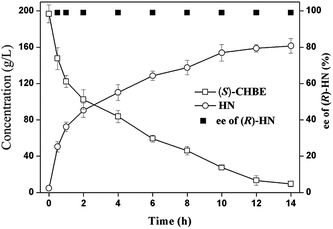
At last we scaled up the process by biotransformation of 200 g L−1 of (S)-CHBE under the optimal conditions (40 °C and pH 8.0) (Fig. 3). The reaction was carried out in a 1 L jacket three-neck bottom containing 400 mL 100 mM PBS buffer (pH 7.5). To it 50% sulfuric acid was added to adjust pH to 4.0 and then 30% of (wt/vol) sodium cyanide solution was added to pH 7.5. The mixture temperature was heated to 40 °C and followed by adding 20 g lyophilized cells of HHDH-PL and 100 g (S)-CHBE (0.6 mol). Subsequently, a pH stat was used to control the pH at 8.0 ± 0.05 by adding 30% of sodium cyanide solution. The reaction process was monitored by taking samples from the mixture for GC analysis. The reaction was completed after 14 h when the conversion of (S)-CHBE reached 95%. To the mixture 8 g CaCl2 was added to stir for 1 h and the cells were removed through filtration. The separated mixture was extracted three times with ethyl acetate (300 mL). The organic extracts were combined and dried over anhydrous sodium sulphate. The ethyl acetate was removed by evaporation under vacuum and remained 80.68 g (0.513 mol and yield 85%) yellow liquid.
In conclusion, a new HHDH gene HHDH-PL was identified and heterologously overexpressed in E. coli in this study. The sequence alignment assay and substrate specificity study indicated that HHDH-PL possessed different properties from the previously characterized HHDHs. In addition, HHDH-PL was applied in biotransformation of (S)-CHBE at a concentration of 200 g L−1, which generated 95% conversion and 85% yield. To the best of our knowledge, this was the first report to prepare HN using a natural HHDH at a high substrate concentration. The high substrate tolerance to (S)-CHBE suggested HHDH-PL was a promising biocatalyst for commercially preparation of HN, as well as for academic research. However, the substrate-to-catalyst ration was high and the catalytic efficiency was inferior to the method reported by Steven K. Ma.2 The large amount of biocatalysts in the process would have an effect on both cost and the product separation. Hence, in our future work we will try to engineer the catalytic activity, stability and enantioselectivity of HHDH-PL for its broader practical application including preparation of HN and the other optically active secondary alcohols and epoxides.
Acknowledgements
This work was financially supported by 973 Program (no. 2011CB710806), National High Technology Research and Development Program of China (no. 2012AA022201B) and Natural Science Foundation of Zhejiang Province of China (no. Z4080032 and R311055).Notes and references
- G. DeSantis, K. Wong, B. Farwell, K. Chatman, Z. L. Zhu, G. Tomlinson, H. J. Huang, X. Q. Tan, L. Bibbs, P. Chen, K. Kretz and M. J. Burk, J. Am. Chem. Soc., 2003, 125, 11476–11477 CrossRef CAS PubMed.
- S. K. Ma, J. Gruber, C. Davis, L. Newman, D. Gray, A. Wang, J. Grate, G. W. Huisman and R. A. Sheldon, Green Chem., 2010, 12, 81–86 RSC.
- J. E. T. V. Vlieg, L. X. Tang, J. H. L. Spelberg, T. Smilda, G. J. Poelarends, T. Bosma, A. E. J. van Merode, M. W. Fraaije and D. B. Janssen, J. Bacteriol., 2001, 183, 5058–5066 CrossRef.
- R. J. Fox, S. C. Davis, E. C. Mundorff, L. M. Newman, V. Gavrilovic, S. K. Ma, L. M. Chung, C. Ching, S. Tam, S. Muley, J. Grate, J. Gruber, J. C. Whitman, R. A. Sheldon and G. W. Huisman, Nat. Biotechnol., 2007, 25, 338–344 CrossRef CAS PubMed.
- G. Hasnaoui-Dijoux, M. M. Elenkov, J. H. L. Spelberg, B. Hauer and D. B. Janssen, ChemBioChem, 2008, 9, 1048–1051 CrossRef CAS PubMed.
- R. M. Haak, C. Tarabiono, D. B. Janssen, A. J. Minnaard, J. G. de Vries and B. L. Feringa, Org. Biomol. Chem., 2007, 5, 318–323 CAS.
- M. M. Elenkov, I. Primozic, T. Hrenar, A. Smolko, I. Dokli, B. Salopek-Sondi and L. X. Tang, Org. Biomol. Chem., 2012, 10, 5063–5072 Search PubMed.
- L. S. Campbell-Verduyn, W. Szymanski, C. P. Postema, R. A. Dierckx, P. H. Elsinga, D. B. Janssen and B. L. Feringa, Chem. Commun., 2010, 46, 898–900 RSC.
- M. M. Elenkov, H. W. Hoeffken, L. Tang, B. Hauer and D. B. Janssen, Adv. Synth. Catal., 2007, 349, 2279–2285 CrossRef CAS.
- F. Xue, Z. Q. Liu, N. W. Wan and Y. G. Zheng, Appl. Biochem. Biotechnol., 2014, 174, 352–364 CrossRef CAS PubMed.
- M. A. Larkin, G. Blackshields, N. P. Brown, R. Chenna, P. A. McGettigan, H. McWilliam, F. Valentin, I. M. Wallace, A. Wilm, R. Lopez, J. D. Thompson, T. J. Gibson and D. G. Higgins, Bioinformatics, 2007, 23, 2947–2948 CrossRef CAS PubMed.
- Z. Y. You, Z. Q. Liu and Y. G. Zheng, Appl. Microbiol. Biotechnol., 2013, 97, 9–21 CrossRef CAS PubMed.
- G. M. Morris, R. Huey, W. Lindstrom, M. F. Sanner, R. K. Belew, D. S. Goodsell and A. J. Olson, J. Comput. Chem., 2009, 30, 2785–2791 CrossRef CAS PubMed.
- R. M. de Jong, J. J. W. Tiesinga, H. J. Rozeboom, K. H. Kalk, L. Tang, D. B. Janssen and B. W. Dijkstra, Embo Journal, 2003, 22, 4933–4944 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental detials, 1H and 13C NMR spectra. See DOI: 10.1039/c4ra13646b |
| This journal is © The Royal Society of Chemistry 2014 |