Chemical sensors and biosensors for the detection of melamine
Ying Li†
,
Jingyue Xu† and
Chunyan Sun*
Department of Food Quality and Safety, Jilin University, Changchun 130062, China. E-mail: sunchuny@jlu.edu.cn; sunchunyan1977@163.com; Fax: +86 431 87836391; Tel: +86 431 87836375
First published on 21st November 2014
Abstract
Melamine is an emerging contaminant in milk, infant formula and pet food. In order to increase the “false” apparent protein content in food products, melamine has been artificially and illegally used as non-protein nitrogen additive. This review focuses on chemical sensors and biosensors for detecting melamine residue. We present the principles, the mechanisms and the performances of the sensors including optical sensors, electrochemical sensors, aptamer-based sensors and immunosensors. We also propose the future perspectives in developing sensors for the detection of melamine.
1. Introduction
Melamine (1,3,5-triazine-2,4,6-triamine, or C3H6N6) contains a substantial amount of nitrogen which accounts for about 66% of its mass. It is commonly used in the production of melamine–formaldehyde polymer resins, which are a component in many plastics, adhesives, glues, fertilizer and laminated products, such as plywood, cement, cleansers and fire retardant paint.1,2 However, the exposure to melamine may be particularly dangerous to human health. Melamine has low acute oral toxicity, but its high concentrations can induce renal pathology and even death in babies and children.3,4 Melamine can be hydrolyzed to cyanuric acid in vitro. Cyanuric acid will in turn associate with melamine to form an insoluble melamine–cyanurate complex which may form stones in the urinary system, probably leading to acute renal failure by obstruction.5,6 Besides, it will also cause a variety of renal toxic effects such as nephrolithiasis, chronic kidney inflammation and bladder carcinoma.7In order to increase the “false” apparent protein content in food products, melamine has been artificially and illegally used as non-protein nitrogen additive. As a nitrogen-rich compound, it was adulterated in pet food and caused many deaths of cats and dogs in the United States and elsewhere.8 The illness of pets firstly made people wonder that melamine ingestion might be very harmful to humans. A lamentable example of the toxic effects of melamine occurred in China in 2008. It has been reported that nearly 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 children ingested an infant formula adulterated with melamine, and at least six of them died.9 After the initial reports of adulterated infant formula, melamine was found in many food items, especially in Chinese-sourced milk powder. Moreover, melamine was also detected in various imported items such as candies, beverages, and cookies.10 The Chinese government promulgated an interim control limit for melamine at 1 mg kg−1 for infant formula and at 2.5 mg kg−1 for other milk products in October 2008, and this interim limit became official for all foodstuffs in April 2011.11 The World Health Organization (WHO) has recommended the tolerable daily intake for melamine to be 0.2 mg kg−1 body weight per day, while, the US Food and Drug Administration (FDA) has updated the maximum residue levels of melamine in infant formula to be 1.0 mg kg−1 and 2.5 mg kg−1 for milk and other milk products, respectively.12
000 children ingested an infant formula adulterated with melamine, and at least six of them died.9 After the initial reports of adulterated infant formula, melamine was found in many food items, especially in Chinese-sourced milk powder. Moreover, melamine was also detected in various imported items such as candies, beverages, and cookies.10 The Chinese government promulgated an interim control limit for melamine at 1 mg kg−1 for infant formula and at 2.5 mg kg−1 for other milk products in October 2008, and this interim limit became official for all foodstuffs in April 2011.11 The World Health Organization (WHO) has recommended the tolerable daily intake for melamine to be 0.2 mg kg−1 body weight per day, while, the US Food and Drug Administration (FDA) has updated the maximum residue levels of melamine in infant formula to be 1.0 mg kg−1 and 2.5 mg kg−1 for milk and other milk products, respectively.12
It is vital to monitor and control the harmful effects of melamine. Thus, various analytical methods for the detection of melamine in feedstuffs and food products have been developed. Most of the published reports have discussed the detection of melamine using chromatographic techniques, such as high performance liquid chromatography (HPLC),13,14 ultra-performance liquid chromatography/tandem mass spectrometry (UPLC/MS/MS),15,16 and gas chromatography/mass spectrometry (GC/MS).17–19 These instrumental methods are widely applied for melamine determination due to their great sensitivity and accuracy. However, they necessarily require various cumbersome sample preparation techniques and lack on-site applicability. More importantly, these methods based on chromatography and mass spectroscopy also require skilled personnel, have high operating costs, and are time-consuming.
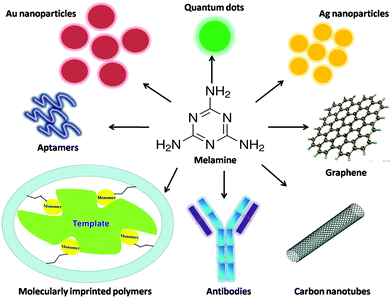
As a kind of novel technique, sensors, especially chemical sensors and biosensors, have been developed rapidly and received considerable attention in recent years. Although a number of reviews about melamine determination in foodstuffs have been reported, the majority of them mainly focus on chromatographic techniques.20–22 The discussion about sensing methods for scanning melamine is relatively rare. Hence, we comprehensively review the chemical sensors and biosensors for melamine detection developed in recent 5 years (Fig. 1).
 | ||
| Fig. 1 Novel recognition and transducer components used for the fabrication of sensors for melamine determination. | ||
2. Chemical sensors for the analysis of melamine
Chemical sensors are devices that transform chemical information, ranging from the concentration of a specific sample component to total composition analysis, into an analytically useful signal. Chemical sensors usually contain two basic components connected in series: a chemical (molecular) recognition system (receptor) and a physico-chemical transducer.23 As chemical devices, they can react with different chemical components and have various applications, such as environmental and security monitoring, medical diagnosis, process control, pollution control and food analysis.24–27 We discuss various chemical sensing methods developed for melamine in the following.2.1 Optical sensors
Optical sensors recognized as analytical tools are of vital importance in the field of chemical sensors. By employing optical transduction techniques, they have been used to provide chemical information ranging from analyte concentration and binding kinetics to microscopic imaging and molecular structure. Based on photonic attributes of optical sensors, a variety of signal transduction pathways are utilized including absorbance, transmission, fluorescence intensity, chemiluminescence, refractive index, polarization, and reflectivity.28 In the following sections, we discuss various optical sensors for melamine analysis including colorimetric sensors, fluorescence sensors, and chemiluminescence sensors.2.1.1.1 AuNPs-based colorimetric sensors. Gold nanoparticles (AuNPs) own particular physical and chemical advantages such as simple synthesis process, unique and readily-tuned optoelectronic properties, excellent biocompatibility, high extinction coefficient, and good suitability for multifunctionalization.30 These unique properties make AuNPs excellent candidates for the fabrication of detection sensors. Particularly, the AuNPs-based colorimetric assays are of great interest because molecular recognition events can be easily transformed into color changes that arise from the interparticle plasmon coupling during AuNPs aggregation (red-to-purple or blue) or redispersion of an AuNPs aggregate (purple-to-red).31,32
With regard to melamine detection, the AuNPs-based colorimetric methods are certainly suitable owing to their simplicity and visibility. Based on the mechanism of color changes of AuNPs mentioned above, the reports of melamine detection could be classified into two types: AuNPs aggregation-based colorimetric sensors and AuNPs nonaggregation-based colorimetric sensors. One is owing to the aggregation of AuNPs, the other is in respect of the non-aggregation of AuNPs. The related assays are illustrated in Table 1.
| Analyte | Analytical Principle | Limit of Determination (LOD) | Analytical Samples | Time | References | |
|---|---|---|---|---|---|---|
| Functionalization | Interaction | |||||
a  AuNPs. AuNPs. |
||||||
| Melamine | Unmodified ( ) ) |
Hydrogen bonds (NH⋯N) | 0.4 ppm | Raw milk | 12 min | 34 |
| Melamine | Unmodified ( ) ) |
Hydrogen bonds (NH⋯N) | 1 ppm | Liquid milk | 10 min | 35 |
| Melamine | Unmodified ( ) ) |
Hydrogen bonds (NH⋯N) | 0.15 ppm | Liquid milk | 30 min | 36 |
| 2.5 ppm | Infant formula | |||||
| Melamine | Unmodified ( ) ) |
Hydrogen bonds (NH⋯N) | ∼25 ppb | Infant milk | Within 5 min | 37 |
| Melamine | Unmodified ( ) ) |
Hydrogen bonds (NH⋯N) | 0.025 ppb | Raw milk and milk powder | — | 38 |
| Melamine | Cyanuric acid derivative (MTT— ) ) |
Hydrogen bonds (NH⋯N, NH⋯O) | 2.5 ppb | Raw milk and infant formula | Within 1 min | 39 |
| Melamine | 3-Mercapto-1-Propanesulfonate-modified (MPS— ) ) |
Hydrogen bonds (N⋯O, NH⋯O) | 1.008 ppb | Milk and infant formula | Within 30 min | 40 |
| Melamine | 18-Crown-6-thiol | Hydrogen bonds (NH⋯O) | 6 ppb | Raw milk | Less than 1 min | 41 |
| Melamine | Cysteamine | Hydrogen bonds | 1 ppm | Milk products, eggs and feeds | — | 42 |
| Melamine | Riboflavin | Hydrogen bonds (NH⋯N, NH⋯O) | 0.1 ppm | — | — | 43 |
| Melamine | 2,4,6-Trinitrobenzenesulfonic acid (TNBS- ) ) |
Charge-transfer | 5 ppb | Infant formula | Within 1 min | 44 |
Uracil-5-carboxylic acid (UCA- ) ) |
Hydrogen bonds (NH⋯N) | 0.1 ppm | ||||
| Melamine | Chitosan | Hydrogen bonds | 6 ppb | Liquid milk | 20 min | 45 |
| Melamine | — | Hydrogen bonds (NH⋯O) | 1 ppb | — | — | 46 |
| Melamine | — | Hydrogen bonds (NH⋯O) | 0.2 ppb | Liquid milk | — | 47 |
| Melamine | — | Hydrogen bonds (NH⋯O) | 0.08 ppb | Liquid milk | — | 48 |
In the AuNPs aggregation-based colorimetric sensors, two patterns have been utilized for the determination of melamine using unmodified AuNPs and ligand-functionalized AuNPs as colorimetric indicators. The first pattern employs the unmodified AuNPs as colorimetric probes which could be readily synthesized by the traditional Frens' method.33 As illustrated in Fig. 2A, the principle of this type of sensor is based on the fact that melamine can strongly bind with AuNPs through the interaction between amine groups of melamine and AuNPs, thus readily displace the stabilizing agents (citrates) from surfaces of AuNPs and cause the aggregation of AuNPs. Besides, the neighbour melamine-coated AuNPs could be cross-linked by NH⋯N hydrogen bonds between melamine molecules. For example, by conducting the control experiment with cyanuric acid, Li et al. have validated that only three amine groups of melamine are responsible for the cross-linking interaction between melamine and AuNPs which could induce the aggregation of AuNPs.34 The sensitivity of two different nanometre-sized AuNPs (13 nm and 2.6 nm) was also studied, demonstrating a simple and rapid colorimetric method for melamine using a relatively more sensitive AuNPs with the particle size of 2.6 nm. The limit of detection for melamine is 0.4 part-per-million (ppm), and the whole process including sample pretreatment takes only 12 min at room temperature. What's more, the method is specially promising for on-site rapid detection of melamine contamination in foods such as eggs and animal feeds. Similarly, Gao et al. reported a label-free AuNPs-based colorimetric kit for the detection of melamine employing 5 nm AuNPs which was more sensitive than 18 nm AuNPs verified in the assay.35 The assay was performed by naked eye in comparison with the standard colorimetric card without the aid of any instrument. Determination of 1 ppm level was achieved by visual detection, and the method was suitable for rapid determination of melamine in most milk products, which could be used in the dairy industry, quality assurance departments, as well as by supermarket managers, customers etc. Guo and cooperators have developed a label-free AuNPs-based colorimetric method for the sensing of melamine using 13 nm AuNPs as the colorimetric indicator.36 The sensing method for melamine in liquid milk and infant formula respectively demonstrated a detection limit of 1.0 and 4.2 ppm by naked eyes, while the detection limit of 0.15 and 2.5 ppm by UV-vis spectrometer. Zhang and cooperators have successfully explored a simple and effective colorimetric visualization of melamine in milk products employing 13 nm citrate-stabilized AuNPs with the aid of NaHSO4 optimization.37 It has been demonstrated that the NaHSO4-optimized AuNPs system exhibits an excellent detection limit as low as ∼25 parts-per-billion (ppb) on account that NaHSO4 could promote the ligand exchange between citrate and melamine at the surface of AuNPs, thus promoting the aggregation of AuNPs. Recently, employing 8.1 nm dual-functional AuNPs with analyte-recognition and peroxidase-like activity, a facile method was proposed for the first time to sensitively detect melamine and highly improve the peroxidase-like activity of bare AuNPs at the same time.38 Bare AuNPs have been demonstrated to possess intrinsic peroxidase-like activity. In this assay, the peroxidase-like activity of the AuNPs is evaluated by the catalysis of 3,3′,5,5′-tetramethlybenzidine (TMB) in the presence of H2O2 to produce a blue color with a maximum absorbance at 652 nm. What's more, the study further revealed that the AuNPs–melamine aggregates formed after the addition of melamine can enhance the peroxidase-like activity of AuNPs to obtain a higher conversion of TMB to oxidized TMB. Consequently, AuNPs–H2O2–TMB detection system for visual melamine determination has been established with the detection limit as low as 0.025 ppb.
