Carbon dots in magnetic colloidal nanocrystal clusters†
Ye Liu,
Ye Tian and
Wuli Yang*
State Key Laboratory of Molecular Engineering of Polymers and Department of Macromolecular Science, Fudan University, Shanghai 200433, China. E-mail: wlyang@fudan.edu.cn; Fax: +86-21-6564-0293; Tel: +86-21-6564-2385
First published on 3rd November 2014
Abstract
In this study, carbon dots are found to be fabricated simultaneously during the typical hydrothermal synthesis process of magnetic colloidal nanocrystal clusters (MCNCs). Their existence in the solvent (ethylene glycol), on the surface and in the interior of MCNCs is also revealed.
Carbon dots (CDs), as a new type of fluorescent nanomaterials, have attracted tremendous attention in recent years because of their potential applications in bioimaging,1,2 medical diagnosis,3 optoelectronic devices,4,5 sensors6 and catalysis.7 Compared to organic dyes and fluorescent semiconductor nanocrystals, CDs provide many promising advantages such as bright fluorescence, low photobleaching, robust chemical inertness and excellent biocompatibility.8 Currently, various methodologies have been developed to prepare CDs, such as arc discharge, laser ablation, electrochemical oxidation, and hydrothermal method.9 Among these strategies, hydrothermal synthesis of CDs has become more and more popular due to low cost, easy operation and abundant resources. Nearly all organics could be used as carbon sources such as citric acid, ethylene diamine, polyethylene glycol, and L-ascorbic acid.10–14
Magnetic colloid nanocrystal clusters (MCNCs) have arouse great interest and desire of exploration in biomedical applications: magnetic resonance imaging, proteins/peptides enrichment and detection, biomarker detection and targeted drug delivery.15 As known, in a typical hydrothermal synthesis process of MCNCs, Fe3+ ions undergo a sodium acetate (NaOAc)-promoted hydrolysis and then are partially reduced to magnetite (Fe3O4) nanoparticles by ethylene glycol with the assistance of trisodium citrate. The magnetite nanoparticles then generally aggregate into clusters (MCNCs) at high temperature.16 It is reasonable to hypothesize that when trisodium citrate and ethylene glycol are hydrothermally treated, CDs are fabricated simultaneously during the synthesis process of MCNCs. In this work, the hypothesis is proved and the existence of CDs in the system is confirmed. Also, the exact locations of CDs and the structure of MCNCs are also discussed: CDs are revealed to be not only dispersed in the solvent, but also stuck on the surface and embedded in the interior of MCNCs. This research offers new insights into the preparation of CDs and the architecture of MCNCs.
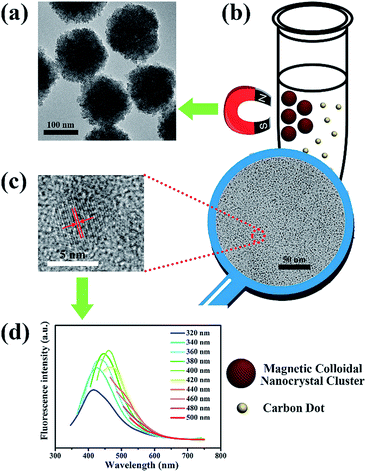
Firstly, MCNCs are fabricated in a typical hydrothermal strategy (Fig. 1a), and numerous CDs are observed in the as-prepared mixture (schemed in Fig. 1b). From the transmission electron microscopy (TEM) image of CDs in the solvent (ethylene glycol), it can be seen that large amounts of CDs with size of about 5 nm are dispersed well in ethylene glycol (Fig. 1b). High-resolution TEM (HRTEM) measurement (Fig. 1c) reveals that CDs are graphitic in nature with a lattice spacing of 0.220 nm consistent with (100) facet of graphite.9 Raman spectroscopy is used to confirm the quality of the CDs (Fig. S1 in the ESI†). Two major features, D band and G band, are observed at around 1385 and 1575 cm−1, respectively. The relative intensity of the “disorder” D-band and the crystalline G-band (ID/IG) for the CDs is about 1.23.4 Moreover, after careful removal of MCNCs with a magnet, nuclear magnetic resonance (NMR, 1H and 13C) of the CDs is employed to distinguish sp2- and sp3-hybridized carbon atoms (Fig. S2 in the ESI†). In the 13C NMR spectrum, signals in the range of 20–70 ppm, which correspond to aliphatic (sp3) carbon atoms, and signals from 100 to 185 ppm, which are indicative of sp2 carbon atoms, are observed. Among them, signals in the range of 170–185 ppm, which correspond to carboxyl groups, are also present.17 The surface groups are also investigated by X-ray photoelectron spectroscopy (XPS) analysis (Fig. S3a and b in the ESI†). C1s analysis reveals several different types of carbon atoms: graphitic or aliphatic (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C–C), C–O, C
C and C–C), C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and O
O and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O.18 In the photoluminescence (PL) emission spectra of the as-prepared mixture, the excitation-dependent PL behaviour is observed, which is common in primarily reported CDs (Fig. 1d).8 Moreover, their ultraviolet/visible (UV/vis) spectrum is similar to that of CDs (Fig. S4 in the ESI†). All the above results prove the existence of CDs in the solvent.
