Preparation of mesoporous nanocrystalline alkali promoted chromium free catalysts (Fe2O3–Al2O3–NiO) for a high temperature water gas shift reaction
Fereshteh Meshkani and
Mehran Rezaei*
Catalyst and Advanced Materials Research Laboratory, Chemical Engineering Department, Faculty of Engineering, University of Kashan, Kashan, 87317, I.R. Iran. E-mail: Rezaei@kashanu.ac.ir; Fax: +98-03155559930; Tel: +98-03155912832
First published on 22nd December 2014
Abstract
The water gas shift reaction is one of the oldest heterogeneous catalytic reactions operating in industry for H2 production with high purity and CO removal from syngas. Typical industrial catalyst for high temperature water gas shift (HTS) reactions is Fe–Cr–Cu, but has environmental and safety concerns related to chromium content, which has been regarded as a hazardous material. In this study, the effect of the addition of alkali metal oxide promoters to chromium free Fe2O3–Al2O3–NiO catalyst was investigated in HTS reaction. Nanocrystalline promoted chromium free catalysts with mesoporous structure were synthesized by coprecipitation and impregnation methods. Brunner–Emmett–Teller (BET), X-ray diffraction (XRD), temperature-programmed reduction (TPR) and desorption (TPD), scanning and transmission electron microscopic (SEM, TEM) techniques were performed to elucidate the HTS catalytic activity based on the influence of the addition of promoters on the catalyst structure. The results indicated that the addition of alkali promoters was effective in suppressing methanation, as well as in promoting HTS reaction activity for CO removal, which was related to the increment of the number of weakly basic sites through the addition of promoter. The results revealed that the Fe2O3–Al2O3–NiO (FAN) catalyst promoted by Na exhibited the highest catalytic activity and the lowest methanation among the investigated catalysts under a low steam/gas molar ratio, which favored methane formation. Furthermore, this catalyst presented higher CO removal activity than the commercial catalyst, which contains chromium. Moreover, the effect of Na content on the structural and catalytic properties of the FANNa catalysts was investigated and the results indicated that the catalyst with 3 wt% Na showed high activity and stability during 50 h time on stream.
Introduction
Recently, a different type of environmental issue is addressed in the area, which is related to the catalyst materials used for hydrogen production (steam or dry reforming, water gas shift reaction and preferential oxidation). The issue begins to be raised as the disposal of hazardous and toxic catalyst materials come under the controlling terms of the environmental regulations of various countries in the EU and US.1–4 The water gas shift reaction for the removal of CO and production of H2 from syngas is a crucial unit reaction in the syngas and/or hydrogen production processes.5–7To produce high purity hydrogen at the highest possible CO conversions, two-stages are used: a high temperature shift (HTS) reactor operating at 320–450 °C with a catalyst based on iron oxide structurally promoted with chromium oxide (Fe2O3–Cr2O3), and a low temperature shift (LTS) reactor operating at a temperature range of 200–250 °C with Cu–ZnO–Al2O3 catalyst.8–10 The conventional high temperature water gas shift (HTS) catalyst is Fe2O3–Cr2O3–CuO, in which chromium is a structural promoter, avoiding the sintering of the active phase of catalyst (Fe3O4) during the reaction.11 In addition, commercial catalysts include 2–4 wt% CuO as a promoter, which may provide additional active sites.12 The fresh and spent commercial HTS Fe–Cr catalysts usually contain levels of about 1–2 wt% hexavalent chromium (Cr6+), a very toxic heavy metal to humans, organisms, or cells, and an undesirable environmental contaminant, which is difficult to properly dispose.13 Especially in Europe, RoHS (Restriction of Hazardous Substances) restricts the use of six hazardous materials including Cr6+ in all the electronic/electrical devices and components.14 Many researches about Cr-free catalysts for HTS reactions have been performed in the last two decades.15–18 Recently, Fe–Al–Cu was known as a highly active catalyst for HTS reactions.16,17 Although the Fe–Al–Cu catalysts showed high activity but reported results also indicated that at higher Cu levels, deactivation of the catalyst with time-on-stream may be an issue.19 Furthermore, nickel was selected as the substitution metal for Cr in the Fe-based catalyst due to its wide use as an active species of catalysts for syngas (CO, H2)-related reactions20–22 and various CO removal reactions.23,24 Moreover, the incorporation of Ni into the Fe oxide structure was reported to enhance the thermal stability and catalytic activity compared to that of the single Fe oxide catalysts.25,26 However, the main drawback is the production of a small amount of CH4 by methanation reaction.24 However, in a standpoint of WGSR operation, any methanation is in principle undesirable due to the associated hydrogen consumption. Therefore, further researches are required to find a proper promoter for the Fe/Ni-based catalysts, which is capable of enhancing the HTS activity while suppressing methanation.4 It is known that the addition of alkali and alkaline earth metals can suppress the methanation activity. On the other hand, previous works have also demonstrated that a Ni catalyst promoted with potassium exhibited high catalytic performance for WGS process.27 The effect of addition of Cs to the Fe–Ni catalysts was investigated by Lee et al.7 They found that the Cs/Ni/Fe catalysts containing 3.9–6.0 Cs wt% showed high CO conversion and low methane formation due to increasing the number of weakly basic sites through Cs promotion. In this paper, the effect of the addition of alkali promoters on CO removal performance and methane formation of chromium free Fe2O3–Al2O3–NiO catalyst was investigated.