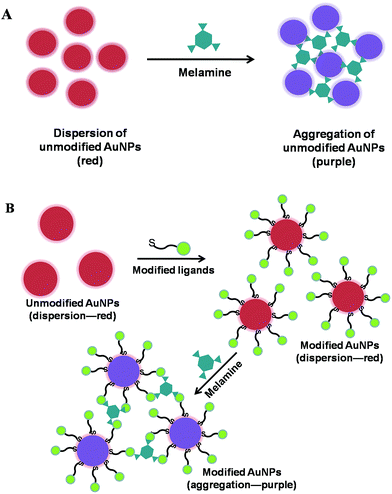 | ||
| Fig. 2 Schematic presentation of the colorimetric mechanism for melamine determination employing unmodified AuNPs (A) and modified AuNPs (B). | ||
To stabilize the AuNPs and improve the sensitivity of the AuNPs-based colorimetric assay, it should be a potential choice to functionalize AuNPs with some suitable ligands. There have been some reports for melamine sensing using ligand-functionalized AuNPs as colorimetric recognition elements. As shown in Fig. 2B, the mechanism of this pattern usually relies on the unique interaction between ligand and melamine allowing the visual sensing of melamine. Lu et al. have developed a MTT-stabilized AuNPs-based colorimetric sensor for visual detection of melamine.39 The first step was synthesis of a thiol-functionalized cyanuric acid derivative 1-(2-mercaptoethyl)-1,3,5-triazinane-2,4,6-trione (MTT). Then, the MTT-stabilized AuNPs (12 nm) was prepared by ligand-exchange reaction using MTT and citrate-stabilized AuNPs. As a result, they demonstrated initially that the color change of AuNPs (from red to blue) was induced by the triple hydrogen-bonding recognition between melamine and MTT, which resulted in excellent selectivity for detection of melamine in milk products with complicated components. Based on this principle, they obtained a detection limit of 2.5 ppb with visual detection within 1 min. In addition, these advantages substantially make this method quite promising for on-site and real-time detection of melamine in raw milk, infant formula, and other milk products. Liu et al. have described a very sensitive colorimetric method for melamine detection employing 3-mercapto-1-propanesulfonate (MPS) modified AuNPs as the probe.40 The functional groups –NH2 of melamine can interact with the sulfo group of MPS via strong hydrogen bonding which could induce the aggregation of the MPS–AuNPs, resulting in a dramatic color change from red to blue. Moreover, the sensitivity of the MPS–AuNPs system could be greatly improved by adding NaCl to the MPS-modified AuNPs solution which leads to a more rapid color change in the NaCl-optimized MPS-modified AuNPs system. Consequently, the melamine determination was achieved with the detection limit as low as 1.008 ppb. Xu and cooperators have synthesized 18-crown-6-thiol-modified AuNPs, and then established a rapid, simple, selective and cost-effective colorimetric method for melamine determination based on the AuNPs aggregation induced by the formation of cavity complexes through the hydrogen bonds between the ether oxygen atoms of the 18-crown-6-thiol and amine groups of melamine.41 With an excellent detection limit of 6 ppb and a wide linear range from 10 to 500 ppb, the proposed method has the potential to sensitively and simply monitor melamine in common products. Using cysteamine-modified AuNPs and an effective sample pretreatment protocol, Zhang et al. have reported a sensitive assay for melamine in complex matrices.42 In this assay, the modification of cysteamine onto citrate-stabilized AuNPs aimed to weaken the electrostatic repulsion force between the AuNPs so that a minute amount of melamine could induce the modified AuNPs to aggregate by hydrogen bonds between melamine and cysteamine. With the limit of detection at 1 ppm, the detection sensitivity of the method was about 100 times higher than that of the method using unmodified AuNPs. Melamine monitoring has been achieved through supramolecular assembly with riboflavin (R) via H-bonding in the platform of R stabilized gold nanoparticles (R-AuNPs), by colorimetric as well as UV-vis techniques.43 In this assay, the ethylene glycol (EG) of the EG stabilized system was replaced by the stronger complexing agent riboflavin so as to form the stable riboflavin-capped AuNPs. Upon addition of melamine to R-AuNPs, R-melamine complexation occurs making the R-AuNPs less stabilized, causing their agglomeration. Based on the principle, the melamine detection was carried out. More importantly, the method is biocompatible as a result of the use of riboflavin (vitamin B2) and sensitive with a detection limit at 0.1 ppm. Raj et al. have described a highly sensitive analytical method based on AuNPs rationally tailored with recognition elements uracil-5-carboxylic acid (UCA) and 2,4,6-trinitrobenzenesulfonic acid (TNBS) for the visual sensing of melamine at the ppb level.44 The different interactions of melamine with these recognition elements induce a rapid visible color change of the tailored AuNPs from red to blue. Due to the aggregation of AuNPs, the results proved that the method employing TNBS-tailored AuNPs reporter based on the charge-transfer interactions is superior to UCA-tailored AuNPs reporter based on multipoint hydrogen-bonding interactions towards melamine sensing. Guan and co-workers have reported a green colorimetric approach for sensing of melamine based on the aggregation of AuNPs stabilized with chitosan which was used as both reducing and stabilizing agent.45 By controlling the concentration of chitosan, chitosan-stabilized AuNPs was prepared by 0.5% chitosan. In the aqueous solution, the as-prepared chitosan-stabilized AuNPs are stable due to the electrostatic repulsion of the negative capping agents, tripolyphosphate (TPP). However, upon the addition of melamine, the as-prepared chitosan-stabilized AuNPs could be aggregated induced by the electrostatic interaction between positively charged melamine and negatively charged chitosan-stabilized AuNPs, leading to a rapid, red-to-blue (dark blue or purple) color change. Based on the mechanism, the sensitive method for melamine sensing was established with a detection limit of 6 ppb. More importantly, the whole process could be accomplished within only 20 min including sample pretreatment allowing the rapid and sensitive detection of melamine.
Up to now, there are a few colorimetric assays for melamine by nonaggregation-based AuNPs as a probe, in which the synthesis of AuNPs was hindered or accelerated by the presence of melamine. Zhao and co-workers have developed a series of AuNPs nonaggregation-based colorimetric sensors realizing the melamine determination during the synthesis of AuNPs.46–48 A simple colorimetric method for melamine monitoring employing nonaggregation-based AuNPs as the probe has been demonstrated.46 In the assay, 3,5-dihydroxybenzoic acid (DBA) was used as a reducer for the reduction of Au3+ ion to form AuNPs. In the absence of melamine, a one-step synthesis of AuNPs was achieved by mixing the Au3+ ion with reducer DBA; in the presence of melamine, the formation of AuNPs was hindered by melamine because the strong hydrogen-binding interaction between melamine and DBA would make DBA not enough for the reduction of Au3+. Thus, the color change of formed AuNPs could be observed from purple to yellow-green with increasing melamine concentration, realizing the sensing of melamine during the formation of AuNPs with the detection limit of 1 ppb. Later, based on the similar principal that melamine could hinder the synthesis of AuNPs, this group reported a colorimetric detection of melamine based on the interruption of the synthesis of AuNPs using ellagic acid (EA) as reducer.47 The addition of melamine could make AuNPs change accompanied by color change from red to pale yellow. As a result, the melamine detection was carried out during the formation of AuNPs with the detection limit of 0.2 ppb. Besides, relying on the different strategy that melamine could accelerated the formation of AuNPs, the group investigated a novel sensitive colorimetric method during the formation of AuNPs based on the principle that melamine could accelerate the synthesis of AuNPs.48 In this study, the reducer 3,5-disodiumsulfonate (PD) may slow the synthesis of AuNPs in that PD can form intramolecular hydrogen-bonding in solution resulting in its weak reducing capacity. However, after the addition of melamine, the interaction of melamine with PD through hydrogen-bonding interrupted the intramolecular hydrogen-bonding of PD while promoted the reduction of Au3+ by PD, resulting in the acceleration of the synthesis of AuNPs. Simultaneously, the melamine determination with a detection limit of 0.08 ppb could be realized via the color change of AuNPs from green to yellow in the process with the addition of different concentration melamine.
2.1.1.2 Silver nanoparticles-based colorimetric sensors. Silver nanoparticles (AgNPs) have been proved to be effective catalytic materials for various applications owing to their large surface-to-volume ratio and electronic properties, and also exhibit higher extinction coefficients than AuNPs at the same size.49 In the metal nanoparticle-based colorimetric assays, AgNPs have been used in less extension than AuNPs, however, a number of colorimetric methods have been established based on color change from yellow to brown during AgNPs dispersion and aggregation.29
Cai et al. have developed a reliable assay for melamine sensing using dopamine-stabilized AgNPs as a colorimetric reader.50 Dopamine alone was used as both reducer to reduce Ag+ and stabilizer to functionalize the generated AgNPs to synthesize monodispersed AgNPs with the color of bright yellow. However, upon the mixture of dopamine with melamine, the AgNPs aggregated and correspondingly the color changed from bright yellow to brown in that melamine could bind dopamine through Michael addition and Schiff base reactions. Consequently, the quantitative monitoring of melamine was achieved at the 0.01 ppm level during the formation of AgNPs. Li et al. demonstrated a novel sensitive and low-cost colorimetric method using the stable ρ-nitroaniline-modified AgNPs, based on the melamine-induced aggregation and color change of AgNPs owing to electron donor–acceptor interaction between melamine and ρ-nitroaniline at the AgNPs interface.51 Through the colorimetric response of AgNPs from yellow to blue, melamine detection was visually distinguished with the detection limit as low as 0.1 ppm. Ping and co-workers have established a visual detection method for melamine in raw milk employing label-free AgNPs as colorimetric probe, with the detection limit of 2.32 μM.52 The mechanism relies on the aggregation of AgNPs induced by the interaction between the three amino groups of melamine and AgNPs corresponding to the color change from yellow to red with the addition of different concentration of melamine. The proposed method with simplicity and rapidness is suitable for on-site screening melamine adulterant in milk products.
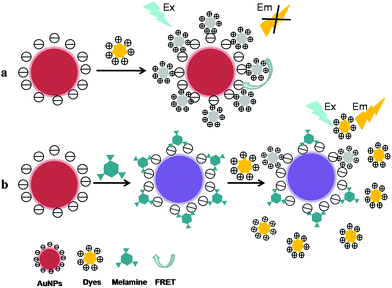
2.1.2.1 Dyes-based sensors. Fluorescence resonance energy transfer (FRET) is a nonradiative process whereby an excited state donor (usually a fluorophore such as dyes, quantum dots, etc.) transfers energy to a proximal ground state acceptor through long-range dipole–dipole interactions, and the rate of energy transfer is highly dependent on the extent of spectral overlap, the relative orientation of the transition dipoles, and especially the distance between the donor and acceptor molecules (typically in the range of 1–10 nm).54 In the AuNPs-based FRET system, AuNPs can be used as excellent acceptors/quenchers due to their extraordinary advantages including high fluorescence quenching efficiency, tunable quenching property, stable optical property, and ease of labeling.55 In addition, the calculated energy-transfer distances are as long as 70–100 nm, about 10 times longer than the typical Förster distances (R0), as the distance dependence changes from R−6 to R−4. So, using a gold metallic nanoparticle as an acceptor for energy transfer distinguishes nanomaterial surface energy transfer (NSET) from FRET in two significant aspects: (1) the distance dependent changes extends the usable distances for the measurement; (2) the same nanoparticle is able to quench dyes of different emission frequencies, spanning the visible range to the near-infrared.54
In recent years, the AuNPs-based FRET assays have been established for the analysis of small organic molecules including melamine which have affinity to bind on the AuNPs surface and could compete with dyes to adsorb onto gold surface. It has been reported that dyes (fluorescein, rhodamine B, etc.) could be adsorbed onto the surface of bare or functionalized AuNPs via electrostatic interaction, resulting in the significant fluorescence quenching through FRET between dyes and the AuNPs. While in the presence of targets, the fluorescence of dyes would recover owing to the competitive binding of dyes and analytes on AuNPs surface. Based on this principle (as illustrated in Fig. 3), Guo et al. have reported a novel sensitive turn-on FRET-based fluorescent senor for melamine detection using fluorescein as donor and AuNPs as acceptor.56 In this work, the fluorescence of fluorescein could be significantly quenched via FRET between fluorescein and the AuNPs. However, when melamine was incubated with AuNPs before fluorescein was added, the fluorescence was recovered as a result of AuNPs aggregation induced by the competitive interaction of AuNPs with melamine and fluorescein. Consequently, the rapid and sensitive detection of melamine was carried out within 15 min with the detection limit at 1 nM level. Relying on the similar strategy, employing rhodamine B (RB) as fluorescent probe accompanied with the AuNPs, an effective and practical method for melamine determination have been proposed based on the fluorescence quenching and recovery of RB through FRET and the competitive binding of RB and melamine on the AuNPs surface.57 Consequently, the melamine detection was obtained with an excellent detection limit of 0.18 ppm. More importantly, through the fluorescence recovery of RB linearly in proportion to melamine concentration, the melamine sensing in milk and powdered infant formula was successfully carried out with excellent recoveries varying from 95.9% to 102.2%. The proposed method appears to be a promising candidate method for rapid on-site screening of melamine contamination in dairy products.
2.1.2.2 Quantum dots-based sensors. Quantum dots (QDs), such as Cd-based QDs and Zn-based QDs, are nanocrystals made of semiconductor materials that are small enough to exhibit quantum mechanical properties. They possess unique optical properties including size-dependent excitation/emission spectrum, broad excitation spectrum and narrow emission spectrum, excellent photostability, good biocompatibility, high fluorescence quantum yields and long fluorescent lifetime. Owing to these superior properties, QDs have become ideal fluorescent probes for signal generation and transduction, and have been widely applied in the field of fluorescence sensing of analytes.58
A number of works for melamine monitoring directly employing QDs as fluorescent probes have been reported. The principle of this strategy is based on the enhancement and decrease of the fluorescence intensity of QDs induced by the interaction between QDs and target analytes. Our group has developed a simple and sensitive fluorescence senor for melamine detection in milk using water-soluble thioglycolic acid (TGA)–CdTe QDs of different sizes.59 Melamine could quench the fluorescence of TGA–CdTe QDs induced by the change of surface properties via hydrogen bonding between the amine groups of melamine and the carboxyl groups of the TGA–CdTe QDs at pH 8.0, which makes the proposed method used to detect melamine with a detection limit of 0.04 ppm. Later, Wang et al. have proved a sensitive and rapid method for melamine determination based on the fluorescence enhancement of TGA–CdS QDs.60 At lower solution pH of 3.0, TGA–CdS QDs exhibited weaker fluorescence intensity in the absence of melamine. However, in the presence of melamine mixed with PBS before addition, the fluorescence intensity was enhanced in that the protonated TGA on the surface of QDs was replaced by the amine group of melamine which can attach onto the surface of QDs via N–Cd bond. Through the increased fluorescence intensity with the increasing melamine concentration, the sensing of melamine was carried out with the detection limit of 1 nM. Then, Zeng et al. have demonstrated an ultrasensitive sensing method for melamine using TGA–CdTe QDs based on the fluorescence intensity change of QDs through QDs aggregation at pH 11.0.61 The pKa of carboxyl group in TGA is 3.53,62 so the TGA–CdTe QDs are negatively charged in strong alkaline aqueous solution. Upon the addition of melamine, TGA on the surface of QDs could be replaced by melamine to form MA-coated CdTe QDs, consequently, the fluorescence of QDs were quenched with the surface change and aggregation of QDs induced by the electrostatic interaction between the negatively-charged TGA–CdTe QDs and positively-charged MA-coated CdTe QDs. Thus, the melamine detection was achieved according to the fluorescence intensity change of QDs. Most importantly, the proposed method possesses advantages of high sensitivity at 5 × 10−12 mol L−1 and good selectivity because the strong alkaline aqueous solution blocked interference from heavy metal ions in the samples. To improve the selectivity, a novel molecularly imprinted polymer-capped CdTe quantum dots (MIP–CdTe QDs)-based fluorescence detection method for melamine has been established relying on the fluorescence quenching of QDs induced by melamine.63 The MIP–CdTe QDs were synthesized by a radical polymerization process among CdTe QDs, a template, 3-aminopropyltriethoxysilane (APTES) and tetraethoxysilane (TEOS). Melamine could be selectively bound onto the surface of MIP–CdTe QDs resulting in the selective sensing of melamine with a detection limit of 0.6 μM, hence, excellent selectivity and high sensitivity of MIP–CdTe QDs toward melamine were observed based on the fluorescence quenching of QDs.