C–O.18 In the photoluminescence (PL) emission spectra of the as-prepared mixture, the excitation-dependent PL behaviour is observed, which is common in primarily reported CDs (Fig. 1d).8 Moreover, their ultraviolet/visible (UV/vis) spectrum is similar to that of CDs (Fig. S4 in the ESI†). All the above results prove the existence of CDs in the solvent.
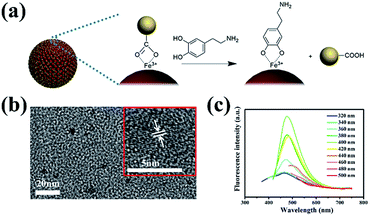
In addition, the PL emission spectra of MCNCs present an excitation-dependent behaviour (Fig. S5 in the ESI†), and washing with deionized water dramatically decreases the fluorescence of MCNCs, indicating the CDs are weakly absorbed on the surface of MCNCs (Fig. S6 in the ESI†). However, in the Raman spectrum of MCNCs after ten times washing, D and G bands can still be observed, suggesting the existence of CDs in the system (Fig. S1 in the ESI†). In this case, to further release CDs on the surface, dopamine is selected to exchange with CDs due to the binding preference of catechol groups toward Fe3+ ions (schemed in Fig. 2a).19,20 After the ligand exchange, the UV/vis absorption spectrum shows an absorption band at 262 nm, which belongs to the absorption of dopamine (Fig. S7 in the ESI†). The Fourier transform infrared (FTIR) spectra of MCNCs and MCNC–dopamine are also recorded (Fig. S8 in the ESI†). Compared to MCNCs, it can be seen that the MCNC–dopamine have an absorption peak at 1268 cm−1 associated with the C–OH stretching mode of the phenolic hydroxyl groups. Thermo gravimetric analysis (TGA) in N2 of MCNC–dopamine shows a larger weight loss of about 15% in the range of 100–800 °C while the MCNCs only have a weight loss of 12%, revealing that the former contains more organic groups (Fig. S9 in the ESI†). Furthermore, the zeta potential of MCNCs changes from −14.8 mV to zero after the dopamine grafting (Table S1 in the ESI†), implying the increase of surface positive charge density of MCNCs attributed to the amine groups. In a word, these results prove that dopamine has been successfully grafted onto the surface of MCNCs.
After the ligand exchange, CDs are observed in the supernatant. Fig. 2b shows the TEM image of CDs with a mean size of about 4 nm. The crystalline structure of CDs with the lattice spacing of 0.205 nm is proved by the HRTEM image shown in Fig. 2b (inset), which corresponds well to the in-plane lattice spacing of graphite (102 facet).9 The supernatant also shows an excitation-dependent behavior in the PL emission spectra (Fig. 2c). The release of CDs in the ligand exchange process confirms that CDs are attached to the surface of MCNCs. As known, CDs contain many –OH and –COOH moieties on their surface, which have strong coordination affinity to Fe3+ ions. As a result, CDs are attached to the surface of MCNCs through coordination in the synthesis process. Whereas the fluorescence of CDs is quenched by Fe3+ ions on account of the nonradiative electron-transfer between Fe3+ ions and –OH, –COOH moieties (Fig. S6 in the ESI†).10 Upon the addition of dopamine, the stronger interaction between Fe3+ ions and catechol groups disturbs the interaction between Fe3+ ions and CDs, which leads to the release of CDs from the surface of MCNCs (Fig. 2a).