Experimental
Materials
The starting materials were Fe (NO3)3·9H2O, Al (NO3)3·9H2O, Ni (NO3)2·3H2O, Na2CO3, KNO3, LiNO3, CsNO3 and NaOH. All the chemicals were of analytical grade and used without further purification. Distilled deionized water was used for the preparation of all aqueous solutions.Catalyst preparation
The prepared catalysts and their abbreviations are listed in Table 1. The unpromoted catalyst and catalysts promoted by Li and Cs were prepared by the coprecipitation method. Depending on the catalyst composition, the desired amounts of metal salt precursors were dissolved in distilled water. After that an aqueous solution of NaOH (1 M) was added dropwise at room temperature to the prepared solution containing metal salt precursors under rapid stirring by careful pH adjustment to 10. After precipitation, the slurry was refluxed at 60 °C for 5 h under continuous stirring. Then, the mixture was cooled to room temperature, filtered and washed with hot deionized water for an effective removal of ions. The final product was dried at 90 °C for 24 h and calcined at 400 °C for 4 h in air atmosphere with a heating rate of 5 °C min−1.| Sample code | Catalyst | Nominal composition (wt%) | Elemental composition (wt%) | Surface area (m2 g−1) | Pore volume (cm3 g−1) | Pore size (nm) | Particle size (nm) | Fe2O3 → Fe3O4 phase transformation (°C) |
|---|---|---|---|---|---|---|---|---|
| F | Fe2O3 | 100% | N.M. | 14.1 | 0.1 | 13.8 | 81.0 | 433 |
| FA | Fe/Al = 10 | 88.3% Fe2O3–11.7% Al2O3 | 88.0% Fe2O3–12% Al2O3 | 152.8 | 0.3 | 4.5 | 7.6 | 400 |
| FAN | Fe/Al = 10, Fe/Ni = 5 | 76.3% Fe2O3–10.1% Al2O3–13.6% NiO | 75.7% Fe2O3–10.5% Al2O3–13.8% NiO | 177.4 | 0.3 | 4.4 | 6.4 | 347 |
| FANNa | Fe/Al = 10, Fe/Ni = 5, 3 wt% Na2O | 74.0% Fe2O3–9.8% Al2O3–13.2% NiO–3% Na2O | 73.1% Fe2O3–10.2% Al2O3–13.6% NiO–3.1% Na2O | 147.7 | 0.3 | 4.7 | 7.8 | 376 |
| FANLi | Fe/Al = 10, Fe/Ni = 5, 3 wt% Li2O | 74.0% Fe2O3–9.8% Al2O3–13.2% NiO–3% Li2O | 74.5% Fe2O3–9.3% Al2O3–13.3% NiO–2.9% Li2O | 192.5 | 0.3 | 4.3 | 6.0 | 341 |
| FANK | Fe/Al = 10, Fe/Ni = 5, 3 wt% K2O | 74.0% Fe2O3–9.8% Al2O3–13.2% NiO–3% K2O | 73.7% Fe2O3–10.0% Al2O3–13.1% NiO–3.2% K2O | 126.4 | 0.2 | 4.6 | 9.1 | 370 |
| FANCs | Fe/Al = 10, Fe/Ni = 5, 3 wt% Cs2O | 74.0% Fe2O3–9.8% Al2O3–13.2% NiO–3% Cs2O | 74.1% Fe2O3–10.1% Al2O3–12.6% NiO–3.2% Cs2O | 182.6 | 0.3 | 4.5 | 6.2 | 347 |
For the K and Na promoted FAN catalysts, first the FAN catalyst was prepared by a coprecipitation method as described above and calcined at 350 °C for 4 h. After that the FAN powder was impregnated with aqueous solutions of K(NO3) and Na2CO3 to prepare K and Na promoted FAN catalysts with desired promoter contents. Then, the impregnated powders were dried at 80 °C and calcined at 400 °C for 4 h in a static air atmosphere.
Characterization
The crystalline structure of the prepared catalysts was determined by X-ray powder diffraction (XRD) using an X-ray diffractometer (PANalytical X'Pert-Pro) and using a Cu-Kα monochromatized radiation source and a Ni filter. The specific surface area was evaluated by the BET method using N2 adsorption at −196 °C using an automated gas adsorption analyzer (Tristar 3020, Micromeritics). The Barrett–Joyner–Halenda (BJH) method was used to determine the pore size distribution from the desorption branch of the isotherm. Temperature-programmed reduction (TPR) was carried out using an automatic apparatus (Chemisorb 2750, Micromeritics) equipped with a thermal conductivity detector. Before the TPR experiment, the fresh sample (ca. 50 mg) was treated under an inert atmosphere at 250 °C for 2 h, and then subjected to a reduction treatment with a heating rate of 10 °C min−1 in a reducing gas flow (20 mL min−1) containing a mixture of H2–Ar (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90).