Very recently, by combining QDs with graphene, Zhu et al. have reported a novel type of rapid and sensitive fluorescence sensing system for melamine using graphene quantum dots (GQDs) as fluorescence probes based on charge transfer quenching of the fluorescence of GQDs in the presence of Hg2+.64 In this study, the synthesized GQDs were strongly luminescent with predominantly aromatic sp2 domains. Melamine could coordinate with Hg2+ through nitrogen atoms in both its amine and triazine groups and bring more Hg2+ to the surface of GQDs through π–π stacking, thus leading to the fluorescence quenching of the of GQDs via charge transfer from the GQDs to Hg2+ with melamine acting as the linkage agent. Consequently, the melamine detection was carried out with a detection limit as low as 0.12 μM. The proposed method exhibits satisfactory advantages such as simple fabrication, convenient operation, high selectivity for melamine against interferences that may exist in real samples, and was suitable for detecting targets in real samples.
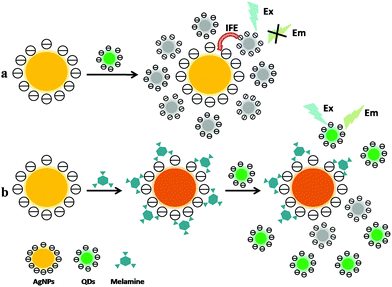
In addition to being directly recognized as fluorescent probes, QDs have also been used in donor–acceptor detection system based on the principle of FRET (mentioned in Section 2.1.2.1) or inner filter effect (IFE) of fluorescence. For example, Cao et al. have developed an efficient and enhanced FRET system between confined quantum dots (QDs) by entrapping CdTe QDs into the mesoporous silica shell (CdTe@SiO2) as donors and gold nanoparticles (AuNPs) as acceptors.65 In this assay, compared with the energy transfer system between unconfined CdTe QDs and AuNPs, the fluorescence quenching efficiency of AuNPs to CdTe@SiO2 could be greatly improved in that AuNPs are noncovalently adsorbed on CdTe@SiO2 via an electrostatic interaction. In the absence of melamine, the CdTe@SiO2–AuNPs assemblies coalesce to form larger clusters due to charge neutralization at pH 6.50, leading to the fluorescence quenching of CdTe@SiO2 as a result of energy transfer. In the presence of melamine, the weak fluorescence system of CdTe@SiO2–AuNPs is enhanced due to the strong interactions between the amino group of melamine and the AuNPs via covalent bond, leading to the release of AuNPs from the surfaces of CdTe@SiO2. Relying on this strategy, the melamine determination was achieved with the detection limit of 0.89 μM.
The mechanism of inner filter effect (IFE) of fluorescence refers to the absorption of the excitation and/or emission light of fluorophores by absorbers (quenchers) in the detection system.66 IFE would occur effectively only if the absorption band of the absorber possesses a complementary overlap with the excitation and/or emission bands of the fluorophore. However, compared with QDs/AuNPs-based FRET systems, the design of IFE processes would be more flexible and simple in that there is no need to modify AuNPs and QDs which aims to involve the intermolecular connection of QDs with AuNPs at a particular distance or geometry to enable the interaction between them. As a result, relying on the principle of IFE (as illustrated in Fig. 4), our group has explored a few IFE-based fluorescence assays for melamine determination in recent years. These assays can be classified into two types: AuNPs/Cd-based QDs IFE system, and AgNPs/Zn-based QDs IFE system. Based on IFE of AuNPs on the fluorescence of Cd-based QDs, two sensitive and rapid fluorescence methods for melamine sensing have been established employing CdTe QDs67 and L-cysteine(L-Cys)-capped CdS QDs68 as fluorescence probes respectively. In the assays, the obtained CdTe QDs and CdS QDs display a maximum fluorescence emission at 553 nm and 537 nm respectively, which is just near the absorption maximum of AuNPs at 522 nm. It is obvious that the absorption spectrum of AuNPs overlaps well with the fluorescence emission spectra of CdTe QDs and CdS QDs. Thus, the fluorescence of Cd-based QDs was obviously quenched upon addition of AuNPs via IFE. While with the presence different concentrations of melamine mixed and incubated with AuNPs before the addition of Cd-based QDs, the fluorescence of Cd-based QDs will gradually recover as a result of melamine-induced AuNPs aggregation. Consequently, the melamine detection was carried out with the detection limit at 0.02 ppm and 17 ppb level respectively. Recently, with eco-friendly L-glutathione-capped ZnSe QDs as fluorophore and citrate-stabilized AgNPs as absorber, a more practical and efficient fluorescence sensing strategy for melamine has been established for the first time based on the efficient IFE of AgNPs on the fluorescence of eco-friendly ZnSe QDs.69 The emission spectrum of ZnSe QDs at 370 nm can overlap well with the absorption spectrum of AgNPs at 395 nm to some extent, suggesting that IFE might take place with ZnSe QDs as fluorophore and AgNPs as absorber. In the absence of melamine, AgNPs could effectively quench the fluorescence emission of ZnSe QDs through IFE. While in the presence of melamine, the fluorescence of QDs will recover due to the AgNPs aggregation induced by interaction between amine groups of melamine and AgNPs via hydrogen bonds. The IFE-based method has been successfully applied in melamine detection in raw milk and egg samples with an excellent detection limit of 0.11 ppb.
2.1.2.3 Metal nonoclusters-based sensors. Metal nanoclusters (NCs), a new type of luminescent nanomaterials, are generally composed of a few to roughly a hundred atoms, and the sizes are below 2 nm which approaches the Fermi wavelength of electrons, leading to the observation of dramatically different optical, electrical and chemical properties as compared to nanoparticles.70 In recent years, metal NCs especially Au and Ag NCs have attracted a great deal of interest on account that they possess very strong, robust, size or scaffold-dependent tunable fluorescence, combine with good photostability and bio-compatibility, and show very high emission rates.71 Due to these excellent features, Au and Ag NCs have been recognized as ideal fluorescent probes in the field of fluorescence sensing for melamine.
Xu et al. have successfully synthesized oligonucleotide-stabilized fluorescent silver nanoclusters (DNA–AgNCs) and applied them in melamine detection for the first time.72 In the study, DNA–AgNCs could exhibit an emission band centered at 665 nm when excited at 597 nm. In the presence of melamine, the fluorescence intensity of DNA–AgNCs could be gradually increased due to the microenvironment change of AgNCs induced by the interaction between the amine groups of melamine and thymine of DNA through strong hydrogen bonds. The proposed method demonstrated good biocompatibility and high sensitivity with the detection limit at 10 nM level. Recently, an environment-friendly and cost-effective turn-on fluorescent assay for melamine has been proposed employing bovine serum albumin stabilized gold nanoclusters (BSA–AuNCs) as the fluorescence probe.73 It has been reported that the fluorescence of AuNCs could be quenched by Hg2+ via high-affinity metallophilic Hg2+ and Au+ interactions.74 Furthermore, melamine has been revealed greater coordination affinity toward Hg2+,75 which may result in the reduction of quenching ability of Hg2+ to AuNCs and accordingly the fluorescence enhancement of AuNCs. Based on the melamine-induced anti-quenching ability of Hg2+ to fluorescent AuNCs, the method with the detection limit as low as 0.15 μM was established and applied in real samples including raw milk and milk powder.
2.1.2.4 Upconverting nanoparticles-based sensors. Upconverting (UC) nanoparticles are lanthanides (Ln3+)-doped materials which are characterized by the conversion of long-wavelength radiation, for instance low energy infrared or near infrared (NIR) radiation, to short-wavelength high-energy radiation, usually in the visible range. UC nanoparticles exhibit high sensitivity because of the lack of autofluorescence background, possess less toxic components, and own high penetration depths in combination with photostability and optical tenability.76 Owing to their outstanding properties, UC nanoparticles have been proposed for applications in chemical sensing,77 bioconjugation and bioimaging,78 etc.
Currently, hexagonal-structured lanthanide-doped NaYF4 has been reported the most promising candidate for detection. For example, Mahalingam et al. have reported for the first time the use of UC nanoparticles to detect melamine up to 2.5 nM.79 In this work, the water dispersible 3,5-dinitrobenzoic acid (DNB) capped NaYF4:Yb3+/Er3+ nanocrystals were prepared by coating the surface of the nanocrystals with electron-deficient groups such as DNB through microwave procedure. Upon 980 nm excitation, the nanocrystals show strong upconversion emissions near 550 nm and 650 nm. Electron-rich melamine can be specifically attached onto the surface of the electron-deficient DNB-coated Er/Yb-NaYF4 nanocrystals via strong charge transfer (CT) interaction (between amine groups of melamine and aromatic nucleus of DNB) and hydrogen bonding interaction (between O atom in –NO2 groups of DNB and H atom in –NH2 groups of melamine). Such interactions essentially decrease the interparticle distance between the nanocrystals and bring the nanocrystals close together resulting in the formation of aggregation. Hence, addition of melamine selectively quenches the luminescence signals from the upconverting nanocrystals because of the aggregation of the nanocrystals. The high selectivity toward melamine determination is verified by the addition of various analytes similar in structure with melamine. In addition, the selective quenching of the upconversion emission is found to be reversible by the addition of HCl, which recovered almost 90% of the initial luminescence intensity. The high robustness, sharp emission peaks and large anti-Stokes shift make Er3+/Yb3+-doped NaYF4 nanocrystals a potential melamine sensing material over other organic fluorophores and nanocrystals.
Song et al. has explored a sensitive luminol–myoglobin CL system for the detection of melamine in milk products.85 It was previously reported that myoglobin (Mb) could react with luminol in alkaline medium and yield strong CL emission.86 While in the presence of melamine, the CL intensity generated from the luminol–Mb reaction would be hindered due to the interaction of melamine with Mb, resulting in the successful detection for melamine in milk products at 3 pg mL−1 level. Later, a simple and rapid flow injection chemiluminescence (FI-CL) method for the melamine sensing has been established using the luminal–H2O2 system in which luminol was used as the CL reagent and H2O2 as the oxidant.87 Based on the principle that melamine can significantly enhance the CL emission of the luminal–H2O2 system in basic medium, the determination of melamine was achieved with the detection limit of 0.12 ppm. The proposed FI-CL method was suitable for high throughput and real-time melamine sensing on account of rapid detection time and excellent sample measurement frequency. Similarly, the luminal–H2O2 system was also used in FI-CL method for melamine monitoring relying on the mechanism that melamine can accelerate the electrons transferring rate of excited 3-aminophthalate with notable enhanced CL intensity of luminol–H2O2 reaction.88 The low detection limit of 0.9 pg mL−1 and wide linear range from 2.5 to 250 pg mL−1 have demonstrated that the method was practical and could be applied in target analysis in complex samples. Another simple and sensitive FI-CL method for melamine determination has been developed based on the luminal–K3Fe(CN)6 system.89 A detection limit of 3.5 ng mL−1 could be achieved relying on the principal that melamine with different concentrations can gradually enhance CL signals from the luminal–K3Fe(CN)6 system in an alkaline medium.
In addition, gold/silver alloy nanoparticles (Au/AgNPs) have been applied for melamine analysis in the field of CL-based sensors. For example, an ultrasensitive CL method has been developed for the determination of low concentrations of melamine employing permanganate–formaldehyde system (KMnO4–HCHO).90 In the absence of Au/AgNPs, a weak CL could be observed in that KMnO4 as CL reagent can oxidize HCHO. While in the presence of small volume of Au/AgNPs solution, the CL of KMnO4–HCHO could be obviously enhanced. However, upon the addition of melamine, the CL intensity of KMnO4–HCHO could be significantly decreased induced by melamine-coated NPs formed through the interaction between the three primary amine groups of melamine and NPs. Furthermore, the CL intensity was proportional to melamine concentration, realizing the determination of melamine with a detection limit of 8 pg mL−1.
Based on this strategy, Kim et al. have developed a rapid, sensitive, and selective PDA sensory system with dual signal capability for convenient melamine detection based on the multiple hydrogen bonding between cyanuric acid (CA) and melamine.95 The PDA liposomes can be conveniently prepared by simple self-assembly and photopolymerization of rationally designed diacetylene molecules, composed of the mixture of 10,12-pentacosadiynoic acid (PCDA) and the CA-carrying diacetylene monomer. Two types of CA-carrying monomers were synthesized and used, including PCDA–CA, and PCDA–EG–CA with the EG (ethyleneglycol) linker. It was demonstrated that the PDA liposomes of PCDA–EG–CA/PCDA was more sensitive to melamine and thus preferred on account that PCDA–EG–CA has the ethyleneglycol linker to match the length of its hydrophobic part with that of PCDA and also has a more balanced amphiphilic structure resulting in the production of intense blue color upon polymerization. The intra/inter liposomal hydrogen bonding between the target melamine and cyanuric acid receptor at the PDA liposome surface induces perturbation of the conjugated PDA backbone and results in rapid and sensitive colorimetric/fluorescence change of the PDA liposomes. The detection limit of the sensory PDA liposome is 1 and 0.5 ppm in the colorimetric and the fluorescence detection schemes, respectively, satisfying the world regulation level.
2.2 Electrochemical sensors
Electrochemical sensors are widely employed because of their instrumental simplicity, moderate cost and portability. They also provide many advantages such as good sensitivity and selectivity, remarkable rapidity and facility which are applicable to most areas of analytical chemistry.96 A number of electrochemical strategies have been explored for the development of electrochemical sensors. Cyclic voltammetry (CV) has received great interest as it can be used for the elucidation of electrode processes and redox mechanisms. Differential pulse voltammetry (DPV) and square wave voltammetry (SWV) are particularly useful in the determination of trace amounts of electroactive compounds. Electrochemiluminescent (ECL) assays are also promising prospective technologies in that they combine the simplicity of electrochemistry with the inherent sensitivity and the wide linear range of the chemiluminescence method. Besides, electrochemical impedance spectroscopy (EIS) is a powerful tool for examining many chemical and physical processes in solution as well as in solids, and can provide information on reaction parameters, corrosion rates, electrode surfaces porosity, coating, mass transport, and interfacial capacitance measurements.As a trimer of cyanamide with a 1,3,5-triazine skeleton, melamine has relatively poor electroactivity. Even though under strong alkaline conditions, it shows a very weak electrochemical response originating from the electrooxidation of the amino group. Thus, it's necessary to fabricate or utilize various electrochemical probes to realize the determination of melamine indirectly.
Zhu and co-workers first explored a reliable and highly sensitive electrochemical sensor for the determination of melamine depending on that melamine could be converted to Cu–melamine complex with excellent electrochemical activity on the surface of a multi-walled carbon nanotubes-modified electrode by coordination of copper salt to melamine.97 In this work, the Cu–melamine complex has been demonstrated to show a sensitive faradaic response, which makes it suitable for melamine detection as low as 0.25 ppb.
Later, based on an idea adopted from the synthesis of melamine formaldehyde resins, Liao et al. successfully developed a simple electrochemical strategy for sensitive and selective detection of melamine in dairy products and pet foods.98 During a preconcentration step (at 1.8 V versus Ag/AgCl), melamine could interact with aldehyde to form a polymer at the preanodized screen-printed carbon electrode. The as-formed polymer was found to be electroactive with a reversible redox peak, and hence SWV was applied to further increase the detection sensitivity to meet the detection limit for application in real sample analysis. Simply with a medium exchange procedure, melamine was selectively detected with a detection limit (S/N = 3) of 0.8 μM (i.e., 98.3 ppb) by the SWV. The recovery tests established for external calibration and standard addition techniques verified that the analysis can be done in a single-run measurement. A single-run approach with the combination of disposable screen-printed carbon electrode and portable electrochemical instrument is actually suitable for on-site, real-time melamine testing.