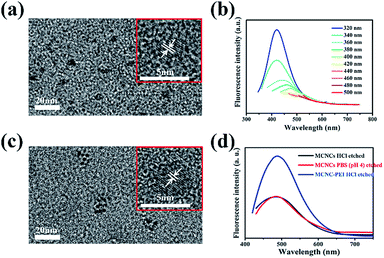
Then Fe3O4 composition in the MCNCs is etched by diluted hydrochloric acid and sodium hydroxide is added to precipitate Fe3+ and Fe2+ ions. CDs are observed in the solution after etching. The TEM image shows CDs with a diameter of about 4 nm (Fig. 3b). HRTEM image reveals the high crystallinity of CDs with the lattice spacing of 0.185 nm which agrees well with the (105) facet of graphite (Fig. 3b, inset).9 The excitation-dependent behavior is displayed in the PL emission spectra of CDs (Fig. 3c). The release of CDs in the etching process brings out the fact that CDs exist in the interior of MCNCs. According to previous reports, the MCNCs undergo a two-stage growth process in which the primary nanoparticles nucleate first in a supersaturated solution and then aggregate into clusters at high temperature.16,21 Considering the strong coordination affinity of –OH, –COOH moieties with Fe3+ ions, CDs are able to anchor on the surface of the primary nanoparticles, and thus embedded in the interior of the MCNCs (Fig. 3a). Namely, the MCNCs are composed of Fe3O4 nanocrystals and CDs. Since the CDs are surrounded by Fe3O4 nanocrystals, their fluorescence is also quenched by Fe3+ ions as mentioned above.10
It should be noted that owing to the different growing environment, the CDs obtained from the solvent, ligand exchange and etching process have various size and lattice fringe. To further investigate the difference, Raman spectroscopy (Fig. S1 in the ESI†) and XPS analysis (Fig. S3 in the ESI†) are conducted. XPS analysis of the three kinds of CDs presents diverse compositions of carbon elements, while the ID/IG values measured in the Raman spectroscopy are similar. With a similar carbon-lattice-structure content (indicated by ID/IG values), the PL emission spectra of the three kinds of CDs are alike.
Inspired by the above results, the MCNCs are dispersed in a pH 4 buffer, which simulates physiological environment. Likewise, CDs can be observed in the solution after the etching process. The TEM image shows CDs with an average size about 4 nm (Fig. 4a). The HRTEM image proves the high crystallinity of CDs with the lattice spacing around 0.210 nm, which corresponds with the (100) diffraction planes of graphite (Fig. 4a, inset).9 The PL emission spectra show a typical fluorescent properties of CDs, while the fluorescence intensity is somewhat weak (Fig. 4b).
To increase the fluorescence intensity, polyethylene imine (PEI) is chosen to graft onto the MCNCs, since PEI contains a great amount of amine groups which can improve the fluorescence quantum yield of CDs.10 To verify the successful grafting of PEI onto the surface of MCNCs, TGA analysis of the composites is conducted. Compared with pure MCNCs, the MCNC–PEI composites have a larger weight loss, implying more organic groups (Fig. S9 in the ESI†). Moreover, the zeta potential (Table S1 in the ESI†) shows the changes from −14.8 mV to 20.8 mV after grafting PEI. These results prove the efficient grafting of PEI onto the surface of MCNCs. When MCNC–PEI composites are etched with hydrochloric acid, CDs are observed in the solution. Fig. 4c shows the TEM and HRTEM images of CDs, which reveal CDs with a diameter of about 4 nm (Fig. 4c). The same lattice spacing of around 0.194 nm agrees well with the (104) spacing of graphite (Fig. 4c, inset).9 As expected, the fluorescence intensity of CDs released from the MCNCs increases drastically after the grafting (Fig. 4d).
In summary, CDs are fabricated simultaneously during the typical hydrothermal synthesis process of MCNCs. Firstly, CDs exist in the solvent and weakly absorb on the surface of MCNCs. Moreover, CDs are attached to the surface of MCNCs due to the complexation between the CDs and Fe3+ ions, and CDs are also embedded in the interior of the MCNCs when magnetic colloidal nanocrystals aggregate into clusters. These results give us a better understanding of the CD preparation and the architecture of MCNCs. Furthermore, upon the removal of Fe3O4, the CDs inside MCNCs could be released under a mild condition and the introduction of PEI could enhance the fluorescence intensity of CDs, which opens up the door for the application of bioresponsive off–on fluorescence in vivo.
Acknowledgements
We acknowledge the support from the National Science Foundation of China (Grant no. 51273047) and the “Shu Guang” Project (12SG07) supported by Shanghai Municipal Education Commission and Shanghai Education Development Foundation.Notes and references
- Y. Fang, S. Guo, D. Li, C. Zhu, W. Ren, S. Dong and E. Wang, ACS Nano, 2012, 6, 400–409 CrossRef CAS PubMed.