90).
The temperature programmed desorption behavior of CO2 and CO was analyzed on the same apparatus as for H2-TPR. Before the experiment, the catalyst was reduced at 400 °C under a reducing gas flow (30 mL min−1) containing a mixture of H2–Ar (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90) for 2 h. After that the reduced catalyst was saturated by CO or CO2 at room temperature for 1 h, and then the saturated sample was purged with He at room temperature for 30 min. TPD was carried out with a ramp of 10 °C min−1 from room temperature to a needed temperature under He stream.
90) for 2 h. After that the reduced catalyst was saturated by CO or CO2 at room temperature for 1 h, and then the saturated sample was purged with He at room temperature for 30 min. TPD was carried out with a ramp of 10 °C min−1 from room temperature to a needed temperature under He stream.
The elemental analysis of various elements was performed using Atomic Absorption Spectrophotometer (AAS) GBC-902. The surface morphology of the catalysts was observed with scanning and transmission electron microscopies techniques (SEM, Vega@Tescan and TEM, Philips CM30).
Catalytic reaction
The high temperature water gas shift reaction tests were performed in a quartz tubular fixed bed flow reactor (i.d. 8 mm) under atmospheric pressure. The thermocouple was inserted in the bottom of the catalyst bed to measure the reaction temperature. The total catalyst charged for each reaction was held constant (100 mg and with particle size of 0.25–0.5 mm). A gaseous mixture of 30% CO, 60% H2, 10% CO2 and a water steam with H2O/dry gas molar ratio of 0.3 were supplied to the catalyst bed. The gas and water flow rates were controlled by the mass flow controllers and a programmable syringe pump, respectively.Prior to reaction, the catalysts were reduced using a gaseous mixture of 30% CO, 60% H2, 10% CO2 and a water steam with H2O/dry ratio of 0.3 at 400 °C for 2 h. The activity tests were carried out at different temperatures ranging from 300 °C to 500 °C in steps of 50 °C. Before each analysis, the effluent passed through a water-trap to remove the water from the product stream. The gas composition was analyzed by a HID YL-6100 gas chromatograph equipped with a Carboxen 1010 column.
Results and discussion
The structural properties of the Fe, Fe–Al, Fe–Al–Ni and promoted Fe–Al–Ni catalysts with alkali metal oxides (Na2O, K2O, Li2O and Cs2O) are presented in Table 1. Pure iron oxide (F) exhibited a low specific surface area and pore volume. The presence of either Al or two promoters i.e. Al and Ni increased the BET surface area and pore volume of the pure iron oxide. Conversely, the addition of these promoters decreased the pore size of the prepared catalysts. As reported in our previous work, aluminum is known as a textural promoter and was employed as a spacer to protect iron oxide from sintering by keeping the particles apart from each other and increasing the specific surface area.28Moreover, increasing the BET surface area of the Fe–Al catalyst by the addition of nickel confirms the role of Ni as a structural promoter.
It is seen that for Li and Cs promoted FAN catalysts, both the BET surface area and pore volume increased possibly due to the differences in pore size and pore volume of the prepared catalysts. However, catalysts promoted by K and Na possessed lower BET surface areas and pore volumes compared to those of the FAN catalyst, which caused by the plugging of the pores with K and Na oxides in the impregnation synthesis step. In addition, the theoretical particle sizes (samples were assumed spherical in particles shape) were calculated from the measured specific surface areas according to the following equation:
| DBET = 6000/(ρS) | (1) |
The pore size distributions of the prepared catalysts are shown in Fig. 1a. The BJH analysis indicates the single modal pore size distribution for the prepared catalysts with major sizes of 2–50 nm. Pure iron oxide showed a broad pore size distribution centered at 22 nm. The pore size distributions of the other catalysts are closely dependent on the type of promoter added to the iron oxide.
 | ||
| Fig. 1 (a) Pore size distributions and (b) N2 adsorption/desorption isotherms of the prepared catalysts calcined at 400 °C. | ||
Addition of Al and Ni significantly affected the pore size distribution of the pure iron oxide, and the FA and FAN catalysts exhibited the narrowest pore size distribution centered at 4–5 nm. Moreover, it is seen that the addition of alkali promoters to the FAN catalyst slightly shifted the pore size distribution to the smaller sizes.
The N2 adsorption/desorption isotherms of the prepared samples are shown in Fig. 1b and imply type IV as classified by IUPAC (International Union of Pure and Applied Chemistry).29 These samples exhibited an H2 type hysteresis loop, which is characteristic of the pores with narrow necks and wide bodies. However, this assignment was considered to be oversimplified, recently, included in a review by Kruk and Jaroniec.30 H2 hysteresis loops were attributed to the relatively uniform channel-like pores. Isotherms with type H2 loops tend to level off when the pressure is close to the saturation pressure.31
In addition, the hysteresis loop of the pure iron oxide was formed at the highest p/p0 relative pressure, indicating that this sample exhibited a broad pore size distribution, as shown in Fig. 1a. For other promoted catalysts the hysteresis loop was observed at lower p/p0 relative pressures, indicating that these samples have narrower pore size distributions.
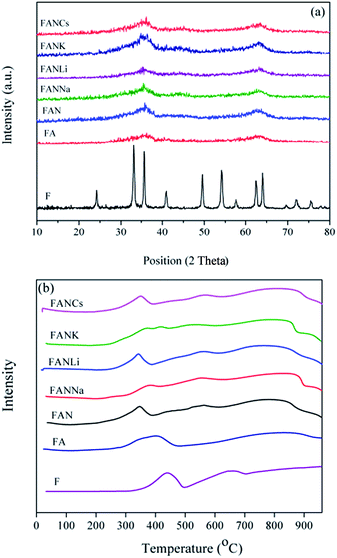
The XRD patterns of the prepared catalysts are presented in Fig. 2a. Pure iron oxide (F) showed a highly crystallized α-Fe2O3 phase, whereas the FA catalyst showed the XRD pattern with a significantly lower degree of crystallinity than that of pure iron oxide. The lower peak intensities of the Al-doped iron oxide catalyst are attributed to the formation of highly small particles in this sample. The effect of alumina could be regarded as an isolator, which prevents the severe sintering of the FeOx phase during the post-treatment processes. As can be seen in the FAN catalyst spectra, the addition of Ni did not affect the degree of crystallinity, and no other phases beside hematite were detected. This may be related to the fact that Ni is expected to enter into the magnetite lattice rather than segregate as another phase due to its similar ionic radius to the iron atom.32,33 For other promoted catalysts no peaks related to promoters were observed due to their low content in the catalyst composition.
TPR analysis was carried out over F, FA, FAN and promoted FAN by alkali metal oxides to provide insight on the reduction behavior of the prepared catalysts, and the obtained results are presented in Fig. 2b. Pure iron oxide displayed three main reduction peaks. The first peak at 433 °C was related to the reduction of Fe2O3 → Fe3O4. The second reduction peak at 650 °C was attributed to the reduction of Fe3O4 → FeO, and finally the transition of FeO → Fe occurred at a temperature higher than 700 °C.
It is seen that the addition of Al to F catalyst (FA) decreased the reduction temperature of hematite to magnetite, Table 1. For this catalyst the reduction of Fe3O4 → FeO and FeO → Fe was observed at a temperature higher than 600 °C.
Comparison of the TPR profile of FA and FAN catalysts and the results given in Table 1 indicated that nickel favors the active phase formation by decreasing the temperature required for the reduction of Fe2O3 → Fe3O4. In the absence of Ni (FA catalyst), the reduction of hematite to magnetite starts at 400 °C. In addition, the dispersed NiO in the FAN catalyst was reduced at 440 °C, and another reduction peak observed at 550 °C was assigned to the reduction of nickel-containing species including NiFe2O4. The last reduction peak at a temperature higher than 700 °C was related to the reduction of FeO to Fe. The TPR profiles of promoted FAN catalysts shown in Fig. 2b confirmed that K and Na promoters make the reduction of Fe2O3 → Fe3O4 difficult, while the addition of Li to the FAN catalyst facilitated this transition. Furthermore, the addition of Cs did not affect the reduction characteristic of the FAN catalyst, as shown in Table 1.
The TEM images of the prepared catalysts with different promoters are shown in Fig. 3. It is seen that all of the prepared catalysts exhibited nanocrystalline structure with a crystallite size smaller than 10 nm, which is in agreement with the particle size determined by the BET area (Table 1). As can be seen, the FANK catalyst showed bigger crystallite sizes than those observed for other prepared catalysts. This catalyst also exhibited the lowest BET surface area.
The amount of CO conversion and the CH4 concentration over the prepared catalysts for the high temperature water gas shift reaction is shown in Fig. 4a. For better comparison the HTS reaction was carried out under a low steam/gas molar ratio, which favors methane formation. As can be seen, methane was produced over all catalysts, and its concentration increased by increasing the reaction temperature up to 450 °C. Further increase in temperature has a negative effect on the methanation due to the exothermic nature of this reaction.
The results indicated that the promoted-FAN catalysts exhibited higher CO conversion and lower methane concentration compared to the FAN catalyst indicating the role of alkali metals for improving HTS reaction and suppression of methanation.
In addition, among the investigated catalysts, the FANNa catalyst possessed the highest activity and the lowest methanation. The activity results showed that the CO conversion of FANNa is surprisingly higher than that of the unpromoted FAN catalyst.
CO2-TPD analysis was employed to investigate the effect of Na addition on the adsorption behavior of CO2 on FAN catalyst. As shown in Fig. 4b, the CO2-TPD profile of FAN catalyst showed a broad CO2 desorption peak at around 50–300 °C indicating the weakly basic sites on the surface of the FAN catalyst, which is similar to the result obtained by Gao et al. that claimed that the weakly basic sites (surface hydroxyl groups) of Ni/Fe oxides are characterized by CO2 desorption peaks around 50–400 °C.34 The CO2-TPD of the FANNa catalyst clearly showed that the addition of Na to the FAN catalyst significantly promoted the CO2 adsorption, indicating the presence of more basic sites over the FANNa catalyst.
The CO-TPD results of FNA and FNANa catalysts are presented in Fig. 4c. As can be seen, a main broad desorption peak at a temperature lower than 300 °C corresponding to the weak CO adsorption was observed for the FAN catalyst. The results demonstrated that the addition of Na significantly affected the CO adsorption and caused a significant increase in the intensity of CO desorption peak. Many results have been reported indicating the improvement of CO adsorption by the addition of alkali metals such as potassium.35–38 Miller and Moskovits39 reported that as the K level increases, the extent of CO adsorption is significantly increased. Wan et al.40 investigated the effect of K addition on the adsorption behavior of iron based catalysts and found that potassium donates electrons to iron and facilitates CO chemisorption because CO tends to accept electrons from iron. Thus, the addition of a K promoter facilitates CO adsorption.
The CO2 and CO methanation test results for the FAN and FAN3Na catalysts at 400 °C are shown in Fig. 4b and c (upper inset), respectively. The results clearly revealed that the addition of Na to the FAN catalyst suppressed both CO2 and CO methanation activity of the FAN catalyst. Considering the water gas shift and methanation activity of FAN and FANNa catalysts, it was expected that the increment of such weakly basic sites of the catalyst would effectively enhance the HTS activity (through formation of format intermediate) and the selectivity of WGSR against methanation.41,42
The structural properties of the FAN catalysts with different Na contents are presented in Table 2. It is seen that the addition of Na to the FAN catalyst decreased the BET surface area and pore volume and increased the average pore size. The decrease in the BET surface area and pore volume after the addition of Na may be caused by a partial blockage of the FAN pores by Na2O clusters and/or a partial collapse of the mesoporous structure. The results showed that increasing in Na content led to a decrease in BET surface area and pore volume.
| Sample code | Composition | Surface area (m2 g−1) | Pore volume (cm3 g−1) | Pore size (nm) | Particle Sizea (nm) |
|---|---|---|---|---|---|
| a Determined by BET area. | |||||
| FAN1.5Na | Fe/Al = 10, Fe/Ni = 5, 1.5 wt% Na2O | 149.7 | 0.3 | 4.6 | 7.6 |
| FAN3Na | Fe/Al = 10, Fe/Ni = 5, 3 wt% Na2O | 147.7 | 0.3 | 4.7 | 7.8 |
| FAN6Na | Fe/Al = 10, Fe/Ni = 5, 6 wt% Na2O | 109.7 | 0.2 | 4.8 | 10.7 |
| Fe–Cr–Cu commercial catalyst | — | 76.0 | 0.2 | 10.4 | 15.0 |
| Reduced-FAN3Na | Fe/Al = 10, Fe/Ni = 5, 3 wt% Na2O | 38.8 | 0.2 | 18.6 | 29.7 |
| Spent-FAN3Na | Fe/Al = 10, Fe/Ni = 5, 3 wt% Na2O | 22.9 | 0.1 | 27.1 | 50.2 |
In addition, as can be seen the particle size also increased by increasing Na content. However, the promoted catalysts exhibited higher surface areas and smaller particle sizes compared to those in the commercial catalyst.
The pore size distributions and N2 adsorption/desorption isotherms are shown in Fig. 5a and b, respectively. It is seen that the promoted catalysts exhibited narrow pore size distributions centered at around 4.5 nm. The results revealed that increasing in Na content did not have a significant effect on the pore size distribution.
 | ||
| Fig. 5 (a) Pore size distributions and (b) N2 adsorption/desorption isotherms of the commercial and FANNa catalysts with different Na contents calcined at 400 °C. | ||
As can be seen, the commercial catalyst exhibited a broad pore size distribution in meso and macro regions. The N2 adsorption/desorption isotherms of the promoted catalysts showed the existence of IV type isotherm with an H2 hysteresis loop. The formation of hysteresis loops at low relative pressures indicates the narrow pore size distribution with small pores as shown in Fig. 5a.
As can be seen, the shape of isotherms did not change after the addition of Na to the FAN catalyst, indicating the similar pore size distributions for the prepared catalysts. The commercial catalyst exhibited a type V isotherm with an H3-type hysteresis loop, which is usually attributed to large mesopores or macropores surrounded by a matrix of considerably smaller pores. For the commercial catalyst the hysteresis loop was formed at a higher p/p0 relative pressure, which shows that this catalyst exhibited a broad pore size distribution, ranging from mesopores to macropores, Fig. 5a (upper inset).
The XRD patterns of the promoted FAN catalysts with different Na contents are shown in Fig. 6a. The XRD results revealed that the promoted samples exhibited a low degree of crystallinity and increasing Na content did not change the XRD patterns. All the promoted catalysts exhibited similar XRD patterns, and no peaks related to Na species were observed. However, the commercial catalyst exhibited higher crystallinity, and the sharp peak at around 26° was related to graphite, which was used during the shaping process of catalyst powder.
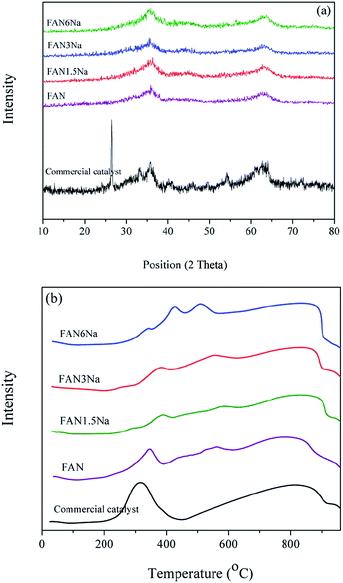 | ||
| Fig. 6 (a) XRD patterns and (b) TPR profiles of the commercial and FANNa catalysts with different Na contents calcined at 400 °C. | ||
The effect of Na2O content on the reduction behaviors of the promoted FAN catalysts is shown in Fig. 6b. The obtained results revealed that increasing in Na2O content caused a slight increase in the reduction temperature of hematite to magnetite. In addition, the TMax of the reduction peaks observed at 550–600 °C (related to reduction of nickel-containing species including NiFe2O4) was shifted to lower temperatures with increasing Na2O content.
For the commercial catalyst, two main reduction peaks were observed in the TPR profile. The reduction peak at lower temperatures (316 °C) was attributed to the reduction of CuO to metallic copper and CrO3 to Cr2O3 and the reduction of Fe2O3 to Fe3O4 and the second broad reduction peak was related to the reduction of Fe3O4 to FeO and metallic iron.
The catalytic activities of the promoted catalysts with different Na contents are shown in Fig. 7a. It is seen that the addition of Na to the FAN catalyst improved the CO conversion. The obtained results showed that increasing Na2O content up to 3 wt% increased the CO conversion, but a further increase in Na2O content has a negative effect on the CO conversion. As can be seen, the FAN3Na catalyst exhibited the highest activity among the prepared catalysts at temperatures lower than 450 °C. The decrease on the CO conversion of FAN6Na could be related to its lower BET area when compared to those of the other promoted catalysts. In addition, the CO conversions of the promoted catalysts were higher than those observed for the commercial catalyst at temperature lower than 450 °C.
 | ||
| Fig. 7 (a) CO conversion of the commercial and prepared catalysts calcined at 400 °C, (b) long term stability of the FAN3Na catalyst at 400 °C, GHSV = 3 × 104 mL h−1 gcat, and steam/gas = 0.3. | ||
The long term stability of the FAN3Na catalyst is shown in Fig. 7b. As can be seen, this catalyst exhibited stable catalytic performance during 50 h time on stream.
The structural properties of the fresh, reduced, and spent FAN3Na catalysts are given in Table 2. It is seen that the structural properties of the fresh catalyst significantly changed after the reduction and reaction steps. In the reduction step, the specific surface area and pore volume of the catalyst decreased from 147.7 to 38.8 m2 g−1 and 0.3 to 0.2 cm3 g−1, respectively. The results showed that the specific surface area and pore volume of the spent catalyst were lower than the fresh and reduced catalysts. The spent catalyst also exhibited the larger pore and particle sizes compared to those of the fresh and reduced catalysts.
The XRD patterns of the fresh, reduced, and spent FAN3Na catalysts are presented in Fig. 8a. As can be seen, the fresh catalyst showed a low degree of crystallinity, while after the reduction it is well crystallized, and all the diffraction peaks in the XRD patterns of the reduced and the spent catalysts can be assigned to the magnetite (Fe3O4), which is an active phase for HTS reaction.
Furthermore, the diffraction peaks of the spent catalyst showed higher intensities compared to those observed for the reduced catalyst, indicating the bigger crystallite size of the spent catalyst.
Fig. 8b shows the pore size distributions and N2 adsorption/desorption isotherms (upper inset) of the fresh, reduced, and spent FAN3Na catalysts. It is seen that in the reduction step, the pore size distribution of the catalyst shifted to larger sizes, and the reduced catalyst showed a broad pore size distribution centered at 23 nm. In addition, after 50 h time on stream, the spent catalyst exhibited a very broad pore size distribution indicating a lower surface area compared to those in the fresh and reduced catalysts.
The N2 adsorption/desorption isotherms for the fresh, reduced, and spent catalysts indicated a type IV isotherm with a H2-type hysteresis loop (upper inset) for fresh and reduced catalysts, while after the reaction the type of hysteresis changed to a type V isotherm with an H3-type hysteresis loop. Moreover, for the fresh catalyst the hysteresis loop occurred at a low relative pressure confirming a narrow pore size distribution of this sample.
For the reduced and spent catalysts the hysteresis loop was observed at higher relative pressure indicating a broader pore size distribution of these samples.
The SEM images of the fresh and spent FAN3Na catalysts are shown in Fig. 8c and d, respectively. It is seen that the particles in the spent catalyst were agglomerated together, and this catalyst exhibited bigger particle size compared to that observed for the fresh catalyst.
The TEM images of the fresh and spent FAN3Na catalysts are also presented in Fig. 9, respectively. As can be seen in the spent catalyst, the crystals are bigger than those observed in the fresh catalyst. The increase in crystal size could be related to sintering of iron crystals under the reaction conditions, which is accompanied by a loss in the BET surface area and an increase in particle size as shown in Table 2.
Conclusion
In this work the effect of alkali promoters (Na, K, Cs and Li) on the CO removal performance of Cr-free Fe–Al–Ni catalysts in high temperature water gas shift reaction is investigated. BET analysis showed that the prepared catalysts possessed a high surface area and mesoporous structure. In the XRD patterns of all prepared catalysts, only a hematite phase was detected, and no separate crystalline phases related to the Al, Ni or other promoters were observed. In addition, from the TPR analysis, it was found that the addition of Li to the Fe–Al–Ni catalyst shifted the reduction temperature of Fe2O3 → Fe3O4 to lower values while the addition of Na and K to the Fe–Al–Ni catalyst increased the reduction temperature of hematite to the magnetite phase. It can be concluded from the HTS activity test that by the addition of alkali promoters to the FAN catalysts, the CO conversion improved and the methanation reaction was suppressed. The results revealed that a Na promoter exhibited the highest positive effect on CO conversion and suppression of methanation. The results of the TPD analysis confirmed that the addition of Na to the FAN catalyst (FANNa) increased the intensity of the CO and CO2 desorption peaks indicating the presence of more basic sites over this catalyst, which is the reason of the higher catalytic performance and methane suppression of this catalyst compared to that in the unpromoted FAN catalyst. In particular, 3 wt% Na promoted Fe–Al–Ni catalyst exhibited higher CO conversion than did the commercial Cr-containing catalyst and showed a high stability during 50 h time on stream during the reaction.The remarkable catalytic performance of the FANNa catalyst is mainly ascribed to the synergistic effect of Al as a textural promoter by increasing the BET surface area and thermal stability of iron oxide. Based on the obtained results, the Fe2O3–Al2O3–NiO–Na2O catalyst can be considered as a promising chromium-free catalyst for a high temperature water gas shift reaction.
Acknowledgements
The authors are grateful to National Petrochemical Company, Research and Technology Company (NPC-RT) for supporting this work.References
- P. Carson and C. Mumford, Hazardous chemicals handbook, Butterworth-Heinemann, Great Britain, 2nd edn, 2002, pp. 148–51 Search PubMed.
- Committee for medicinal products for human use (CHMP), Guideline on the specification limits for residues of metal catalysts or metal reagents, Doc. no. EMEA/CHMP/SWP/4446/2000, European Medicines Agency, London, U.K., February 2008 Search PubMed.
- M. Marafi, A. Stanislaus and E. Furimsky, Handbook of spent hydroprocessing catalysts, Elsevier, Amsteram and Oxford, 2010, pp. 93–120 Search PubMed.
- J. Y. Lee, D.-W. Lee, Y.-K. Hong and K.-Y. Lee, Int. J. Hydrogen Energy, 2011, 36, 8173–8180 CrossRef CAS PubMed.
- F. A. de Bruijn, B. Rietveld and R. W. van den Brink, in Catalysis for Renewables, ed. G. Centi and A. van Santen, Wiley-VCH, Weinheim, 2007, pp. 299–336 Search PubMed.
- K. Babita, S. Sridhar and K. V. Raghavan, Int. J. Hydrogen Energy, 2011, 36, 6671–6688 CrossRef CAS PubMed.
- J. Y. Lee, D.-W. Lee, M. S. Lee and K.-Y. Lee, Catal. Commun., 2011, 15, 37–40 CrossRef CAS PubMed.
- F. Bustamante, R. M. Enick, R. P. Killmeyer, B. H. Howard and K. S. Rothenberger, AIChE J., 2005, 51, 1440–1454 CrossRef CAS.
- E. B. Quadro, M. de Lourdes, R. Dias, A. M. M. Amorim and M. do Carmo Rangel, J. Braz. Chem. Soc., 1999, 10, 51–59 CrossRef CAS PubMed.
- A. Haryanto, S. D. Fernando, S. D. Filip To, P. H. Steele, L. Pordesimo and S. Adhikari, J. Thermodyn. Catal., 2011, 2, 1000106 Search PubMed.
- F. Meshkani and M. Rezaei, J. Ind. Eng. Chem., 2014, 20, 3297–3302 CrossRef CAS PubMed.
- A. Andreev, V. Idakiev, D. Mihajlova and D. Shopov, Appl. Catal., 1986, 22, 385–387 CrossRef CAS.
- K. Kochloefl, in Handbook of Heterogeneous Catalysis, ed. G. Ertl, H. Knözinger and J. Weitkamp, Wiley-VCH, Weinheim, 1997, vol. 4, pp. 1831–1843 Search PubMed.
- D.-W. Lee, M. S. Lee, J. Y. Lee, S. Kim, H.-J. Eom, D. J. Moon and K.-Y. Lee, Catal. Today, 2013, 210, 2–9 CrossRef CAS PubMed.
- F. M. Gottschalk, R. G. Copperthwaite, M. V. D. Riet and G. J. Hutchings, Appl. Catal., 1988, 38, 103–108 CrossRef CAS.
- G. C. Araújo and M. C. Rangel, Catal. Today, 2000, 62, 201–207 CrossRef.
- S. Natesakhawat, X. Wang, L. Zhang and U. S. Ozkan, J. Mol. Catal. A: Chem., 2006, 260, 82–94 CrossRef CAS PubMed.
- F. M. Gottschalk and G. J. Hutchings, Appl. Catal., 1989, 51, 127–139 CrossRef CAS.
- L. Zhang, J. M. M. Millet and U. S. Ozkan, Appl. Catal., A, 2009, 357, 66–72 CrossRef CAS PubMed.
- Y. S. Oh, H. S. Roh, K. W. Jun and Y. S. Baek, Int. J. Hydrogen Energy, 2008, 28, 1387–1392 CrossRef.
- D. Harshini, Y. Kwon, J. Han, S. P. Yoon, S. W. Nam and T. H. Lim, Korean J. Chem. Eng., 2010, 27, 480–486 CrossRef CAS PubMed.
- S. H. Park, B. H. Chun and S. H. Kim, Korean J. Chem. Eng., 2001, 28, 402–408 CrossRef.
- S. H. Kim, S. W. Nam, T. H. Lim and H. I. Lee, Appl. Catal., B, 2008, 81, 97–104 CrossRef CAS PubMed.
- K. Watanabe, T. Miyao, K. Higashiyama, H. Yamashita and M. Watanabe, Catal. Commun., 2009, 10, 1952–1955 CrossRef CAS PubMed.
- L. Huang, J. Xie, R. Chen, D. Chu, W. Chu and A. T. Hsu, Int. J. Hydrogen Energy, 2008, 33, 7448–7456 CrossRef CAS PubMed.
- M. Feyzi, A. A. Mirzael and H. R. Bozorgzadeh, J. Nat. Gas Chem., 2010, 19, 341–353 CrossRef CAS.
- K.-R. Hwang, C.-B. Lee and J.-S. Park, J. Power Sources, 2011, 196, 1349 CrossRef CAS PubMed.
- F. Meshkani and M. Rezaei, Renewable Energy, 2015, 74, 588–598 CrossRef CAS PubMed.
- K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquérol and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 603 CrossRef CAS.
- M. Kruk and M. Jaroniec, Chem. Mater., 2001, 13, 3169 CrossRef CAS.
- L. Zhang, X. Wang, J.-M. M. Millet, P. H. Matter and U. S. Ozkan, Appl. Catal., A, 2008, 351, 1–8 CrossRef CAS PubMed.
- M. E. Dry and L. C. Ferreira, J. Catal., 1967, 7, 352 CrossRef CAS.
- P. S. Sidhu, R. J. Gilkes and M. Posner, J. Inorg. Nucl. Chem., 1978, 40, 429 CrossRef CAS.
- Z. Gao, Y. Feng, F. Cui, Z. Hua, J. Zhou, Y. Zhu and J. Shi, J. Mol. Catal. A: Chem., 2011, 336, 51–57 CrossRef CAS PubMed.
- X. Wang, G. Li and U. S. Ozkan, J. Mol. Catal. A: Chem., 2004, 217, 219 CrossRef CAS PubMed.
- M. E. Dry and G. J. Oosthuizen, J. Catal., 1968, 11, 18 CrossRef CAS.
- J. W. Niemantsverdriet, A. M. vander Kraan, W. L. van Dijk and H. S. vander Baan, J. Phys. Chem., 1980, 84, 3363 CrossRef CAS.
- H. Kölbel, Kalium als strucktureller und Energetischer Promotor in Eisenkatalysatoren, in Actes du Deuxieme Congress International de Catalyse, Tchnip, Paris, 1960, vol. II, p. 2075 Search PubMed.
- D. G. Miller and M. Moskovits, J. Phys. Chem., 1988, 92, 6081 CrossRef CAS.
- H. Wan, B. Wu, C. Zhang, H. Xiang and Y. Li, J. Mol. Catal. A: Chem., 2008, 283, 33–42 CrossRef CAS PubMed.
- K. R. Hwang, C. B. Lee and J. S. Park, J. Power Sources, 2011, 196, 1349–1352 CrossRef CAS PubMed.
- S. H. Kim, J. H. Chung, Y. T. Kim, J. Han, S. P. Yoon, S. W. Nam and H. I. Lee, Int. J. Hydrogen Energy, 2011, 35, 3136–3140 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2015 |