Zhao et al. have explored a sensitive and environment-friendly electrochemical determination of melamine by DPV utilizing a glassy carbon (GC) electrode coated with the multi-wall carbon nanotube/chitosan (MWCNT/CS) composite.100 A MWCNT/CS composite containing abundant groups (–OH, hydrogen bond) was prepared by electrostatic self-assembly with MWCNTs and CS. [Fe(CN)6]3−/4− was used as the electrochemical probe to characterize the behaviors of the MWCNT/CS-modified GC electrode by CV and EIS. The modified GC electrode has a small electron-transfer resistance due to good conductivity of MWCNT, thus MWCNT/CS composite can greatly increase the conduction of electrons providing an improved electroanalytical response for ferricyanide ion. The interactions (electrostatic attraction and hydrogen-bonding interaction) between melamine and the MWCNT/CS composite could promote the electrochemical reaction of [Fe(CN)6]3−/4− at the MWCNT/CS modified GC electrode, suggested by the DPV of electrochemical probe [Fe(CN)6]3−/4−. Thus, the melamine detection was achieved through the interaction between melamine and MWCNT/CS with the detection limit of 3 nM. Furthermore, this method can be directly applied to the determination of melamine in real milk, and did not need many complicated pretreatments such as centrifugation, filtration, and the use of toxic solvent (e.g. trichloroacetic acid and methanol).
Up to now, in addition to ferricyanide used as the electroactive probe for facile voltammetric determination of melamine, melamine could also interact with some other electrochemical indicators and affect their electrochemical signals, which can therefore be used to build up novel electrochemical detection schemes. With 3,4-dihydroxyphenylacetic acid (DOPAC) as the electrochemical probe and the recognition element, a new electrochemical sensor for melamine in milk products has been established at a GC electrode relying on the interaction between melamine and DOPAC.101 Melamine has a good H-bonding ability and can spontaneously bind to the carboxyl group and two phenolic-OH groups of DOPAC to form a non-electroactive DOPAC–melamine complex.102 The diffusion of the DOPAC–melamine complex is smaller than that of free DOPAC. Meanwhile, the hydrogen-bonding interaction restricts the electroactivity of the phenolic-OH group. Therefore, the redox peak currents of DOPAC decrease with the increasing concentrations of melamine. The anodic peak currents of DOPAC obtained by differential pulse voltammetry are linear with the logarithm of melamine concentrations in the range from 1.0 × 10−8 to 5.0 × 10−6 M with the detection limit of 3 nM.
Liao et al. have successfully developed a simple and easy electrochemical approach for sensitive detection of non-electroactive melamine utilizing a disposable preanodized screen-printed carbon electrode (SPCE*) with uric acid as the recognition element.103 An improved electrochemical activity of uric acid was especially observed through the hydrogen bond between oxo-surface groups and the oxygen atom of the carbonyl group at C-2 in uric acid.104,105 The oxo-surface groups at the SPCE* also provoke the adsorption of melamine that noticeably reduced the hydrogen bonding sites where uric acid reacted. Consequently, this assay is based on the competitive adsorption behavior of melamine at the SPCE* causing suppression in the oxidation current of uric acid. A linear range up to 126 ppb with a detection limit of 1.6 ppb (S/N = 3) is achieved at the SPCE* by DPV. The established electrochemical method was successfully applied to detect the melamine content in tainted milk powder and dog food.
In addition to the hydrogen bonding recognition effect mentioned above, recently, the charge-transfer interaction between melamine and quinones has also been applied to develop a new voltammetric method for the determination of melamine at the glassy electrode.106 For the three types of quinones employed, i.e., tetrachloro-p-benzoquinone (TCBQ), benzoquinone (BQ), and vitamin K1 (VK1), TCBQ exhibits the highest interaction activity with melamine due to its four electron-withdrawing chloro groups. Such a property was further employed for the voltammetric determination of melamine based on the decrease in the redox peak currents of TCBQ/TCBQ− caused by the pre-consumption of TCBQ with melamine. Under the conditions employed in this study, the decrease in the peak current of TCBQ was linear with the concentration of melamine within a concentration range from 10 μM to 1.0 mM.
An ECL enhancement method combined with solid-phase extraction has been developed for the determination of melamine in dairy products.109 The principle is that melamine could enhance the ECL of Ru(bpy)32+ at GC electrode in a strong base solution (pH = 13). Melamine and Ru(bpy)32+ could be oxidized on the electrode, and the active neutral free radical intermediate produced by the oxidation of melamine would react with the electrogenerated Ru(bpy)33+. As a result, the excited Ru(bpy)32+* would be produced, which leads to the strong enhancement of the ECL signal. The enhanced ECL intensity was linearly proportional to the logarithm of melamine concentration with the detection limit of 0.003 ppb.
Liu et al. have studied the ECL of Ru(bpy)32+ in the pH = 10 borate buffer at the bare GC electrode and the single-wall carbon nanotubes (SWNTs) modified GC electrode for the determination of melamine.110 It was confirmed that melamine can be a candidate as an amine co-reactant and react with Ru(bpy)32+ instead of TPA. In addition, the planar electron-rich melamine molecule can be stacked on the surface of SWNTs through strong π–π interaction. Notably, it was found that after addition of melamine, some increasing effect on both the ECL and anodic current intensity at SWNTs-modified GC electrode can be observed in comparison with that at bare GC electrode, owing to the collection and enrichment function of the SWNTs. Thus, the simultaneous immobilization of Ru(bpy)32+ and melamine on the surface of the SWNTs provides a possibility to alter the interaction between the Ru(bpy)32+ and melamine reductant from intermolecular to something like ‘both intra- and inter-molecular’ on the electrode surface. Furthermore, due to the increased effective area of the electrode, the electron transfer reaction on the SWNTs surfaces would be promoted. As a result, the ECL could be increased significantly. The detection limit for melamine can be reduced from 1.0 × 10−10 M at the bare GC electrode to 1.0 × 10−13 M after modification of the GC electrodes by SWNTs.
A novel ECL approach for the melamine monitoring has been established by immobilizing Ru(bpy)32+ onto the electrode surface.111 The Ru(bpy)32+ was first loaded into the sulfhydryl modified MCM-41 mesoporous silica nanoparticles (MSN) through the electrostatic absorption. Then the Ru(bpy)32+-doped MSN was immobilized onto the surface of Au electrode by Au–S interaction. As mentioned above, melamine could replace the TPA in the Ru(bpy)32+–TPA ECL system and then realize the detection of melamine. The proposed solid-state ECL sensor provides a new strategy possessing the advantages of simplicity and low cost. Compared with the solution-phase Ru(bpy)32+ ECL system, it will reduce the consumption of expensive reagent and simplify the experimental design. Particularly, it is a regenerable sensor based on Ru(bpy)32+ recycled at the electrode surface during the ECL reaction.
Jia et al. also developed a solid-state ECL sensor for melamine based on mesoporous SiO2/Ru(bpy)32+/Nafion modified GC electrodes.112 The homogeneous mesoporous silica nanospheres were synthesized using modified Stöber sol–gel process. Since Nafion was known to contain a hydrophobic domain or phase composed of fluorocarbon skeleton, a hydrophobic cation Ru(bpy)32+ can be easily incorporated into the composite films composed of cation-exchangeable Nafion and mSiO2 nanospheres via both an ion-exchange process and hydrophobic interactions. The ECL and electrochemistry of the modified electrodes were investigated in detail with TPA as the coreactant. In the presence of TPA, the oxidation current increases but the reduction current decreases, at the same time, the enhanced ECL signal was observed, which was consistent with the Ru(bpy)32+–TPA reaction mechanism.107 The modified electrodes were applied in testing melamine because of the similar structure of TPA and melamine. Furthermore, it has been successfully applied to determine melamine in milk powder. The proposed detection method has shown advantages including high sensitivity, stability and wide linear range, resulting from the high surface area and special structure of the mesoporous silica nanospheres for loading Ru(bpy)32+ species.
Similarly, another ECL sensor for melamine based on Ru(bpy)32+-doped silica (Ru(bpy)32+@SiO2) nanoparticles and graphene composite has been fabricated recently.113 Spherical Ru(bpy)32+@SiO2 nanoparticles with uniform size about 55 nm were prepared by the reverse microemulsion method. An emerging carbon material, graphene, was adopted to improve the conductivity, resulting in greatly enhanced ECL signal. Due to its extraordinary electric conductivity, graphene improved the conductivity and accelerated the electron transfer rate. In addition, graphene could work as electronic channel improving the efficient luminophor amount participating in the ECL reaction, which further enhanced the ECL signal. Combined with the high Ru(bpy)32+ loading capacity of Ru(bpy)32+@SiO2 nanoparticles and the high conductivity of graphene, the developed solid-state ECL sensor exhibited excellent performance and high sensitivity for melamine detection with a detect limit as low as 1 × 10−13 M.
MIP-based potentiometric sensors, which rely on generating a potential difference across a membrane placed between two charged solutions with different activity without the extraction of template from the membrane, have been reported to have unique advantages that they do not require the template molecules to diffuse through the electrode membranes for generation of membrane potentials, thus obviously reducing the response time.115 Based on this strategy, Liang et al. have explored a potentiometric sensor employing a new polymeric membrane ion-selective electrode based on a MIP for the selective analysis of melamine in milk.116 In this work, the MIP was prepared through the non-covalent imprinting process by using melamine as a template molecule, methacrylic acid (MAA) as a functional monomer, ethylene glycol dimethacrylate (EGDMA) as a cross-linking agent, and 2,2′-azobisisobutyronitrle (AIBN) as an initiator. Melamine could bind with MAA through the triple hydrogen bonds. Removal of the template and unreacted monomers was done by washing the polymer successively in methanol/acetic acid solution. Melamine is readily protonated in aqueous solution at pH lower than 5.0, which could lead to the near-Nernstian response (54 mV per decade) of membrane electrode, realizing the determination of the melamine with the detection limit of 6 μM.
The major drawback for MIP-based sensors is the difficulty in regenerating the MIP films. MIP films obtained by in situ polymerization are easily destroyed because of the collapse of special recognition cavities during the extraction template process, which in turn affects the sensor reproduction ability. In addition, the extraction process of template molecules is tedious and time-consuming. Pizzariello et al. proved that a renewable MIP sensor can be constructed by embedding MIP particles in a carbon paste.117 Inspired by this strategy, a novel strategy for constructing highly sensitive and easily renewable MIP sensors for melamine detection was proposed.118 MIP particles with melamine recognition cavities were designed and prepared based on the non-covalent method, and the imprinting process and removal of template were similar to the procedure mentioned in ref. 118. Film renewability was achieved by embedding MIP particles in a carbon paste using solid paraffin as an adhesive, and the high sensitivity was obtained using a highly sensitive enzyme amplifier. Melamine was indirectly determined from the competition between the reactions of melamine and horseradish peroxidase-labeled melamine with the vacant cavities. Thus the detection signals were amplified because of enzymatic reaction to the H2O2 catalytic oxidation. Based on voltammetric monitoring and amplification of detection signals, sensitivity for the melamine determination was markedly improved with the detection limit of 0.7 nM. After each use, the sensor was easily renewed by simple mechanical polishing.
With [Fe(CN)6]3−/4− as an electroactive probe, the MIPs sensor was successfully applied to the selective determination of melamine in milk products. For example, [Fe(CN)6]3−/4− was used for the indirect determination of melamine by Jin et al. on a molecularly imprinted nano-porous film based electrochemical sensor.119 The molecularly imprinted nano-porous sensing film was prepared by casting melamine–MIP on a nano-Ag polyquercetin modified electrode for the adsorption of melamine. The film displays excellent and highly selective sorption of melamine in the 3-dimensional porous nanomaterial. The electrode responds linearly to melamine in the concentration range of 1 × 10−8 to 9 × 10−7 M, with a detection limit of 1.3 × 10−9 M (at 3σ) in real samples. The interaction between the porous film and melamine was also studied by using [Fe(CN)6]3−/4− as an electrochemical indicator.
In addition to the traditional MIPs synthesized by in situ polymerization, molecular imprinting has been approached using the electrosynthesis of conducting polymers through galvanostatic, potentiostatic and cyclic voltammetric methods.120 Electrosynthesis of MIP-based sensors allows the generation of a rigid, uniform and compact molecularly imprinted layer with good adherence to a transducer surface of any shape and size. Moreover, the thickness and the density of the polymer layer can also be well controlled with the electropolymerization conditions (e.g., applied potential, the number of CV cycles). This feature gives the possibility of creating a direct communication between the coating and the surface of the transducer in a very simple way for the development of electrochemical sensors.121,122 Recently, MIPs prepared by electropolymerization have been applied in the electrochemical sensors for melamine monitoring as reported in the following assays.
A novel and simple electrochemical sensor has been developed for the determination of melamine in milk, which is based on the electropolymerized MIP of poly(para-aminobenzoic acid) (P-p-ABA) modified glassy carbon electrode using [Fe(CN)6]3−/4− as the electrochemical indicator.123 p-ABA was chosen as the monomer for electropolymerization because it has been shown that it can be easily electropolymerized on various substrate materials and form films with good chemical and mechanical stability.124–127 Meanwhile, p-ABA can establish hydrogen bonds with melamine, which facilitates the recognition and selectivity for the analyte. The (P-p-ABA) film was deposited in a p-ABA solution by potentiodynamic cycling of potential with the template (melamine) on a GC electrode. The surface feature of the modified electrode was characterized by EIS and CV. DPV was employed for the quantitative determination of melamine with the detection limit of 0.36 μM due to its higher sensitivity compared with CV. The results of this research demonstrate that it is feasible to use the molecular imprinting methodology when preparing sensing devices for analytes that are electrochemically inactive.
A novel molecularly imprinted impedimetric sensor was promoted for selective detection of melamine with a low detection limit down to 3.0 × 10−9 M.128 The Au electrode modified with MIP of poly (2-mercaptobenzimidazole) (PMBI) was prepared by electrochemical polymerization of 2-mercaptobenzimidazole (2-MBI) using CV in the presence of template molecule melamine. 2-Mercaptobenzimidazole (2-MBI) contained a mercapto group and might consequently improve the polymer–gold binding characteristics. The obtained poly(2-mercaptobenzimidazole) (PMBI) is very stable even under harsh conditions. The properties of the sensor were investigated by impedance spectroscopy, CV and DPV using [Fe(CN)6]3−/4− as the electrochemical probe. The tailor-made cavities formed in the imprinted film showed good selectivity toward melamine, and the main driving force of recognition is the p-donor–acceptor interaction between melamine and PMBI. The effective method has a potential application to monitor nonelectrochemically active substances in complicated real samples.