- S. Sahu, B. Behera, T. K. Maiti and S. Mohapatra, Chem. Commun., 2012, 48, 8835–8837 RSC.
- B. Kong, A. Zhu, C. Ding, X. Zhao, B. Li and Y. Tian, Adv. Mater., 2012, 24, 5844–5848 CrossRef CAS PubMed.
- Y. Li, Y. Hu, Y. Zhao, G. Shi, L. Deng, Y. Hou and L. Qu, Adv. Mater., 2011, 23, 776–780 CrossRef CAS PubMed.
- V. Gupta, N. Chaudhary, R. Srivastava, G. D. Sharma, R. Bhardwaj and S. Chand, J. Am. Chem. Soc., 2011, 133, 9960–9963 CrossRef CAS PubMed.
- J. Zhao, G. Chen, L. Zhu and G. Li, Electrochem. Commun., 2011, 13, 31–33 CrossRef CAS PubMed.
- S. Zhuo, M. Shao and S.-T. Lee, ACS Nano, 2012, 6, 1059–1064 CrossRef CAS PubMed.
- Y. P. Sun, B. Zhou, Y. Lin, W. Wang, K. A. S. Fernando, P. Pathak, M. J. Meziani, B. A. Harruff, X. Wang, H. F. Wang, P. J. G. Luo, H. Yang, M. E. Kose, B. L. Chen, L. M. Veca and S. Y. Xie, J. Am. Chem. Soc., 2006, 128, 7756–7757 CrossRef CAS PubMed.
- S. N. Baker and G. A. Baker, Angew. Chem., Int. Ed., 2010, 49, 6726–6744 CrossRef CAS PubMed.
- S. J. Zhu, Q. N. Meng, L. Wang, J. H. Zhang, Y. B. Song, H. Jin, K. Zhang, H. C. Sun, H. Y. Wang and B. Yang, Angew. Chem., Int. Ed., 2013, 52, 3953–3957 CrossRef CAS PubMed.
- W. Li, Z. Zhang, B. Kong, S. Feng, J. Wang, L. Wang, J. Yang, F. Zhang, P. Wu and D. Zhao, Angew. Chem., Int. Ed., 2013, 52, 8151–8155 CrossRef CAS PubMed.
- Y. Dong, H. Pang, H. B. Yang, C. Guo, J. Shao, Y. Chi, C. M. Li and T. Yu, Angew. Chem., Int. Ed., 2013, 52, 7800–7804 CrossRef CAS PubMed.
- B. Zhang, C.-y. Liu and Y. Liu, Eur. J. Inorg. Chem., 2010, 2010, 4411–4414 CrossRef.
- M. Chen, W. Wang and X. Wu, J. Mater. Chem. B, 2014, 2, 3937 RSC.
- J. Guo, W. Yang and C. Wang, Adv. Mater., 2013, 25, 5196–5214 CrossRef CAS PubMed.
- J. Liu, Z. Sun, Y. Deng, Y. Zou, C. Li, X. Guo, L. Xiong, Y. Gao, F. Li and D. Zhao, Angew. Chem., Int. Ed., 2009, 48, 5875–5879 CrossRef CAS PubMed.
- H. Liu, T. Ye and C. Mao, Angew. Chem., Int. Ed., 2007, 46, 6473–6475 CrossRef CAS PubMed.
- S. Liu, J. Tian, L. Wang, Y. Zhang, X. Qin, Y. Luo, A. M. Asiri, A. O. Al-Youbi and X. Sun, Adv. Mater., 2012, 24, 2037–2041 CrossRef CAS PubMed.
- H. B. Na, G. Palui, J. T. Rosenberg, X. Ji, S. C. Grant and H. Mattoussi, ACS Nano, 2012, 6, 389–399 CrossRef CAS PubMed.
- Y. Liu, T. Chen, C. Wu, L. Qiu, R. Hu, J. Li, S. Cansiz, L. Zhang, C. Cui, G. Zhu, M. You, T. Zhang and W. Tan, J. Am. Chem. Soc., 2014, 136, 12552–12555 CrossRef CAS PubMed.
- S. Xuan, Y.-X. J. Wang, J. C. Yu and K. Cham-Fai Leung, Chem. Mater., 2009, 21, 5079–5087 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, Fig. S1 to S9, and Table S1. See DOI: 10.1039/c4ra13140a |
| This journal is © The Royal Society of Chemistry 2014 |