Compared to the reported indirect electrochemical methods, direct detection of melamine should be much more simple and convenient. The imprinted sol–gel electrochemical sensor for direct recognition and detection of melamine in real samples has been explored without the need of indirect signal reporter such as [Fe(CN)6]3−/4−.129 Thin film of molecularly imprinted sol–gel polymers with specific binding sites for melamine was fabricated on GC electrode surface by electropolymerization method. Electrochemical behavior of melamine on GCE surface was investigated in an acidic electrolyte, demonstrating that a pair of reversible redox peaks (0.65 V for reduction current and 0.72 V for oxidized current) could be seen, which was assumed to be the formation of a radical cation through electrochemical oxidation.130 Then melamine could be detected by its reversible redox peaks instead of other electroactive probe. A change of oxidation peak of melamine could be seen corresponding to the incorporation of melamine onto the surface of MIPs/GCE. Based on this strategy, melamine could be detected in the wider range of 6.3 × 10−7 M to 1.1 × 10−4 M with a detection limit of 6.8 × 10−8 M.
Recently, based on the synergetic effect of the electrochemical accumulation process and the signal amplification of enzymes, a new sensitive method has been developed for the detection of subnanomolar melamine in infant milk powders and fish feed samples.133 There are two steps involved in the sensor construction process: (1) accumulation of melamine on an electrode by cyclic voltammetric method and (2) chemical coupling of horseradish peroxidase (HRP) with the accumulated melamine through the linkage of glutaraldehyde. The coupled HRP catalyzes the oxidation of guaiacol to generate an amber-colored product. Quantitative analysis of melamine is performed by measuring the absorption intensities of the colored product.
3. Biosensors for the analysis of melamine
A biosensor is an analytical device, used for the detection of an analyte, which combines a biological component with a physicochemical detector. A biosensor typically consists of a bio-recognition component, a biotransducer component, and an electronic system which includes a signal amplifier, processor, and display. According to the specific bio-recognition principle, biosensors are classified into immunochemical, enzymatic, non-enzymatic receptor, tissues, organelles, microbes or whole-cell and DNA biosensors, usually transformed by electrical, thermal or optical signals.134 Compared with conventional analytical techniques, biosensors offer the main advantages such as possibility of portability, miniaturisation and working on-site, and the ability to measure pollutants in complex matrices with minimal sample preparation.135 Various biosensing methods developed for detection of melamine have been discussed in the following sections.3.1 Aptamer-based sensors
Aptamer is a synthetic single-stranded nucleic-acid or peptide molecules binding to particular target with high affinity and specificity, which is normally screened with a combinatorial technique called systematic evolution of ligands by exponential enrichment (SELEX).136,137 Aptamers offer advantages over antibodies as they are readily synthesized, possess desirable storage properties and could be modified easily. Because of their inherent superiorities, they are widely used as recognition elements in biosensor construction and are quite helpful in the detection of a wide range of substances from metal ions, organic molecules, peptides, protein to cells.138,139The ability of pyrimidine to form hydrogen bonds with purines has been well documented, and the hydrogen bonding between thymine and 2,6-diamino-5-methylpyrimidin-4-one, which has a similar molecular structure as melamine, was reported.140 Thus, the single stranded oligonucleotide containing thymines may also bind to melamine via hydrogen bonding. Based on this, numbers of aptamer-based biosensors for melamine detection have been developed. Generally, the selected aptamers are the single stranded oligonucleotide of thymines with the difference on the length of the sequence.
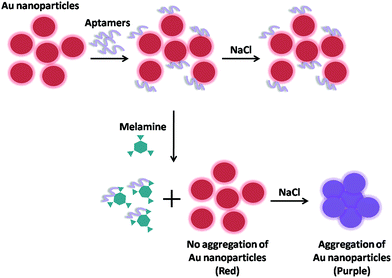
Huang et al. has firstly reported visual and light scattering spectrometric detections of melamine with polythymine-stabilized AuNPs.141 Citrate-coated AuNPs get aggregated obviously in high ionic strength medium. While, polythymine can be adsorbed onto the surfaces of AuNPs and make nanoparticles well-dispersed and stable owing to the repulsion force of the neighboring nanoparticles resulting from the negative charges of polythymine. If melamine, which might form triple H-bonds with polythymine in aqueous medium and decrease the negative charges of the surface of AuNPs, is added, then aggregation of AuNPs might occur, resulting in visual changes from red to blue owing to the variation of localized plasmon resonance absorption and light scattering. Notably, it has been proved that single-stranded DNA oligomers including polyAn, polyCn, polyGn and polyTn could make AuNPs stable without aggregation owing to enough negative charges on their surface. However, the presence of melamine in the medium only makes the polyTn-stabilized AuNPs aggregate, indicating that specific interactions occur between melamine and polyTn. And the stability of a single base unit in polyTn decreases with the length increase of the polyTn. It was the first report which demonstrated the novel specific recognition of melamine with thymine and this triple H-bonds motif in aqueous medium.
Later, He et al. has developed an aptamer-AuNPs-based colorimetric sensor using label-free and labeled AuNPs.142 In the labeled-free AuNPs procedure (as shown in Fig. 5), AuNPs were coated by the negative-charged citrate ions which could prevent AuNPs from aggregation in aqueous solution, while induce the AuNPs aggregation in the presence of high concentration of salt. However, aptamers (poly-T10) could strongly adsorb on AuNPs and enhance the stability of AuNPs against the NaCl-induced aggregation. In the presence of melamine, aptamers will competitively bind with melamine by the stronger affinity which will decrease the salt tolerance of AuNPs and will result in the subsequent aggregation of AuNPs. The color of AuNPs solution quickly changed from wine red to blue as a result of aggregation that provides a rapid and on-site detection of melamine by naked eyes or UV-Vis spectrometer. However, in the labeled-based AuNPs procedure, the single-stranded oligonucleotide labeled AuNPs was used for melamine determination, which was also based on the combination of thymine with melamine. The HSssDNA (HS-(CH2)6-TAGCTATGGAATTCCTCGTAGGCATTTTTT) was first attached to the surface of AuNPs. The DNA functionalized AuNPs can assemble when the oligonucleotides hybridize, which causes the changes of colors. Melamine could induce the hybridization. When melamine was added in, the functionalized AuNPs assembled upon binding of the oligonucleotide to melamine, which resulted in the red-to-blue color change. Particularly, this assembly process was reversible. The dissociation would occur upon thermal denaturation, leading to a blue-to-red color change. Both assays were high selectivity and high sensitivity with the detection limit as low as 41.7 nM and 46.5 nM, respectively. Notably, compared with label-free method, the label-based method provides a better stability, a better accuracy and a larger response range.
Similarly, an AuNPs-based colorimetric aptasensor for melamine was developed using salt-induced aggregation of AuNPs.143 In this paper, they used 5′-TTTTTTTTTTTTTTTTTTTT-3′(dT20) as melamine aptamer. It was used to protect AuNPs under conditions of high salt concentrations. After adding melamine, melamine would competitively combine with the aptamer and induced the release of aptamer from the surface of AuNPs. Without the protection of aptamer, the aggregation of AuNPs will cause the color change. The detection limit was 1.5 mg L−1 in naked eyes and 0.5 mg L−1 with UV-Vis spectrometer.
Recently, Zhou et al. reported an AuNPs-based colorimetric aptasensor for melamine which is different from others.144 They indicated that some approaches using modified nanoparticles and high concentrations of NaCl as the sensor had a shortcoming that the interference of other salts cannot be ignored. This study exhibited the great advantages of cationic polymers in the aggregation of AuNPs. Poly (diallyldimethylammonium chloride) (PDDA), a water soluble cationic polymer, exhibited two features in the present biosensor. First, it can be used to aggregate citrate-stabilized AuNPs. As a positively charged polyelectrolyte, PDDA can be used to aggregate the AuNPs or stabilize the AuNPs–ssDNA, depending on whether or not melamine is present. Second, it can interact with an aptamer to form a complex structure. In the absence of melamine, the aptamer can combine with both the AuNPs by intermolecular and hydrophobic forces and PDDA to form a complex structure, so that the AuNPs do not aggregate and change the solution color. In the presence of melamine, the T base and melamine could form many hydrogen bonds, thus, the ssDNA and melamine could form stable conjugates. As a result the aptamer lost the ability to protect the AuNPs from PDDA resulting in the aggregation of AuNPs. Hence, they used PDDA to control the aggregation of the AuNPs instead of NaCl which makes the system more stable, selective and sensitive.
The resonance scattering (RS) analysis is a new, rapid, simple, convenient and sensitive analytical technique and was applied to the analysis of trace nucleic acid, protein, drug and so on.145 The RS effect of nanoparticles was combined with a highly selective aptamer reaction to assay Hg2+ and K+ with satisfactory results.146–148 A RS spectral assay was developed using aptamer-modified AgNPs as probe.149 AgNPs were modified by a single-strand DNA to fabricate an Ag-aptamer probe for melamine. In the presence of NaCl, the probe was stable. While, upon the addition of melamine, it interacted with the probe to aggregate big clusters, which caused a significant increase of the RS intensity at 470 nm. The increased RS intensity is linear to melamine concentration in the range of 6.31–378.4 μg L−1 with a detection limit of 3.1 μg L−1.
Later, a similar but more sensitive strategy has been established by the same group with the AuNPs instead of AgNPs.150 The aptamer was used to label AuNPs to fabricate an aptamer-AuNPs probe for melamine. The probe was stabile in buffer solutions and in the presence of high concentration of electrolyte. Upon addition of melamine, it interacted with the probe to form big aptamer-AuNPs–melamine aggregations that led to the RS intensity at 566 nm increased greatly. The increased RS intensity is linear to melamine concentration in the range of 1.89–81.98 μg L−1, with a detection limit of 0.98 μg L−1 melamine. In addition, in this paper, the method has been improved based on the high affinity between aptamer and AuNPs. The unreacted probe in the aptamer reaction solution exhibited strong catalytic effect on the slow Cu2O particle reaction between glucose and Fehling reagent, but the catalytic activity of AuNPs aggregations is very weak. In addition, the cubic Cu2O particles produced and exhibited a RS peak at 614 nm. When melamine concentration increased, the unreacted aptamer-AuNPs probe decreased, and the RRS intensity decreased. The decreased RS intensity is linear to melamine concentration in the range of 0.63–47.30 ng L−1 melamine, with a detection limit of 0.38 ng L−1. The proposed aptamer-modified AuNPs catalytic RS assay was more sensitive and selective for melamine determination.
3.2 Immunosensors
Immunoassay is a kind of analytical technique which mainly depends on the immunoaffinity of an antibody towards its antigen, and the extent of binding can be converted to readable output signals using an appropriate transducer. Immunoassay-based biosensors are usually fabricated using monoclonal, polyclonal or recombinant antibodies as biorecognition elements.151 These immunosensors have been widely applied in pharmaceutical analysis, toxicological analysis, bioanalysis, clinical chemistry, and environmental analysis, due to their advantages such as high sensitivity, high selectivity, rapid detection and possible analysis of difficult matrices without extensive pretreatment.Wang et al. proposed an icELISA for the simultaneous determination of melamine and cyromazine.157 Cyromazine is a triazine pesticide used for fly control in crop production and animal feed to inhibit insect growth, whereas melamine is the major metabolite of cyromazine. Thus, cyromazine and melamine can exist simultaneously in animal-derived food. In this paper, they obtained the polyclonal antibody showing broad specificities to melamine and cyromazine by immunising three rabbits with the melamine–BSA conjugate. Another melamine conjugate, melamine–OVA was also produced to be the coating antigen for the icELISA. HRP labeled goat anti-rabbit IgG and TMB were added as the secondary antibody and the substrate, respectively. This is the first paper reporting the development of an icELISA method for the simultaneous detection of melamine and cyromazine in animal muscle tissues. The limit of detection for melamine and cyromazine was 1.8 ng g−1 and 4.5 ng g−1, respectively, and the cross-reactivity (CR) was 59%.
Immunoassay with high CR is suitable for multi-residue analysis, however, it is not expected in a single-analyte specific analysis. Due to the similar structure to melamine, cyromazine is a major potential matrix factor which is not expected in the immunochemical analysis of melamine. Sun et al. developed icELISAs for melamine detection by synthesizing several melamine haptens with different spacer-arms and coupling them to BSA and OVA for immunogens and coating antigens, respectively.158,159 Polyclonal antibodies were obtained for evaluating homogeneous and heterogeneous assay formats. In both studies, the specificity and sensitivity of icELISAs was greatly improved using the heterologous combination of coating antigen and antibody, while the CR for cyromazine was significantly decreased. Notably, it is interesting to find that spacer arm effects and electronic effects appear to be two important factors with regard to the antibody binding to the haptens.
A monoclonal antibody (mAb)-based icELISA has been developed for the analysis of melamine.160 Melamine was conjugated with BSA and OVA for immunogen and coating antigen, respectively. Melamine–BSA immunogen was used to immunise the mice and then the mAb against melamine was obtained. Goat anti-mice IgG peroxidase conjugate and TMB were added as the secondary antibody and the substrate, respectively. The cross-reactivity with cyromazine or other compounds was not studied in this paper. While the proposed method presents high sensitivity with a detection limit of 0.01 ng mL−1, and it has been successfully applied in the liquid milk, powder milk, dog food and cat food indicating that it is a potential method for the rapid and reliable monitoring of melamine in real samples.
Another mAb based icELISA for the determination of melamine has been established based on rational hapten modification and heterogeneous antibody/coating antigen combinations.161 Three melamine derivatives with different length of carboxylic spacer at the end were synthesized and linked to carrier proteins for the production of immunogens (melamine–BSA) and coating antigens (melamine–OVA). Mice were immunized with immunogen and mAb against melamine was produced. The results showed that, in heterogeneous format, the antibody will have a higher affinity toward the analyte in comparison to the coating antigen, leading to a higher sensitivity. Thus a highly sensitive and specific ELISA using heterogenous antibody/coating antigen combinations was established. The CRs of the assays with cyromazine were within 8.2% and no cross-reactivity with other structurally related and unrelated compounds was found.
A novel immunoassay using 2 types of sensors (QDs and an enzyme) was simultaneously used for detecting multiple structurally different molecules in milk, including melamine, sulfonamides and quinolones.162 The method integrates the indirect competitive fluorescence-linked immunosorbent assay (icFLISA) using QD605 and QD655 as probes and an icELISA using HRP labeled secondary antibody. Compared to icELISA, icFLISA is the assay which utilizes fluorescent material as the only sensitive fluorescent label of the sensor system instead of enzyme. In this study, the icFLISA was produced by anti-sulfonamide and anti-quinolone broad-specificity mAbs for simultaneously detecting 6 sulfonamides and 11 quinolones. Combined with the icFLISA, an icELISA was utilized for detecting melamine from the same milk samples. The cross-reactivity of the mAbs was retained while binding the QDs by using avidin and a secondary antibody as bridges. Milk samples were detected using this hybrid immunoassay, with limits of detection of the quinolones (0.18 ng mL−1), sulfonamides (0.17 ng mL−1) and melamine (7.5 ng mL−1), respectively. The results demonstrated that the detection limits of the integrated methods were better than required and simplified the sample pretreatment process. The developed novel immunoassay based on icFLISA in conjunction with the traditional icELISA is suitable for high-throughput screening of low-molecular weight contaminants.
Later, Sanz-Medel et al. established a competitive FLISA for melamine analysis based on the synthesis and characterization of a new immunoprobe, melamine–BSA–QDs conjugates.163 Melamine was firstly conjugated with BSA for the production of melamine–BSA conjugates. The carbodiimide chemistry was further used to synthesize the immunoprobe melamine–BSA–QD forming a chemical bond between the carboxylic groups from the polymeric coating of the QDs and the amino groups from the BSA. Thus, QDs were conjugated to the antigen and a competitive immunoassay format was selected for determination of melamine, where the hapten and the immunoprobe (melamine–BSA–QDs) compete for the limited binding sites of the immobilized antibody. The competition is established by the addition of a mixture of the standard (or sample) and a known amount of the immunoprobe (melamine–BSA–QD). The fluorescence emission of the photoactivated QDs from the melamine–BSA–QDs recognised by the antibody is measured as the analytical signal of this competitive immunoassay. This method combined the advantages of an ELISA immunoassay (simple sample preparation and high throughput) with the unique properties of fluorescent QDs to develop a convenient quantitative analysis of melamine in infant formula milk.
An immunogold chromatographic strip tests have been developed for detecting melamine.165 The capture reagents, melamine–BSA antigen and goat anti-mouse IgG, were immobilized on the nitrocellulose (NC) membrane in the test line and control line, respectively. mAb was labeled with colloidal gold and used as detection reagents. The principle of test strips is based on competitive binding immunoassay. If melamine was absent in the sample, the detection reagent would then be trapped by the capture reagent to form a visible test line. If melamine was present in the sample, then it would compete with the capture reagent for the limited amount of detection reagent. When enough melamine was present, it would then prevent the detection reagent from binding the capture reagent, and the test line signal would decrease to a nonvisible line and the results would be positive. When the test procedure was properly performed, the control line was always visible. The limit of detection was estimated to be 0.05 μg mL−1 in raw milk, since the detection test line on the strip test completely disappeared at this concentration. The limit of detection was 2 μg mL−1 (or 2 μg g−1) for milk drinks, yogurt, condensed milk, cheese, and animal feed and 1 μg g−1 for milk powder. Compared with the ELISA, the immunogold chromatographic assay requires the least sample pretreatment, without the need for expensive equipment, and the results can be obtained within 3–5 min. It is the first report concerned with the method of immunogold chromatographic assay for detection of melamine in raw milk, milk products and animal feed.
A rapid and sensitive lateral flow immunoassay (LFIA) based on competitive format was developed and validated for simultaneous detection of cyromazine and melamine in foods of animal origin.166 The principle of test strips is based on competitive binding immunochromatographic assay, in which the reporter reagents in the conjugate pad were anti-cyromazine mAbs and anti-melamine mAbs coated with colloidal gold particles. The cyromazine–BSA, melamine–BSA and goat anti-mouse IgG were separately immobilised on nitrocellulose membranes as the capture reagents. This paper first reported an immunogold chromatographic method for the simultaneous determination of melamine and cyromazine in foods of animal origin. The sample preparation was simple and the sensitivity was high with the lower detection limit of 1.75 and 2.04 ng g−1 for cyromazine and melamine, respectively. Therefore, the lateral flow immunoassay can be used as a simple, semiquantitative, quantitative and sensitive screening tool for routine monitoring the residues of cyromazine and/or melamine in large number of animal food products.
In contrast with a colloidal gold test strip for melamine, the lateral flow test strip based on colloidal selenium immunoassay for rapid detection of melamine was easy to prepare and more cost-efficient.167 Colloidal selenium was synthesized by L-ascorbic acid to reduce seleninic acid at room temperature. The mAb was conjugated with colloidal selenium instead of colloidal gold because of the relatively complicated preparation process and high cost of gold particles. The test line-immobilized melamine–BSA can react with mAb, thus capturing adequate colloidal selenium particles to show an orange test line. If there is elevated melamine in the tested sample, the melamine will react with mAb first, leaving insufficient mAb for melamine–BSA to bind, hence no visible test line can be observed due to lack of colloidal selenium particles. The intensity of the test line color is proportionate to the quantity of melamine in the sample. Similarly, the immobilized goat anti-mouse IgG in the control area will always react with mAb to show an orange line. The positive result is determined by the appearance of an orange line in the control area, but the absence of it in the test area. The negative result is determined by an orange line exhibited in both the test area and the control area. If no visible orange line is present in the control area, the test strip is considered invalid regardless of whether an orange line appears in the test area or not. The detection limit of the test strip reached 150 μg kg−1, 1000 μg kg−1, and 800 μg kg−1 in liquid milk, milk powder, and animal feed, respectively. No cross-reactions with homologues cyanuric acid, cyanurodiamide, or ammelide were found. Moreover, the melamine test strip can remain stable after storage for 1 year at room temperature. This study firstly employed a colloidal selenium-based chromatographic immunoassay for detection of melamine in milk, milk powder, and animal feed.
Fodey et al. have generated a polyclonal antibody based SPR biosensor for detecting melamine.170 As a result of its low molecular weight, melamine must be coupled to a large carrier protein so that the conjugate will illicit an immune response in the host animal. Preparation of such a conjugate is complicated by the small chemical structure of melamine and the three amine groups that are available for reaction with a protein. A structural mimic (6-hydrazino-1,3,5-triazine-2,4-diamine) for melamine was used as a hapten for antibody production, removing the need for chemical manipulation of melamine itself. A suitable bifunctional cross-linker was attached to the carrier protein to allow direct reaction with the hydrazide group of the hapten. The resulting polyclonal antibody was then incorporated into a SPR optical biosensor immunoassay. The antibody did not cross-react with any of the byproducts of melamine manufacture; however, significant cross-reactivity was observed with the insecticide cyromazine of which melamine is a metabolite. When sample matrix was applied to the assay, a limit of detection of <0.5 μg mL−1 was determined in both infant formula and infant liquid milk.
Li et al. have also developed a portable miniaturized SPR biosensor for rapid detection and quantification of melamine through immunoassay based on the binding between melamine and anti-melamine antibody.171 With sensing surface comprising of an avidin monolayer and biotinylated anti-melamine antibody, three types of immunoassay have been successfully performed for the detection and quantification of melamine, namely direct assay, displacement assay and competitive assay. In addition to direct assay based on antibody–antigen binding, BSA–melamine conjugate is used to induce more significant changes in displacement assay and competitive assay. The displacement assay involves the introduction of an excess of the BSA–melamine conjugate over the sensor surface to occupy as many binding sites as possible. Upon addition of molecular melamine, displacement of the melamine–BSA conjugate occurs. On the other hand, the competitive assay involves the introduction of a mixture of the molecular melamine and melamine–BSA over the sensor surface for them to compete for the binding sites. Compared to direct assay, the displacement assay and competitive assay using BSA–melamine conjugate enhance the sensitivity by about 14 times and 60 times, respectively. The competitive assay can be finished in 15 min with detection limit of 0.02 μg mL−1. The feasibility of testing real samples is proven to be good for infant formula after simple pretreatment. The SPR biosensor with proposed analysis assays is rapid, convenient and low-cost for effective detection of melamine.
In addition, several fluorescence immunosensors for detecting melamine have been established. Lei et al. have designed a fluorescence polarization immunoassay (FPIA) based on a polyclonal antibody for the determination of melamine in milk and milk powder.172 FPIA is one of the most extensively used homogeneous techniques, and meets the requirements of a simple, reliable, fast, and cost effective analysis. FPIA is a competitive immunoassay method based on the increase in the polarization of the fluorescence of a small fluorescein-labeled hapten (tracer) when it is bound by a specific antibody. Recently, the use of FPIA for the determination of pesticides, biological toxins, mycotoxins, and veterinary drugs in agricultural products and environmental samples has been reported. The assay in this paper is based on an improved polyclonal antibody produced by a melamine hapten (6-hydrazinyl-1,3,5-triazine-2,4-diamine, hapten A) coupled to BSA as the immunogen for the rabbit immunization. Three fluorescein-labeled melamine tracers with different structures and spacer bridges were synthesized. Without any additional coupling reagent, hapten A was linked directly to FITC (fluorescein isothiocyanate isomer I) for use as fluorescein-labeled tracer A. Tracers B and C were synthesized using the active ester method based on hapten B (3-(4,6-Diamino-1,6-dihydro-1,3,5-triazin-2-ylthio) propanoic acid) and hapten C (6-(4,6-diamino-1,6-dihydro-1,3,5-triazin-2-ylamino) hexanoic acid) respectively. Thus, tracers A, B, and C differed in not only the spacer length, but also in chemical structure between the hapten and the fluorophore. All three tracers were used as the competing molecules in the FPIA. The results showed that tracer C, with a six-carbon spacer arm as a heterogeneous competitor, gave better assay sensitivity. This FPIA method could realize the detection without complicated cleanup and it is the first development of an FPIA for the detection of melamine.
Another fluorescence immunosensor has been developed by Shi et al.173 They developed an indirect competitive immunoassay using the planar waveguide fluorescence immunosensor (PWFI) based on the principle of immunoreaction and total internal reflect fluorescence (TIRF). The objective of achieving spatially resolved excitation and collecting fluorescence from fluorescently labeled antibodies locally bound at a planar interface can be met by the evanescent field excitation of fluorophore. Excitation light was guided by total internal reflection within transducer structure resulting in an evanescent wave which allows the excitation fluorophore bound to the transducer surface. The TIRF principle allows selective detection of surface bound fluorophore and, therefore, on line monitoring of binding events, which was superior than that with direct illumination of the active area of transducers. A planar transducer was also preferred to a fiber-based system, as being manufactured as an integrated part to fluidics systems. For the detection of melamine, an inhibition immunoassay was used. The Cy5.5 labeled melamine–antibody was firstly mixed with analyzed samples for pre-incubation. The antibody binding sites were occupied depending on the concentration of the melamine. Then the mixture will pump through the sensor instrument and the free melamine–antibody-Cy5.5 will bind the surface with immobilized melamine–BSA conjugate. Since the melamine inhibited the antibodies binding to the immobilized conjugate, the PWFI signal declined when concentrations of analyte increased. The chip of the sensor immobilized by melamine–BSA was reusable and highly resistive to non-specific binding of proteins.
4. Perspective and conclusion
Several novel methods have been exhibited in above discussion including optical sensors, electrochemical sensors and biosensors. These sensors possess the advantages of sensitivity, rapidity, cheapness and are available for detection of melamine in real samples such as raw milk, infant formula, meat, and animal feeds. Nevertheless, they still require improvements in order to obtain robust and further application.Metal nanoparticles-based colorimetric assays, which are simple and rapid, have several drawbacks, including complicated pretreatment of real samples to remove the interference components, instability of some functionalized metal nanoparticles, poor selectivity and availability. Fluorescence sensors are still needed to be improved by means of synthesising inorganic nanomaterials with homogeneous optical properties, proposing novel modification strategies for nanomaterials to achieve better water solubility and surface functionalization, enhancing the characterization methods, and improving the purifying methods to increase the stability and chemical purity of nanomaterials. Chemiluminescence sensors are promising methods for melamine detection due to the rapidness and controllable emission rate. However, many chemiluminescence reactions exhibit low selectivity as a result of the interference effects, and show low robustness for many experimental conditions such as pH, temperature, reagent concentrations, nature of solvent, ionic strength, etc. Hence, the practical applications of chemiluminescence sensors in real samples are in great demand. Electrochemical sensors are endowed with low energy consumption, rapid response time, enhanced selectivity, and refreshability. However, the poor electroactivity of melamine limits the application of electrochemical sensors. Thus, it's necessary to fabricate or utilize various electrochemical probes to realize the determination of melamine indirectly. Immunosensors are based on the binding properties of antibodies with antigens. The main drawbacks of this technique are that a known reciprocal antigen or antibody must be generated to detect a given antibody or antigen, and nonspecific binding of the antibody or antigen might lead to a false result. It is difficult to prepare antibodies with sufficiently high specificity to distinguish compounds that are structurally closely related. For melamine screening, the cross-reactivity to the insecticide cyromazine couldn't be ignored. Particularly, melamine, as a kind of hapten, has no immunogenicity and should be conjugated to a protein to add immunogenicity. Therefore, it is one of the key steps to produce a complete antigen for the development of immunoassays for the melamine detection.
It can be found that the establishment of various methods for melamine detection is mainly based on the specific structure of melamine. For example, aptamer-based sensors are developed on the basis of the formation of H-bonds between melamine and T-base group of aptamer. Moreover, due to the similar structure, melamine could replace the TPA in the Ru(bpy)32+–TPA ECL system and then realize the detection of melamine by electrochemical sensing.
It is evident that the novel materials, such as metal nanoparticles, aptamers, quantum dots, graphene and carbon nano tube have good potential to be used for the fabrication of chemical and biosensors. Nanomaterials could realize the signal amplification of sensors like optical sensors and electrochemical sensors, and then improve the sensitivity of the method. Aptamers have the inherent characteristics of high recognition towards specific molecular targets, which could increase the selectivity of the assay. Thus, aptasensors are increasingly replacing conventional immuno-based sensing systems. Recently, relying on the specific indentification of aptamer and the advantages of nanomaterials including the unique optical and electrochemical properties, our group has explored a variety of aptamer-based nanosensors for the determination of noxious substances in food. Furthermore, our group has made important progress and gradual achievement. Importantly, the proposed methods would pave promising ways for the establishment of more novel and effective strategies available for on-site screening, greatly expanding the practical application of aptamer-based sensors.
Compared to traditional method like chromatographic technique, novel sensors may help the regulatory agencies and other people to monitor and to screen real samples for the presence of melamine with less effort. Nevertheless, in spite that the development of the sensors is rapidly and convincing, improvements in real sample analysis still need to be focused on designing ideal sensing systems with better sensitivity and selectivity towards melamine. Since the fabrication of sensors involves various streams of science, multidisciplinary effort is necessary in designing ideal sensors.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (no. 20905031), the Natural Science Foundation of Jilin Province (no. 201215024) and the Excellent Youth Talent Cultivation Project of Heping Campus of Jilin University.References
- V. D. M. Finete, M. M. Gouvea, F. F. D. Marques and A. D. P. Netto, Talanta, 2014, 123, 128–134 CrossRef CAS PubMed.
- W. C. Andersen, S. B. Turnipseed, C. M. Karbiwnyk, S. B. Clark, M. R. Madson, C. M. Gieseker, R. A. Miller, N. G. Rummel and R. Reimschuessel, J. Agric. Food Chem., 2008, 56, 4340–4347 CrossRef CAS PubMed.
- E. Y. Y. Chan, S. M. Griffiths and C. W. Chan, Lancet, 2008, 372, 1444–1445 CrossRef CAS.
- N. Guan, Q. F. Fan, J. Ding, Y. M. Zhao, J. Q. Lu, Y. Ai, G. B. Xu, S. N. Zhu, C. Yao, L. N. Jiang, J. Miao, H. Zhang, D. Zhao, X. Y. Liu and Y. Yao, N. Engl. J. Med., 2009, 360, 1067–1074 CrossRef CAS PubMed.
- F. Sun, W. Ma, L. Xu, Y. Zhu, L. Liu, C. Peng, L. Wang, H. Kuang and C. Xu, TrAC, Trends Anal. Chem., 2010, 29, 1239–1249 CrossRef CAS PubMed.
- T. Kobayashi, A. Okada, Y. Fujii, K. Niimi, S. Hamamoto, T. Yasui, K. Tozawa and K. Kohri, Urol. Res., 2010, 38, 117–125 CrossRef CAS PubMed.
- A. K. Hau, T. H. Kwan and P. K. Li, J. Am. Soc. Nephrol., 2009, 20, 245–250 CrossRef CAS PubMed.
- C. A. Brown, K. S. Jeong, R. H. Poppenga, B. Puschner, D. M. Miller, A. E. Ellis, K. Kang, S. Sum, A. M. Cistola and S. A. Brown, J. Vet. Diagn. Investig., 2007, 19, 525–531 CrossRef PubMed.
- J. R. Ingelfinger, N. Engl. J. Med., 2008, 26, 2745–2748 CrossRef PubMed.
- S. A. Tittlemier, Food Addit. Contam., 2010, 27, 129–145 CrossRef CAS.
- Y. N. Wu and Y. Zhang, Food Chem. Toxicol., 2013, 56, 325–335 CrossRef CAS PubMed.
- W. Chansuvarn, S. Panich and A. Imyim, Spectrochim. Acta, Part A, 2013, 113, 154–158 CrossRef CAS PubMed.
- G. Venkatasami and J. R. Sowa Jr, Anal. Chim. Acta, 2010, 665, 227–230 CrossRef CAS PubMed.
- H. W. Sun, L. X. Wang, L. F. Ai, S. X. Liang and H. Wu, Food Control, 2010, 21, 686–691 CrossRef CAS PubMed.
- S. Goscinny, V. Hanot, J.-F. Halbardier, J.-Y. Michelet and J. Van Loco, Food Control, 2011, 22, 226–230 CrossRef CAS PubMed.
- X. Xia, S. Y. Ding, X. W. Li, X. Gong, S. X. Zhang, H. Y. Jiang, J. C. Li and J. Z. Shen, Anal. Chim. Acta, 2009, 651, 196–200 CrossRef CAS PubMed.
- X. L. Zhu, S. H. Wang, Q. Liu, Q. Xu, S. X. Xu and H. L. Chen, J. Agric. Food Chem., 2009, 57, 11075–11080 CrossRef CAS PubMed.
- B. R. Shang, Y. Q. Chen, Z. Y. Wang, W. J. Yang and L. Y. Zhang, J. Anim. Vet. Adv., 2001, 1, 73–80 Search PubMed.
- X. M. Xu, Y. P. Ren, Y. Zhu, Z. X. Cai, J. L. Han, B. F. Huang and Y. Zhu, Anal. Chim. Acta, 2009, 650, 39–43 CrossRef CAS PubMed.
- Y. Liu, E. E. D. Todd, Q. Zhang, J. R. Shi and X. J. Liu, J. Zhejiang Univ., Sci., B, 2012, 13, 525–532 CrossRef CAS PubMed.
- F. X. Sun, W. Ma, L. G. Xu, Y. Y. Zhu, L. Q. Liu, C. F. Peng, L. B. Wang, H. Kuang and C. L. Xu, TrAC, Trends Anal. Chem., 2010, 29, 1239–1249 CrossRef CAS PubMed.
- Y. C. Tyan, M. H. Yang, S. B. Jong, C. K. Wang and J. Shiea, Anal. Bioanal. Chem., 2009, 395, 729–735 CrossRef CAS PubMed.
- D. R. Thevenot, K. Toth, R. A. Durst and G. S. Wilson, Biosens. Bioelectron., 2001, 16, 121–131 CrossRef CAS.
- J. H. Kim, S. C. Mun, H. N. Ko, G. Y. Yun and J. H. Kim, Nanotechnology, 2014, 25, 1–7 Search PubMed.
- J. Janata, Anal. Chem., 2001, 73, 151–153 CrossRef.
- M. Frost and M. E. Meyerhoff, Anal. Chem., 2006, 78, 7370–7377 CrossRef CAS.
- J. Janata, Anal. Chem., 1990, 62, 33–44 CrossRef PubMed.
- M. S. Luchansky and R. C. Bailey, Anal. Chem., 2012, 84, 793–821 CrossRef CAS PubMed.
- D. Vilela, M. C. Gonzalez and A. Escarpa, Anal. Chim. Acta, 2012, 751, 24–43 CrossRef CAS PubMed.
- K. Saha, S. S. Agasti, C. Kim, X. N. Li and V. M. Rotello, Chem. Rev., 2011, 112, 2739–2779 CrossRef PubMed.
- W. A. Zhao, M. A. Brook and Y. F. Li, ChemBioChem, 2008, 9, 2363–2371 CrossRef CAS PubMed.
- D. B. Liu, Z. Wang and X. Y. Jiang, Nanoscale, 2011, 3, 1421–1433 RSC.
- K. C. Grabar, R. G. Freeman, M. B. Hommer and M. J. Natan, Anal. Chem., 1995, 67, 735–743 CrossRef CAS.
- L. Li, B. X. Li, D. Cheng and L. H. Mao, Food Chem., 2010, 122, 895–900 CrossRef CAS PubMed.
- Q. Q. Zhou, N. Liu, Z. W. Qie, Y. Wang, B. A. Ning and Z. X. Gao, J. Agric. Food Chem., 2011, 59, 12006–12011 CrossRef CAS PubMed.
- L. Q. Guo, J. H. Zhong, J. M. Wu, F. F. Fu, G. N. Chen, X. Y. Zheng and S. Lin, Talanta, 2010, 82, 1654–1658 CrossRef CAS PubMed.
- H. Chi, B. H. Liu, G. J. Guan, Z. P. Zhang and M. Y. Han, Analyst, 2010, 135, 1070–1075 RSC.
- P. J. Ni, H. C. Dai, Y. L. Wang, Y. J. Sun, Y. Shi, J. T. Hu and Z. Li, Biosens. Bioelectron., 2014, 60, 286–291 CrossRef CAS PubMed.
- K. L. Ai, Y. L. Liu and L. H. Lu, J. Am. Chem. Soc., 2009, 131, 9496–9497 CrossRef CAS PubMed.
- H. C. Su, H. Fan, S. Y. Ai, N. Wu, H. M. Fan, P. C. Bian and J. C. Liu, Talanta, 2011, 85, 1338–1343 CrossRef CAS PubMed.
- H. Kuang, W. Chen, W. J. Yan, L. G. Xu, Y. Y. Zhu, L. Q. Liu, H. Q. Chu, C. F. Peng, L. B. Wang, H. Q. Chu, C. F. Peng, L. B. Wang and N. A. Kotov, Biosens. Bioelectron., 2011, 26, 2032–2037 CrossRef CAS PubMed.
- X. S. Liang, H. P. Wei, Z. Q. Cui, J. Y. Deng, Z. P. Zhang, X. Y. You and X. E. Zhang, Analyst, 2011, 136, 179–183 RSC.
- B. Roy, A. Saha and A. K. Nandi, Analyst, 2011, 136, 67–70 RSC.
- R. K. Bera and C. R. Raj, Analyst, 2011, 136, 1644–1648 RSC.
- H. A. Guan, J. Yu and D. F. Chi, Food Control, 2013, 32, 35–41 CrossRef CAS PubMed.
- Q. Cao, H. Zhao, Y. J. He, X. J. Li, L. X. Zeng, N. Ding, J. A. Wang, J. Yang and G. W. Wang, Biosens. Bioelectron., 2010, 25, 2680–2685 CrossRef CAS PubMed.
- Z. J. Wu, H. Zhao, Y. Xue, Q. A. Cao, J. Yang, Y. J. He, X. J. Li and Z. B. Yuan, Biosens. Bioelectron., 2011, 26, 2574–2578 CrossRef CAS PubMed.
- X. F. Zhang, Z. J. Wu, Y. Xue, Y. Zhang, H. Zhao, Y. J. He, X. J. Li and Z. B. Yuan, Anal. Methods, 2013, 5, 1930–1934 RSC.
- J. Li, W. Li, W. B. Qiang, X. Wang, H. Li and D. K. Xu, Anal. Chim. Acta, 2014, 807, 120–125 CrossRef CAS PubMed.
- Y. R. Ma, H. Y. Niu, X. L. Zhang and Y. Q. Cai, Analyst, 2011, 136, 4192–4196 RSC.
- C. P. Han and H. B. Li, Analyst, 2010, 135, 583–588 RSC.
- H. Ping, M. W. Zhang, H. K. Li, S. G. Li, Q. S. Chen, C. Y. Sun and T. H. Zhang, Food Control, 2012, 23, 191–197 CrossRef CAS PubMed.
- W. W. Zhong, Anal. Bioanal. Chem., 2009, 394, 47–59 CrossRef CAS PubMed.
- G. W. Chen, F. L. Song, X. Q. Xiong and X. J. Peng, Ind. Eng. Chem. Res., 2013, 52, 11228–11245 CrossRef CAS.
- J. Ling and C. Z. Huang, Anal. Methods, 2010, 2, 1439–1447 RSC.
- L. Q. Guo, J. H. Zhong, J. M. Wu, F. F. Fu, G. N. Chen, Y. X. Chen, X. Y. Zheng and S. Lin, Analyst, 2011, 136, 1659–1663 RSC.
- X. Y. Cao, F. Shen, M. W. Zhang, J. J. Guo, Y. L. Luo, J. Y. Xu, Y. Li and C. Y. Sun, Dyes Pigm., 2014, 111, 99–107 CrossRef CAS PubMed.
- R. Gill, M. Zayats and I. Willner, Angew. Chem., Int. Ed., 2008, 47, 7602–7625 CrossRef CAS PubMed.
- M. W. Zhang, H. Ping, X. Y. Cao, H. K. Li, F. R. Guan, C. Y. Sun and J. B. Liu, Food Addit. Contam., 2012, 29, 333–344 CAS.
- G. L. Wang, H. J. Jiao, X. Y. Zhu, Y. M. Dong and Z. J. Li, Talanta, 2012, 93, 398–403 CrossRef CAS PubMed.
- X. F. Li, J. Li, H. Y. Kuang, L. Feng, S. J. Yi, X. D. Xia, H. W. Huang, Y. Chen, C. R. Tang and Y. L. Zeng, Anal. Chim. Acta, 2013, 802, 82–88 CrossRef CAS PubMed.
- H. Zhang, Z. Zhou, B. Yang and M. Y. Gao, J. Phys. Chem. B, 2003, 107, 8–13 CrossRef CAS.
- B. T. Huy, M. H. Seo, X. F. Zhang and Y. I. Lee, Biosens. Bioelectron., 2014, 57, 310–316 CrossRef CAS PubMed.
- L. L. Li, G. H. Wu, T. Hong, Z. Y. Yin, D. Sun, E. S. Abdel-Halim and J. J. Zhu, Interfaces, 2014, 6, 2858–2864 CAS.
- F. Gao, Q. Q. Ye, P. Cui and L. Zhang, J. Agric. Food Chem., 2012, 60, 4550–4558 CrossRef CAS PubMed.
- P. Yuan and D. R. Walt, Anal. Chem., 1987, 59, 2391–2394 CrossRef CAS.
- M. W. Zhang, X. Y. Cao, H. K. Li, F. R. Guan, J. J. Guo, F. Shen, Y. L. Luo, C. Y. Sun and L. G. Zhang, Food Chem., 2012, 135, 1894–1900 CrossRef CAS PubMed.
- X. Y. Cao, F. Shen, M. W. Zhang, J. J. Guo, Y. L. Luo, X. Li, H. Liu, C. Y. Sun and J. B. Liu, Food Control, 2013, 34, 221–229 CrossRef CAS PubMed.
- X. Y. Cao, F. Shen, M. W. Zhang and C. Y. Sun, Sens. Actuators, B, 2014, 202, 1175–1182 CrossRef CAS PubMed.
- L. Shang, S. J. Dong and G. U. Nienhaus, Nano Today, 2011, 6, 401–418 CrossRef CAS PubMed.
- L. B. Zhang and E. K. Wang, Nano Today, 2014, 9, 132–157 CrossRef CAS PubMed.
- S. Han, S. Y. Zhu, Z. Y. Liu, L. Z. Hu, S. Parveen and G. B. Xu, Biosens. Bioelectron., 2012, 36, 267–270 CrossRef CAS PubMed.
- H. C. Dai, Y. Shi, Y. L. Wang, Y. J. Sun, J. T. Hu, P. J. Ni and Z. Li, Biosens. Bioelectron., 2014, 53, 76–81 CrossRef CAS PubMed.
- J. P. Xie, Y. G. Zheng and J. Y. Ying, Chem. Commun., 2010, 46, 961–963 RSC.
- J. J. Du, S. Y. Yin, L. Jiang, B. Ma and X. D. Chen, Chem. Commun., 2013, 49, 4196–4198 RSC.
- M. Haase and H. Schafer, Angew. Chem., Int. Ed., 2011, 50, 5808–5829 CrossRef CAS PubMed.
- D. E. Achatz, R. Ali and O. S. Wolfbeis, Top. Curr. Chem., 2011, 300, 29–50 CrossRef CAS.
- H. S. Mader, P. Kele, S. M. Saleh and O. S. Wolfbeis, Curr. Opin. Chem. Biol., 2010, 14, 582–596 CrossRef CAS PubMed.
- C. Hazra, V. N. K. B. Adusumalli and V. Mahalingam, ACS Appl. Mater. Interfaces, 2014, 6, 7833–7839 CAS.
- N. Li, D. Q. Liu and H. Cui, Anal. Bioanal. Chem., 2014, 406, 5561–5571 CrossRef CAS PubMed.
- Z. Lin, H. Chen and J. M. Lin, Analyst, 2013, 138, 5182–5193 RSC.
- J. A. Ocaña-González, M. Ramos-Payán, R. Fernández-Torres, M. V. Navarro and M. A. Bello-López, Talanta, 2014, 122, 214–222 CrossRef PubMed.
- M. L. Liu, Z. Lin and J. M. Lin, Anal. Chim. Acta, 2010, 670, 1–10 CrossRef CAS PubMed.
- D. L. Giokas, A. G. Vlessidis, G. Z. Tsogas and N. P. Evmiridis, TrAC, Trends Anal. Chem., 2010, 29, 1113–1126 CrossRef CAS PubMed.
- Z. M. Wang, D. H. Chen, X. Gao and Z. H. Song, J. Agric. Food Chem., 2009, 57, 3464–3469 CrossRef CAS PubMed.
- Z. H. Song, L. Wang and S. Hou, Anal. Bioanal. Chem., 2004, 378, 529–535 CrossRef CAS PubMed.
- H. J. Zeng, R. Yang, Q. W. Wang, J. J. Li and L. B. Qu, Food Chem., 2011, 127, 842–846 CrossRef CAS PubMed.
- J. J. Zhang, M. Wu, D. H. Chen and Z. H. Song, J. Food Compos. Anal., 2011, 24, 1038–1042 CrossRef CAS PubMed.
- X. S. Tang, X. Y. Shi, Y. H. Tang, Z. J. Yue and Q. Q. He, Luminescence, 2012, 27, 229–233 CrossRef CAS PubMed.
- J. L. Manzoori, M. Amjadi and J. Hassanzadeh, Microchim. Acta, 2011, 175, 47–54 CrossRef CAS.
- K. Lee, L. K. Povlich and J. Kim, Analyst, 2010, 135, 2179–2189 RSC.
- C. Y. Sun and J. H. Li, Advances in Planar Lipid Bilayers and Liposomes, Elsevier, 2006, vol. 4, ch. 7, pp. 229–252 Search PubMed.
- M. A. Reppy and B. A. Pindzola, Chem. Commun., 2007, 42, 4317–4338 RSC.
- D. J. Ahn and J. M. Kim, Acc. Chem. Res., 2008, 41, 805–816 CrossRef CAS PubMed.
- J. Lee, E. J. Jeong and J. Kim, Chem. Commun., 2011, 47, 358–360 RSC.
- A. C. Chen and S. Chatterjee, Chem. Soc. Rev., 2013, 42, 5425–5438 RSC.
- H. Zhu, S. X. Zhang, M. X. Li, Y. H. Shao and Z. W. Zhu, Chem. Commun., 2010, 46, 2259–2261 RSC.
- C. W. Liao, Y. R. Chen, J. L. Chang and J. M. Zen, J. Agric. Food Chem., 2011, 59, 9782–9787 CrossRef CAS PubMed.
- Q. A. Cao, H. Zhao, L. X. Zeng, J. Wang, R. Wang, X. H. Qiu and Y. J. He, Talanta, 2009, 80, 484–488 CrossRef CAS PubMed.
- T. K. Zhao, L. H. Liu, G. M. Li, A. L. Dang and T. H. Li, J. Electrochem. Soc., 2012, 159, 141–145 CrossRef PubMed.
- Q. A. Cao, H. Zhao, Y. J. He, N. Ding and J. A. Wang, Anal. Chim. Acta, 2010, 675, 24–28 CrossRef CAS PubMed.
- A. Saha, B. Roy, A. Garai and A. K. Nandi, Langmuir, 2009, 25, 8457–8461 CrossRef CAS.
- C. W. Liao, Y. R. Chen, J. L. Chang and J. M. Zen, Electroanalysis, 2011, 23, 573–576 CAS.
- K. S. Prasad, G. Muthuraman and J. M. Zen, Electrochem. Commun., 2008, 10, 559–563 CrossRef PubMed.
- J. L. Chang, K. H. Chang, C. C. Hu, W. L. Cheng and J. M. Zen, Electrochem. Commun., 2010, 12, 596–599 CrossRef CAS PubMed.
- L. M. Zhang, T. Li, P. Yu, T. Ohsaka and L. Q. Mao, Electrochem. Commun., 2013, 26, 89–92 CrossRef CAS PubMed.
- M. M. Richter, Chem. Rev., 2004, 104, 3003–3036 CrossRef CAS PubMed.
- X. B. Yin, S. Dong and E. Wang, TrAC, Trends Anal. Chem., 2004, 23, 432–441 CrossRef CAS.
- Z. Y. Guo, P. P. Gai, T. T. Hao, S. Wang, D. Y. Wei and N. Gan, Talanta, 2011, 83, 1736–1741 CrossRef CAS PubMed.
- F. Y. Liu, X. Yang and S. G. Sun, Analyst, 2011, 136, 374–378 RSC.
- Y. H. Wang, X. X. He, K. M. Wang, J. Su, Z. F. Chen and G. P. Yan, Chem. J. Chin. Univ., 2012, 33, 1162–1166 CAS.
- H. M. Cao, X. Q. Hu, C. Y. Hu, Y. Zhang and N. Q. Jia, Biosens. Bioelectron., 2013, 41, 911–915 CrossRef CAS PubMed.
- L. M. Zhou, J. S. Huang, L. Yang, L. B. Li and T. Y. You, Anal. Chim. Acta, 2014, 821, 57–63 CrossRef PubMed.
- S. A. Piletsky and A. P. F. Turner, Electroanalysis, 2002, 14, 317–323 CrossRef CAS.
- M. C. Blanco-López, M. J. Lobo-Castanón, A. J. Miranda-Ordieres and P. Tunón-Blanco, Trends Anal. Chem., 2004, 23, 36–48 CrossRef.
- R. N. Liang, R. M. Zhang and W. Qin, Sens. Actuators, B, 2009, 141, 544–550 CrossRef CAS PubMed.
- A. Pizzariello, M. Stred, S. Stred'anska and S. Miertus, Sens. Actuators, B, 2001, 76, 286–294 CrossRef CAS.
- J. P. Li, Z. Q. Chen and Y. P. Li, Anal. Chim. Acta, 2011, 706, 255–260 CrossRef CAS PubMed.
- P. Jin, B. Yu, S. Z. Yang and H. H. Ma, Microchim. Acta, 2011, 174, 265–271 CrossRef.
- L. özcan and Y. Sahin, Sens. Actuators, B, 2007, 127, 362–369 CrossRef PubMed.
- A. Gómez-Caballero, M. A. Goicolea and R. J. Barrio, Analyst, 2005, 130, 1012–1018 RSC.
- A. Gómez-Caballero, A. Ugarte, A. Sanchez-Ortega, N. Unceta, M. A. Goicolea and R. J. Barrio, J. Electroanal. Chem., 2010, 638, 246–253 CrossRef PubMed.
- Y. T. Liu, J. Deng, X. L. Xiao, L. Ding, Y. L. Yuan, H. Li, X. T. Li, X. N. Yan and L. L. Wang, Electrochim. Acta, 2011, 56, 4595–4602 CrossRef CAS PubMed.
- F. Xu, M. N. Gao, G. Y. Shi, L. Wang, W. Zhang, J. Xue, L. T. Jin and J. Y. Jin, Anal. Chim. Acta, 2001, 439, 239–246 CrossRef CAS.
- A. Benyoucef, F. Huerta, J. L. Vázquez and E. Morallon, Eur. Polym. J., 2005, 41, 843–852 CrossRef CAS PubMed.
- K. J. Huang, C. X. Xu, W. Z. Xie and W. Wang, Colloids Surf., B, 2009, 74, 167–171 CrossRef CAS PubMed.
- Y. Z. Zhang, J. Wang and M. L. Xu, Colloids Surf., B, 2010, 75, 179–185 CrossRef CAS PubMed.
- B. W. Wu, Z. H. Wang, D. X. Zhao and X. Q. Lu, Talanta, 2012, 101, 374–381 CrossRef CAS PubMed.
- G. L. Xu, H. L. Zhang, M. Zhong, T. T. Zhang, X. J. Lu and X. W. Kan, J. Electroanal. Chem., 2014, 713, 112–118 CrossRef CAS PubMed.
- S. Baskar, C. W. Liao, J. L. Chang and J. M. Zen, Electrochim. Acta, 2012, 88, 1–5 CrossRef PubMed.
- T. H. Tsai, S. Thiagarajan and S. M. Chen, J. Agric. Food Chem., 2010, 58, 4537–4544 CrossRef CAS PubMed.
- Ü. T. Yilmaz and Z. Yazar, Food Anal. Methods, 2012, 5, 119–125 CrossRef.
- Q. Xu, H. P. Wei, S. Du, H. B. Li, Z. P. Ji and X. Y. Hu, J. Agric. Food Chem., 2013, 61, 1810–1817 CrossRef CAS PubMed.
- M. Farré, R. Brix and D. Barceló, TrAC, Trends Anal. Chem., 2005, 6, 532–545 CrossRef PubMed.
- S. Rodriguez-Mozaz, M.-J. Lopez de Alda and D. Barceló, Anal. Bioanal. Chem., 2006, 386, 1025–1041 CrossRef CAS PubMed.
- A. D. Ellington and J. W. Szostak, Nature, 1990, 346, 818–822 CrossRef CAS PubMed.
- M. Citartan, S. C. B. Gopinath, J. Tominaga, S. C. Tan and T. H. Tang, Biosens. Bioelectron., 2012, 34, 1–11 CrossRef CAS PubMed.
- J. W. Liu, Z. H. Cao and Y. Lu, Chem. Rev., 2009, 109, 1948–1998 CrossRef CAS PubMed.
- S. Song, L. Wang, J. Li, J. Zhao and C. Fan, TrAC, Trends Anal. Chem., 2008, 27, 108–117 CrossRef CAS PubMed.
- D. L. Chen, Meena, S. K. Sharma and L. W. McLaughlin, J. Am. Chem. Soc., 2004, 126, 70–71 CrossRef CAS PubMed.
- W. J. Qi, D. Wu, J. Ling and C. Z. Huang, Chem. Commun., 2010, 46, 4893–4895 RSC.
- H. Huang, L. Li, G. H. Zhou, Z. H. Liu, Q. Ma, Y. Q. Feng, G. P. Zeng, P. Tinnefeldc and Z. K. He, Talanta, 2011, 85, 1013–1019 CrossRef CAS PubMed.
- W. Yun, H. Li, S. Q. Chen, D. W. Tu, W. Y. Xie and Y. Huang, Eur. Food Res. Technol., 2014, 238, 989–995 CrossRef CAS PubMed.
- H. B. Xing, S. S. Zhan, Y. G. Wu, L. He and P. Zhou, RSC Adv., 2013, 3, 17424–17430 RSC.
- Z. G. Chen, G. L. Liu, M. H. Chen, Y. R. Peng and M. Y. Wu, Anal. Biochem., 2009, 384, 337–342 CrossRef CAS PubMed.
- W. Cai, Y. Y. Fan, Z. L. Jiang and J. E. Yao, Talanta, 2010, 81, 1810–1815 CrossRef CAS PubMed.
- G. Q. Wen, A. H. Liang, Y. Y. Fan, Z. L. Jiang and C. N. Jiang, Plasmonics, 2010, 5, 1–6 CrossRef CAS.
- Z. L. Jiang, Y. Y. Fan, M. L. Chen, A. H. Liang, X. J. Liao, G. Q. Wen, X. C. Shen, X. C. He, H. C. Pang and H. S. Jiang, Anal. Chem., 2009, 81, 5439–5445 CrossRef CAS PubMed.
- A. H. Liang, L. P. Zhou and Z. L. Jiang, Plasmonics, 2011, 6, 387–392 CrossRef CAS.
- A. H. Liang, L. P. Zhou, H. M. Qin, Y. Zhang, H. X. Ouyang and Z. L. Jiang, J. Fluoresc., 2011, 21, 1907–1912 CrossRef CAS PubMed.
- M. Farré, L. Kantiani and D. Bàrceló, TrAC, Trends Anal. Chem., 2007, 26, 1100–1112 CrossRef PubMed.
- S. D. Gan and K. R. Pate, J. Invest. Dermatol., 2013, 133, 1–3 CrossRef PubMed.
- L. Asensio, I. González, T. Garcıía and R. Martín, Food Control, 2008, 19, 1–8 CrossRef CAS PubMed.
- H. Y. Zhang and S. Wang, J. Immunol. Methods, 2009, 350, 1–13 CrossRef CAS PubMed.
- H. Y. Zhang, S. Wang and G. Z. Fang, J. Immunol. Methods, 2011, 368, 1–23 CrossRef CAS PubMed.
- E. Watanabe, S. Miyake and Y. Yogo, J. Agric. Food Chem., 2013, 61, 12459–12472 CrossRef CAS PubMed.
- J. X. Liu, Y. B. Zhong, J. Liu, H. C. Zhang, J. Z. Xi and J. P. Wang, Food Control, 2010, 21, 1482–1487 CrossRef CAS PubMed.
- H. T. Lei, Y. D. Shen, L. J. Song, J. Y. Yang, O. P. Chevallier, S. A. Haughey, H. Wang, Y. M. Sun and C. T. Elliott, Anal. Chim. Acta, 2010, 665, 84–90 CrossRef CAS PubMed.
- H. T. Lei, R. Su, S. A. Haughey, Q. Wang, Z. L. Xu, J. Y. Yang, Y. D. Shen, H. Wang and Y. M. Sun, Molecules, 2011, 16, 5591–5603 CrossRef CAS PubMed.
- Y. Zhou, C. Y. Li, Y. S. Li, H. L. Ren, S. Y. Lu, X. L. Tian, Y. M. Hao, Y. Y. Zhang, Q. F. Shen, Z. S. Liu, X. M. Meng and J. H. Zhang, Food Chem., 2012, 135, 2681–2686 CrossRef CAS PubMed.
- B. Y. Cao, H. Yang, J. Song, H. F. Chang, S. Q. Li and A. P. Deng, Talanta, 2013, 116, 173–180 CrossRef CAS PubMed.
- K. Zhu, J. C. Li, Z. H. Wang, H. Y. Jiang, R. C. Beierc, F. Xu, J. Z. Shen and S. Y. Ding, Biosens. Bioelectron., 2011, 26, 2716–2719 CrossRef CAS PubMed.
- L. Trapiella-Alfonso, J. M. Costa-Fernandez, R. Pereiro and A. Sanz-Medel, Talanta, 2013, 106, 243–248 CrossRef CAS PubMed.
- B. B. Dzantiev, N. A. Byzova, A. E. Urusov and A. V. Zherdev, TrAC, Trends Anal. Chem., 2014, 55, 81–93 CrossRef CAS PubMed.
- X. M. Li, P. J. Luo, S. S. Tang, R. C. Beier, X. P. Wu, L. L. Yang, Y. W. Li and X. L. Xiao, J. Agric. Food Chem., 2011, 59, 6064–6070 CrossRef CAS PubMed.
- T. Le, P. F. Yan, J. Xu and Y. J. Hao, Food Chem., 2013, 138, 1610–1615 CrossRef CAS PubMed.
- Z. Z. Wang, D. J. Zhi, Y. Zhao, H. L. Zhang, X. Wang, Y. Ru and H. Y. Li, Int. J. Nanomed., 2014, 1699–1705 CrossRef PubMed.
- J. Homola, Chem. Rev., 2008, 108, 462–493 CrossRef CAS PubMed.
- K. V. Ragavan, N. K. Rastogi and M. S. Thakur, TrAC, Trends Anal. Chem., 2013, 52, 248–260 CrossRef CAS PubMed.
- T. L. Fodey, C. S. Thompson, I. M. Traynor, S. A. Haughey, D. G. Kennedy and S. R. H. Crooks, Anal. Chem., 2011, 83, 5012–5016 CrossRef CAS PubMed.
- H. N. Wua, H. Y. Li, F. Z. H. Chu, S. Fong and Y. Li, Sens. Actuators, B, 2013, 178, 541–546 CrossRef PubMed.
- Q. Wang, S. A. Haughey, Y. M. Sun, S. A. Eremin, Z. F. Li, H. Liu, Z. L. Xu, Y. D. Shen and H. T. Lei, Anal. Bioanal. Chem., 2011, 399, 2275–2284 CrossRef CAS PubMed.
- H. L. Guo, X. H. Zhou, Y. Zhang, B. D. Song, L. H. Liu, J. X. Zhang and H. C. Shi, Sens. Actuators, B, 2014, 194, 114–119 CrossRef CAS PubMed.
Footnote |
| † First two authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |