Facile synthesis of unsaturated polyester-based double-network gels via chemoselective cross-linking using Michael addition and subsequent UV-initiated radical polymerization†
Tang Tang and
Akinori Takasu*
Department of Frontier Materials, Graduate School of Engineering, Nagoya Institute of Technology, Gokiso-cho, Showa-ku, Nagoya 466-8555, Japan. E-mail: takasu.akinori@nitech.ac.jp; Fax: +81-52-735-5342; Tel: +81-52-735-7159
First published on 20th November 2014
Abstract
Michael addition and UV-initiated radical polymerization of vinyl monomers were used for a one-pot synthesis of polyester-based double-network (DN) gels by chemoselective cross-linking at room temperature. Unsaturated copolyesters containing itaconate and maleate units were prepared via ternary polycondensation of maleic anhydride and itaconic anhydride with 3-methyl-1,5-pentanediol under mild conditions. The addition of a diamine to a mixture of unsaturated copolyester, methyl methacrylate (MMA), and UV-initiator in toluene at room temperature yielded the first network via chemoselective cross-linking of maleate double bonds. This network, containing MMA, was next irradiated under UV-light for 3 h to obtain a polyester/poly(MMA) DN gel with high mechanical strength and shape recovery properties. A poly(ester-co-ether)/poly(acryl amide) DN hydrogel, which swelled with 70 wt% of water after immersion in water, was also synthesized by a similar procedure.
Introduction
Aliphatic polyesters, especially unsaturated ones, are well-known for their biodegradability and biocompatibility.1 They are employed in many fields such as coating and insulating materials,2 biomedical applications,3 and lightweight fiber-reinforced composites for the automotive industry.4 We have published a series of papers dealing with the synthesis of polyesters using rare-earth catalysts5 or Brϕnsted acids6 under mild conditions. However, enhancing their mechanical strength in order to meet the demands of further applications remains a challenge. This barrier was temporarily broken by the concept of interpenetrating polymer networks (IPNs) by Millar in 1960.7 IPNs, combining two or more polymer networks, are prepared as the strategy that at least one polymer cross-linked in the presence of the other network(s). They can be used for numerous applications due to their mechanical strength, thermal stability, and swelling property. Many papers, including reviews, have been published8 for the continuous development of IPNs. However, the related concept of double network (DN) gels has recently arisen, in which two interpenetrating networks such as poly(ethylene oxide)/polyacrylic acid DN gels9 or hydraluronan/poly(N,N-dimethylacrylamide) DN hydrogels,10 show improved mechanical properties and balance rigidity with toughness by controlling the structures of the macromolecules. Gong and her coworkers reported a series of modified DN hydrogels11 with excellent mechanical properties. However, multi-step procedures and tedious extractions have so far been required for preparing IPNs or DN gels. The synthesis of agar/poly(acrylamide) DN hydrogels12 was reported as a one-pot method, using agar as the first network. The fact that we cannot modify the structure of agar prompted us to explore facile strategies for preparing the main chain structure of the first network. Even though some papers referring to polyester-based IPNs have been reported,8d–g there is no report of a polyester-based DN gel under best of our survey. In this article, an unsaturated polyester (containing itaconate and maleate units)-based double-network (DN) gel was synthesized by the selective cross-linking of maleate units (endo-type double bonds) with diamines and subsequent UV initiated radical polymerization of vinyl monomers via the itaconate unit (exo-type double bonds).The first report of hetero-Michael addition reactions was published by Sokoloff and Latschinoff.13 The Michael and hetero-Michael addition reactions have been reported as the most promising bond forming strategies for carbon–carbon as well as carbon–heteroatom bonds in organic chemistry.14 In particular, the aza-Michael addition's great strength lies in its mild reaction conditions (stable in air or water), high functional group tolerance as well as high conversions and favorable reaction rates, in which amines can act as both nucleophiles and bases, no additional catalyst is typically needed. Thus, these commonly termed conjugate additions have gained much attention as strategies for N-containing polymer synthesis or modification of macromolecular architectures.15 In conjugate addition including the Michael addition, only a few examples concerning the influence of the double-bond geometry are reported,16 in which they observed that there is an isomerization of the double in favor of the thermodynamically more stable (E) substrate since, in reactions that do not go to completion.16d As an exception, internal cyclo-Michael addition of methyl 2-amino-2-octenate is reported, in which trans-isomer (86% yield) was more reactive than cis-isomer (73% yield) because latter has not chair-like transition state but boat-like transition state.16e Recently, it was also found that in comparison to other common aza-Michael acceptors, dimethyl maleate, maleic acid dimethyl ester, is a high versatile Michael acceptor, having two electron withdrawing groups in α- and β-positions.17 It is more reactive than acceptors with terminal double bonds, such as methyl acrylate and acrylonitrile, which are considered to be some of the most reactive Michael acceptors. On the other hand, dimethyl maleate is also highly selective towards the mono-adduct in contrast to these acceptors.17
On the other hand, Coates and colleagues converted the maleate polyester to fumarate polyester, prepared by ring-opening polymerization of maleic anhydride (MAn) with epoxides, via a diethylamine (HNEt2)-catalyzed cis–trans isomerization reaction.18 We also used this method to demonstrate the isomerization in unsaturated polyester gels prepared via ring-opening ternary polycondensation of MAn, itaconic anhydride (IAn), and 3-methyl-1,5-pentanediol (MPD).6d In this paper, we unexpectedly found that the aza-Michael reactions of fumarate or itaconate double bonds in the unsaturated polyester with primary amines did not proceed at room temperature, nor was isomerization of these double bonds observed, even though dimethyl fumarate (neat)17 and dimethyl itaconate (in methanol)19 react with aliphatic primary amine at room temperature to afford the mono-adduct. Furthermore, we investigated the UV initiated cross-linking of itaconate units in the presence of methyl methacrylate (MMA). The itaconate polyester was cross-linked by MMA, however, the fumarate and maleate polyesters were not. These two unexpected chemoselectivities prompted us to challenge the one-pot preparation of unsaturated polyester-based DN gels by chemoselective cross-linking of maleate and itaconate units, sequentially, using aza-Michael addition and radical polymerization.
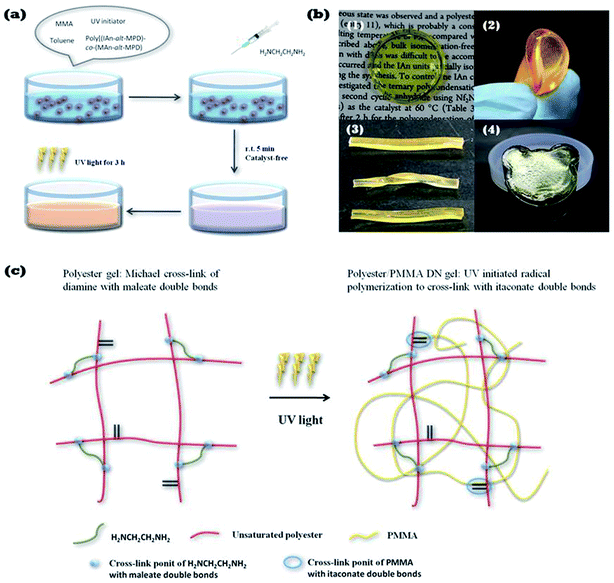
In this article, first, after poly[(IAn-alt-MPD)-co-(MAn-alt-MPD)], MMA, and a UV initiator were mixed in toluene, 1,2-ethanediamine was added to the mixture to form the first network. For the second step, the gel was cured by UV light to polymerize the MMA and cross-link the itaconate double bonds. We compared the mechanical strength of the polyester/poly(MMA) DN gel with the parent single network (SN) gels, i.e., poly[(IAn-alt-MPD)-co-(MAn-alt-MPD)] cross-linked by H2NCH2CH2NH2 or poly[(IAn-alt-MPD)-co-(MAn-alt-MPD)] cross-linked by MMA].
Experimental section
Materials
Maleic anhydride (MAn), dimethyl maleate, itaconic anhydride (IAn) were purchased from Tokyo Chemical Industry Co., Ltd (Tokyo, Japan). 1,4-Dioxane, tetrahydrofuran (THF), dimethylformamide (DMF), toluene and chloroform were purchased from Nacalai Tesque (Kyoto, Japan), and were distilled before use. Bis(nonafluorobutanesulfonyl)imide (Nf2NH) was purchased from Mitsubishi Materials Electronic Chemicals Co., Ltd. (Akita, Japan), and was dried under reduced pressure before use. MPD, triethylene glycol (TEG), MMA, acrylamide (AM), diethylamine (Et2NH), 1,2-ethanediamine, 1,4-butanediamine, n-butylamine, isobutylamine, and benzylamine were purchased from Nacalai Tesque (Kyoto, Japan). 1-Hydroxycyclohexyl phenyl ketone (Irgacure 184) was purchased from Tokyo Chemical Industry Co., Ltd (Tokyo, Japan).Measurements
1H-NMR spectra were recorded at 27 °C using a Bruker Analytik DPX200 spectrometer (200 MHz for 1H). 13C-NMR spectra were recorded at 27 °C using a Bruker Analytik DRX600 spectrometer (150 MHz for 13C). Number average molecular weight (Mn) and polydispersity index (Mw/Mn) of products were determined using size-exclusion chromatography (SEC) calibrated with polystyrene standards. The chromatographic system included a RI detector (Tosoh RI-8020), a Tosoh DP8020 pump system, and a TSK gel Super Multipore HZ-M column. Chromatography was performed using chloroform as the eluent. The flow rate was 0.35 mL min−1, and the temperature was maintained at 40 °C. FT-IR spectra were recorded in KBr disks using a JASCO FT/IR-430 spectrometer. Differential scanning calorimetry (DSC), using a DSC6220S calorimeter (Seiko Instruments Inc., Chiba, Japan), was investigated from −80 to 120 °C, with the temperature increased or decreased at a rate of 10 °C min−1. The instrument was calibrated using indium and tin samples. For all samples, a complete temperature cycle from −80 to 120 °C and back to −80 °C was obtained. Each sample weighted between 6 to 10 mg and was placed into an aluminum pan that was covered with a lid within the calorimeter. The glass transition temperature (Tg) was taken as the inflection point of the corresponding heat capacity jump of the DSC trace. The compressive properties and tensile strengths of products were investigated by a universal testing machine (SHIMADZU, AGS-G). Cuboidal samples with a height of 3 mm, a length of 10 mm, and width of 10 mm were used for compression test. The compression rate was 0.1 mm min−1. For tensile strengths, the gel samples were cut into stripe shapes with a gauge length of ∼15 mm, a width of ∼5 mm and a thickness of ∼1 mm. The loading rate was 0.1 mm min−1 for single gels and 1 mm min−1 for DN gels.Preparation of unsaturated polyesters6b,d
A typical procedure for ternary polycondensation under bulk conditions at low temperature was as follows (Table 1, entry 1). Nf2NH (23.36 mg, 0.04 mmol), MAn (784 mg, 8.00 mmol), and MPD (945 mg, 8.00 mmol) were mixed. This mixture was stirred at 60 °C for 1 h. The pressure was gradually decreased to 0.3–3 mm Hg keeping at 60 °C for 12 h. The products were dissolved in CHCl3 and then precipitated into an excess amount of n-hexane. The precipitated polymers were then dried under reduced pressure (82%, yield). The structure was confirmed by 1H NMR (Fig. S1†).| Entrya | Cyclic anhydride ([M1]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [M2]0) [M2]0) |
Diol | Catalystb (mol%) | Inhibitorc (mol%) | Temp. (°C) | Yieldd (%) | Mne × 10−3 | Mw/Mne |
|---|---|---|---|---|---|---|---|---|
| a Polycondensations were carried out for 12 h. In entries 2–5, the time needed for homogeneous state was 10–12 h.b Catalyst ratio (mol% relative to total monomers).c Radical inhibitor (mol% relative to cyclic anhydrides).d Yields were calculated after purification by reprecipitation.e Determined by SEC. The column had been calibrated with poly(styrene)s. | ||||||||
| 1 | MAn | MPD | 0.25 | None | 60 | 82 | 12.8 | 2.3 |
| 2 | IAn | MPD | 1 | 0.1 | 80 | 81 | 3.0 | 2.4 |
| 3 | IAn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MAn (1 MAn (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
MPD | 1 | 0.1 | 80 | 79 | 10.1 | 2.3 |
| 4 | IAn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MAn (1 MAn (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) 8) |
MPD | 1 | 0.1 | 80 | 81 | 13.0 | 2.3 |
| 5 | IAn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MAn (1 MAn (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) 6) |
TEG | 1 | 0.1 | 60 | 83 | 4.7 | 1.9 |
Isomerization of poly(MAn-alt-MPD) to poly[fumaric acid (FA)-alt-MPD]
To investigate its isomerization, maleate polyester, poly(MAn-alt-MPD), was dissolved in DMF, dioxane, CHCl3, THF, or toluene, and then 1% (vol/vol) HNEt2 (0.2 mol/mol relative to maleate units) was added. The mixtures were stirred at room temperature for 12 h and the isomerization monitored by 1H NMR spectroscopy. After the isomerization was complete, all volatiles were removed under vacuum. The polyester was dissolved in chloroform and precipitated into n-hexane and methanol, sequentially. The structure of fumarate polyester, was confirmed by 1H NMR (Fig. S2†).Reactions of poly(MAn-alt-MPD) with primary amines in different solvents at room temperature
The poly(MAn-alt-MPD) was dissolved in DMF, dioxane, CHCl3, THF, or toluene (polymer concentration: 0.15 g mL−1). The primary amine (n-butylamine, isobutylamine, or benzylamine), was added to the solution at a ratio of 1/1 ([maleate unit]0/[amine]0, mol/mol) and reacted at room temperature with stirring. After the reaction was completed, the product was dissolved in chloroform and precipitated into n-hexane and methanol, subsequently.Preparation of single gel via cross-linking of poly(MAn-alt-MPD) with 1,2-ethanediamine in different solvents
The poly(MAn-alt-MPD) was dissolved in DMF, dioxane, CHCl3, THF, or toluene (polymer concentration: 0.3 g mL−1). 1,2-Ethanediamine was added to the solution at room temperature, at a ratio of 1/4 ([diamine]0/[maleate unit]0, mol/mol). The reaction was allowed go to completion yielding the gel (Scheme 1). | ||
| Scheme 1 Cis–trans isomerization of carbon–carbon double bonds by secondary amine and Michael addition of primary amines with poly(MAn-alt-MPD). | ||
One-pot preparation of unsaturated polyester-based double-network gels
The unsaturated copolyester, poly[(IAn-alt-MPD)-co-(MAn-alt-MPD)], with a unit ratio of [IAn]0/[MAn]0/[MPD]0 = 1/4/5 (mol/mol/mol) was dissolved with MMA ([IAn]0/[MMA]0 = 1/10, mol/mol) and photoinitiator Irgacure 184 (4 wt% relative to polyester and MMA) in toluene [polymer concentration: 0.3 g mL−1]. Then 1,2-ethanediamine (feed ratio is ca. 1/4, [diamine]0/[maleate unit]0, mol/mol) was added to the mixture at room temperature. The first network was prepared after 5 min, and the gel was then placed under 250–400 nm UV light for 3 h to give the DN gel. DN gels with unit ratios of [IAn]0/[MAn]0/[MPD]0 = 1/8/9 ([IAn]0/[MMA]0 = 1/18) and [IAn]0/[MAn]0/[MPD]0 = 1/8/9 ([IAn]0/[MMA]0 = 1/27) were also prepared by same procedure.One-pot preparation of unsaturated polyester-based double-network hydrogel and swelling property in water
The copolyester, poly[(IAn-alt-TEG)-co-(MAn-alt-TEG)], with a unit ratio of [IAn]0/[MAn]0/[TEG]0 = 1/4/5 (mol/mol/mol) was dissolved with AM (IAn]0/[AM]0 = 1/2, mol/mol) and photoinitiator Irgacure 184 (4 wt% relative to polyester and AM) in THF [polyester concentration: 0.3 g mL−1]. Then 1,4-butanediamine ([diamine]0/[maleate unit]0 = 1/4, mol/mol) was added to the mixture at room temperature. The first network was prepared within 5 min, and the gel was then placed under 250–400 nm UV light for 3 h. The prepared DN hydrogel was immersed in water for 1 day after which a swelling test in water was carried out. The weight of dried DN hydrogel was 85 mg, and after immersion in water for 1 day, the weight of DN hydrogel swollen with water was 290 mg. The water content was calculated to be 70 wt%.Model reaction
Dimethyl 2-(butylamino)butanedioate was prepared by Michael addition of dimethyl maleate (0.40 mmol) with n-butylamine (0.40 mmol) at room temperature without catalyst. The expected product was obtained with high yield (>98%). Prepared dimethyl 2-(butylamino)butanedioate was mixed with dimethyl maleate at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (mol/mol). Chloroform was used as solvent (0.3 g mL−1). The reaction was carried out at room temperature for 24 h. The degree of isomerization calculated by 1H NMR measurement was trace (<4%).
1 (mol/mol). Chloroform was used as solvent (0.3 g mL−1). The reaction was carried out at room temperature for 24 h. The degree of isomerization calculated by 1H NMR measurement was trace (<4%).
Results and discussion
Preparation of unsaturated polyesters under mild conditions
We have already reported the synthesis of unsaturated polyesters and copolyesters containing maleate and itaconate units (carbon–carbon double bonds) using Nf2NH as catalyst via ring-opening polycondensation of the cyclic anhydrides with diols in the presence of a radical inhibitor.6d Herein, we also used this method to prepare poly(MAn-alt-MPD) (Fig. S1†), poly(IAn-alt-MPD) (Fig. S3†), poly[(IAn-alt-MPD)-co-(MAn-alt-MPD)] (Fig. S4†), and poly[(IAn-alt-TEG)-co-(MAn-alt-TEG)] (Fig. S5†) under mild conditions (See Fig. 1 and Table 1).Isomerization of poly(MAn-alt-MPD) in some organic solvents
As reported by Coates,18 isomerization of maleate to fumarate units in the unsaturated polyesters, prepared via ring-opening polymerization of MAn with epoxides, was catalyzed by HNEt2 in CHCl3. To synthesize fumarate polyester, we also reported isomerization-free polycondensation of MAn and MPD, and then carried out HNEt2-catalyzed cis–trans isomerization of the maleate polyester units in CHCl3.6d Herein we continued to investigate the effect of solvent polarity on the isomerization. DMF, 1,4-dioxane, CHCl3, THF, and toluene were selected, and HNEt2-catalyzed cis–trans isomerization of maleate polyester was carried out in each of these solvents. The results are summarized in Table 2 and indicated that the degree of isomerization increased with polarity of the solvents. As shown in entry 1, the isomerization was completed within 12 h in DMF. Thus the rate of isomerization was highest in DMF (Table 2). In the 1H NMR spectrum, after reaction with diethylamine (1 vol% to solvent) in DMF, the signal at 6.20–6.22 ppm of the cis-alkene disappeared completely, and instead a new signal ascribed to the trans-alkene was observed at 6.82–6.84 ppm (Fig. S2†). The Mn hardly differed before and after the isomerization [before isomerization, Mn = 12.8 kDa, Mw/Mn = 2.3; after isomerization, Mn = 12.3 kDa, Mw/Mn = 2.3, Fig. S6†]. The mechanism of this reaction was reported in previous papers20 and we supported the mechanism shown in Fig. S7.†| Entrya | Solvent | Polarity indexb | Time | Conversion |
|---|---|---|---|---|
| a All the entries were catalyzed by 1% (v/v) Et2NH (0.2 mol/mol relative to maleate carbon–carbon double bond units).b The polarity index is a measure of the relative polarity of a solvent and is useful for identifying suitable mobile phase solvents. The polarity index increases with polarity.21 | ||||
| 1 | DMF | 6.4 | 12 | 100 |
| 2 | 1,4-Dioxane | 4.8 | 12 | 73 |
| 3 | CHCl3 | 4.1 | 12 | 66 |
| 4 | THF | 4.0 | 12 | 64 |
| 5 | Toluene | 2.4 | 12 | 46 |
Michael additions of carbon–carbon double bonds in unsaturated polyesters with primary amine at room temperature
When we used fumarate (Fig. S8†) and itaconate polyesters (Fig. S9†), Michael addition with n-butylamine did not occur within 20 min at room temperature (entries 1 and 2 in Table 3). Entries 3–9 in Table 3 summarise the reactions of maleate polyester with n-butylamine (Fig. S10†), isobutylamine (Fig. S11†) or benzylamine (Fig. S12†) in DMF, 1,4-dioxane, CHCl3, THF or toluene respectively. The 1H-NMR spectra of these reactions revealed that the maleate double bonds are versatile for chemoselective aza-Michael addition to afford polyesters having pendent imino groups and simultaneously, and fumarate units produced via isomerization of the maleate units remained (unreacted). The ratios of double bonds participating in Michael addition and isomerization were calculated from 1H-NMR spectra. From entries 3–7, it is concluded that the isomerizations and Michael additions were faster in DMF than in other solvents. In toluene (run 6), because of the low degree of isomerization as shown in Table 1, the Michael reaction predominated compared with in other solvents. The low degree of isomerization agreed with the results of isomerization catalyzed by secondary amine (HNEt2) shown in Table 2. Isobutylamine (run 8) and benzylamine (run 9) showed similar tendency with n-butylamine.| Entry | Solventa | Polyester | Amineb | Time (min) | Addition![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isomerization isomerization![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) unreactedc (% unreactedc (%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) % %![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) %) %) |
|---|---|---|---|---|---|
a Solvent concentration: 0.15 g mL−1, polymer weight/solvent volume.b The feed ratio is about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (maleate unit 1 (maleate unit![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) amine, mol/mol).c The ratio of conversion was calculated by 1H NMR spectra. amine, mol/mol).c The ratio of conversion was calculated by 1H NMR spectra. |
|||||
| 1 | CHCl3 | Poly(FA-alt-MPD) | n-Butylamine | 20 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
| 2 | CHCl3 | Poly(IAn-alt-MPD) | n-Butylamine | 20 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
| 3 | CHCl3 | Poly(MAn-alt-MPD) | n-Butylamine | 20 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 44 |
| 4 | 1,4-Dioxane | Poly(MAn-alt-MPD) | n-Butylamine | 20 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13 |
| 5 | THF | Poly(MAn-alt-MPD) | n-Butylamine | 20 | 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
| 6 | Toluene | Poly(MAn-alt-MPD) | n-Butylamine | 20 | 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
| 7 | DMF | Poly(MAn-alt-MPD) | n-Butylamine | 5 | 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 39 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
| 15 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
||||
| 8 | DMF | Poly(MAn-alt-MPD) | Isobutylamine | 20 | 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 30 | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
||||
| 9 | DMF | Poly(MAn-alt-MPD) | Benzylamine | 20 | 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
| 50 | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
||||
We inferred herein two possible reaction routes for this phenomenon (Scheme 2). Route 1 is via the secondary amine group, created by Michael addition of maleate double bonds with primary amines, catalyzing isomerization of maleate double bonds to fumarate double bonds. We therefore prepared the model reaction shown in Scheme 3. Dimethyl 2-(butylamino)butanedioate was synthesized (Fig. S13†) and used as catalyst for isomerization of dimethyl maleate in CHCl3 under the same conditions as entry 3, Table 3. Even after 24 h, the degree of isomerization was only 4%, determined from the 1H NMR data (Fig. S14†) compared with 66% for entry 3 in Table 2 using Et2NH (in CHCl3). From these results, we can eliminate route 1. In route 2, Michael addition and isomerization by the primary amine occur simultaneously and the trans double bonds in the polyester remain unreacted.
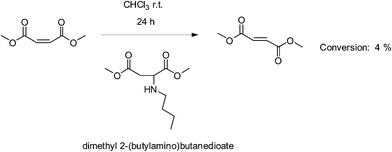 | ||
| Scheme 3 Isomerization of dimethyl fumarate catalyzed by dimethyl 2-(butylamino)butanedioate as the model compound. | ||
One-pot preparation of polyester/poly(MMA) double-network gels via chemoselective Michael addition and UV promoted cross-linking at room temperature
As described above, we found that primary amines react chemoselectively with maleate units via Michael reaction in the presence of fumarate and itaconate units of unsaturated copolyester. As a proof of concept, poly(MAn-alt-MPD), poly(FA-alt-MPD), and poly(IAn-alt-MPD) were used as unsaturated polyesters leading to gelation using 1,2-ethanediamine as cross-linker at room temperature (0.25 mol% relative to maleate double bonds). As shown in Fig. 2, after 5 min, the maleate polyester, poly(MAn-alt-MPD), both before and after reprecipitation, was quickly cross-linked by 1,2-ethanediamine to give a gel, confirming that cross-linking occurred even in the presence of the polycondensation catalyst residue. On the contrary, when we used the fumarate polyester [poly(FA-alt-MPD)] and itaconate polyester [poly(IAn-alt-MPD)], gelation did not occur. These results prompted us to design of polyester-based gel by selective cross-linking of maleate in the presence of fumarate and itaconate units. DMF, 1,4-dioxane, chloroform, THF and toluene were used as solvents respectively (Table 4). The cross-linking in DMF was faster than in other solvents, because the high polarity of solvent increases the reaction rate of the Michael addition. Initially, protic solvents were desirable in the carbon-Michael reaction to promote rapid proton transfer and to stabilize charged intermediates, however, Schlessinger's group has shown that high yielding reactions were also achieved using aprotic solvents.22 | ||
| Fig. 2 Selective cross-linking of maleate, fumarate, and itaconate-based unsaturated polyesters with 1,2-ethanediamine. | ||
The FT-IR spectra of the mixtures before and after cross-linking are shown in Fig. S15,† in which the peaks at 1377–1408 cm−1 and 1261–1298 cm−1 are ascribed to cis and trans double bonds, respectively. During the gelation, cis double bonds were partially transformed to trans, which indicated isomerization induced by 1,2-ethanediamine.
Subsequently, UV induced cross-linking of the three kinds of carbon–carbon double bonds were investigated using poly(MAn-alt-MPD), poly(FA-alt-MPD), and poly(IAn-alt-MPD). We irradiated each polyester under 250–400 nm UV light with MMA and photoinitiator Irgacure 184 (Fig. 3). After 30 min, the poly(IAn-alt-MPD) had gelled, however, poly(MAn-alt-MPD) and poly(FA-alt-MPD) had not, indicating chemoselective cross-linking by the UV-initiated radical polymerization of MMA (in CHCl3). Poly[(IAn-alt-MPD)-co-(MAn-alt-MPD)] with different unit ratios ([IAn]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [MAn]0 = 1
[MAn]0 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, [IAn]0
4, [IAn]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [MAn]0 = 1
[MAn]0 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) were also irradiated in UV light in the presence of MMA and Irgacure 184, and after 1.5 h, it was confirmed that both of the polyesters were cross-linked.
8) were also irradiated in UV light in the presence of MMA and Irgacure 184, and after 1.5 h, it was confirmed that both of the polyesters were cross-linked.
 | ||
| Fig. 3 Selective cross-linking of different double bonds with MMA under UV light for preparation of polyester-based DN gel. | ||
Based on these results, a new one-pot procedure to prepare double-network gels was developed via chemoselective cross-linking. As shown in Fig. 4(a), poly[(IAn-alt-MPD)-co-(MAn-alt-MPD)], MMA, and photoinitiator Irgacure 184 were mixed in toluene. After the mixture was completely dissolved, an appropriate amount of 1,2-ethanediamine was added to the solution. The first network was prepared after 5 min, followed by irradiation under 250–400 nm UV light for 3 h to yield the DN gel. The structure of the DN gel is represented in Fig. 4(c). The first network was synthesized from selective cross-linking of 1,2-ethanediamine with maleate double bonds at room temperature without catalyst. After that, a second network of free radical polymerized poly(MMA) was synthesized by irradiation with UV light, which interpenetrated into the first network via the itaconate double bonds. The polyester/PMMA double-network gel [Fig. 4(b)-(1)] exhibited excellent mechanical properties. It was tough and withstood high-levels of deformation by bending [Fig. 4(b)-(2)] and twisting [Fig. 4(b)-(3)]. Particularly, once the deformation force was removed, the DN gel recovered its initial shape [Fig. 4(b)-(3)]. Furthermore, the DN gel could be processed in a desired shape because of the one-pot method (Fig. 4(b)-(4), bear head).
To compare mechanical strength, the single network gel, SN gel 1, [poly(MAn-alt-MPD) cross-linked by 1,2-ethanediamine], SN gel 2 (poly[(MAn-alt-MPD)-co-(IAn-alt-MPD)] ([IAn]0/[MAn]0 = 1/8) cross-linked by MMA, [IAn]0/[MMA]0 = 1/27, mol/mol), SN gel 3 [poly[(MAn-alt-MPD)-co-(IAn-alt-MPD)] ([IAn]0/[MAn]0 = 1/8) cross-linked by MMA, [IAn]0/[MMA]0 = 1/18, mol/mol], and three polyester/PMMA DN gels with different MMA ratios [[IAn]0/[MMA]0 = 1/18 (DN gel 1), [IAn]0/[MMA]0 = 1/10 (DN gels 2) or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 (DN gel 3) mol/mol] and different double bond unit ratios [IAn]0/[MAn]0 = 1/4 (DN gel 2), or 1
27 (DN gel 3) mol/mol] and different double bond unit ratios [IAn]0/[MAn]0 = 1/4 (DN gel 2), or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (DN gels 1 and 3), mol/mol] were prepared. The structures are summarized in Table 5. The compression properties of the SN and DN gels are depicted in Fig. 5(1). The SN gel 1 was easily broken by compression [Fig. 5(1)], and its compressive strain and compressive stress (at break) were about 30% and 4.98 MPa [red line in Fig. 5(2)]. The SN gel 2 [green line in Fig. 5(1)], cross-linked by MMA, exhibited a higher compressive strain (77%) and compressive stress (at break) (21.1 MPa) than SN gel 1. The DN gel 1, polyester/poly(MMA) DN gel ([IAn]0/[MMA]0 = 1/18, mol/mol; [IAn]0/[MAn]0 = 1/8, mol/mol) exhibited more robust mechanical properties [yellow line in Fig. 5(1)], however, we observed a significant change in the curve after a compressive strain of 36% and compressive stress (at break) of 21.3 MPa, indicating collapse under compression. After we increased the cross-linking density of the DN gel, by changing the [IAn]0/[MAn]0 ratio from 1/8 to 1/4 (mol/mol), and the ratio of [IAn]0/[MMA]0 = 1/10, mol/mol (DN gel 2), the destruction of DN gel 2 under compression was less obvious [purple line in Fig. 5(1)]. In the stress–strain curve we observed DN gel 2 collapse at a compression strain of 42% and compressive stress (at break) of 33.4 MPa. This indicated that the DN gel 2 exhibited higher mechanical strength than DN gel 1 because of its higher cross-linking density. In order to prepare a much stronger DN gel, we increased the ratio of MMA (the [IAn]0/[MMA]0 was increased from 1
8 (DN gels 1 and 3), mol/mol] were prepared. The structures are summarized in Table 5. The compression properties of the SN and DN gels are depicted in Fig. 5(1). The SN gel 1 was easily broken by compression [Fig. 5(1)], and its compressive strain and compressive stress (at break) were about 30% and 4.98 MPa [red line in Fig. 5(2)]. The SN gel 2 [green line in Fig. 5(1)], cross-linked by MMA, exhibited a higher compressive strain (77%) and compressive stress (at break) (21.1 MPa) than SN gel 1. The DN gel 1, polyester/poly(MMA) DN gel ([IAn]0/[MMA]0 = 1/18, mol/mol; [IAn]0/[MAn]0 = 1/8, mol/mol) exhibited more robust mechanical properties [yellow line in Fig. 5(1)], however, we observed a significant change in the curve after a compressive strain of 36% and compressive stress (at break) of 21.3 MPa, indicating collapse under compression. After we increased the cross-linking density of the DN gel, by changing the [IAn]0/[MAn]0 ratio from 1/8 to 1/4 (mol/mol), and the ratio of [IAn]0/[MMA]0 = 1/10, mol/mol (DN gel 2), the destruction of DN gel 2 under compression was less obvious [purple line in Fig. 5(1)]. In the stress–strain curve we observed DN gel 2 collapse at a compression strain of 42% and compressive stress (at break) of 33.4 MPa. This indicated that the DN gel 2 exhibited higher mechanical strength than DN gel 1 because of its higher cross-linking density. In order to prepare a much stronger DN gel, we increased the ratio of MMA (the [IAn]0/[MMA]0 was increased from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 to 1
18 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27, mol/mol) [DN gel 3, blue line in Fig. 5(1)]. The DN gel 3 exhibited the expected mechanical properties. Even with the compressive stress up to 41.8 MPa, the DN gel 3 still exhibited a smooth increase in stress in the diagram (blue line in Fig. 5), and the compressive strain was about 51%. Interestingly, DN gel 3 is softer than DN gel 1 and DN gel 2, but the compression resulting in collapse was higher than those of DN gel 1 and DN gel 2. This may be due to the higher poly(MMA) content of the second network, meaning the DN gel 3 has longer spacers between the polymer chains in the gel. At first, the gel is easily compressed, however, after compression, due to its high poly(MMA) content, the DN gel 3 showed the highest mechanical strength. When the spacer was shorter (DN gel 1 or 2), the gels collapsed at lower compressive strain and compressive stress. Fig. 5(3) shows DN gel 3 before and after compression. After removing the stress, the shape of the DN gel was obviously changed. However, after 12 h, the shape looks to be recovered (>99%).
27, mol/mol) [DN gel 3, blue line in Fig. 5(1)]. The DN gel 3 exhibited the expected mechanical properties. Even with the compressive stress up to 41.8 MPa, the DN gel 3 still exhibited a smooth increase in stress in the diagram (blue line in Fig. 5), and the compressive strain was about 51%. Interestingly, DN gel 3 is softer than DN gel 1 and DN gel 2, but the compression resulting in collapse was higher than those of DN gel 1 and DN gel 2. This may be due to the higher poly(MMA) content of the second network, meaning the DN gel 3 has longer spacers between the polymer chains in the gel. At first, the gel is easily compressed, however, after compression, due to its high poly(MMA) content, the DN gel 3 showed the highest mechanical strength. When the spacer was shorter (DN gel 1 or 2), the gels collapsed at lower compressive strain and compressive stress. Fig. 5(3) shows DN gel 3 before and after compression. After removing the stress, the shape of the DN gel was obviously changed. However, after 12 h, the shape looks to be recovered (>99%).
| Entrya | Gel | Cross-linker for MAn unitsb | [IAn]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [MAn]0 [MAn]0 |
[IAn]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [MMA]0 [MMA]0 |
|---|---|---|---|---|
| a The polyester of entry 1 was poly(MAn-alt-MPD). The polyesters of other entries were poly[(MAn-alt-MPD)-co-(IAn-alt-MPD)].b Cross-linker for MAn units via aza-Michael addition. | ||||
| 1 | SN gel 1 | 1,2-Ethanediamine | — | — |
| 2 | SN gel 2 | None | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 27 |
| 3 | SN gel 3 | None | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
| 4 | DN gel 1 | 1,2-Ethanediamine | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
| 5 | DN gel 2 | 1,2-Ethanediamine | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| 6 | DN gel 3 | 1,2-Ethanediamine | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 27 |
As shown in Fig. 6(1), the DN gel 1 (yellow line) also showed much higher tensile strength than single gels. The SN gel 1 is the weakest (red line). Even the SN gel 3[poly[(MAn-alt-MPD)-co-(IAn-alt-MPD)] ([IAn]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [MAn]0 = 1
[MAn]0 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) cross-linked by MMA, [IAn]0/[MMA]0 = 1/18, mol/mol] (black line) exhibited lower tensile strength than DN gel 1 but showed much greater elongation. However, the DN gel 1 has the highest stress as well as strain.
8) cross-linked by MMA, [IAn]0/[MMA]0 = 1/18, mol/mol] (black line) exhibited lower tensile strength than DN gel 1 but showed much greater elongation. However, the DN gel 1 has the highest stress as well as strain.
 | ||
| Fig. 6 (1) Tensile strengths of DN gel 1, SN gel 1, SN gel 3. (2) DSC measurements of SN gel 1, SN gel 3 and DN gel 1. | ||
As to the thermal properties, the Tg of SN gel 1 (cross-linked diamine) was −23 °C. However the Tg of SN gel 3 (−7 °C) (cross-linked by MMA under UV-light) exhibited a higher glass transition temperature than SN gel 1, which is probably due to the miscibilities of the poly(MMA) segments. The Tg of the DN gel 1 was detected at −10 °C which was similar to that of SN gel 3. It seems that the poly(MMA) chain significantly affects the Tg in the DN gel. From these results, we concluded that a polyester-based DN gel with excellent mechanical properties was obtained, in which the poly(MMA) content influences both its thermal as well as mechanical properties. Based on these results, we expect to be able to produce a wide range of DN gels prepared by a facile procedure.
Conclusion
In this paper, we first investigated the isomerization of maleate to fumarate units in unsaturated polyesters by secondary amines in a variety of organic solvents. The isomerization in DMF was the most efficient. When primary amines were used to react with maleate polyester at room temperature, the Michael addition of the amines with maleate double bonds proceeded rapidly and, simultaneously, maleate double bonds partially isomerized to fumarate double bonds. Interestingly, the Micheal addition of fumarate or itaconate double bonds with primary amines were not observed at room temperature. We also investigated the UV cross-linking of unsaturated polyester by MMA in the presence of UV initiator. Itaconate polyester was cross-linked by MMA, but, the fumarate and maleate polyesters not. These results enables us to develop a one-pot preparation of DN gels by chemoselective cross-linking. The polyester/poly(MMA) DN gel showed much more robust mechanical properties, compared with the parent SN gels. We believe that this new facile strategy with the advantages of one-pot, low temperature, simple procedure, structural diversity and tolerance of air and water, can have widespread applications in various fields.Acknowledgements
The authors acknowledge the double-degree project of Nagoya Institute of Technology and Beijing University of Chemical Technology. T.T. is grateful for the scholarship provided by China Scholarship Council (CSC). A.T. was funded by the Ministry of Education, Science, and Culture of Japan (Grant-in-Aid for Development Scientific Research, no. 24550132). The authors are grateful to Dr Tadashi Hayakawa (Henkel Japan Ltd) for valuable financial support and fruitful discussions.References and Notes
- (a) O. Dechy-Cabaret, B. Martin-Vaca and D. Bourissou, Chem. Rev., 2004, 104, 6147–6176 CrossRef CAS PubMed; (b) H. M. Muller and D. Seebach, Angew. Chem., Int. Ed. Engl., 1993, 32, 477–502 CrossRef; (c) O. Coulembier, P. Degee, P. J. L. Hedrick and P. Dubois, Prog. Polym. Sci., 2006, 31, 723–747 CrossRef CAS PubMed.
- (a) M. Malik, V. Choudhary and I. K. Varma, J. Macromol. Sci., Rev. Macromol. Chem. Phys., 2000, 40, 139–165 CrossRef PubMed; (b) M. Andreis, Z. Meic and Z. Veksli, Polymer, 1983, 24, 2611–2616 CrossRef.
- (a) R. A. Hakala, H. Korhonen, V. V. Meretoja and J. V. Seppala, Biomacromolecules, 2011, 12, 2806–2814 CrossRef CAS PubMed; (b) B. C. Guo, Y. W. Chen, Y. D. Lei, L. Q. Zhang, W. Y. Zhou, A. B. M. Rabie and J. Q. Zhao, Biomacromolecules, 2011, 12, 1312–1321 CrossRef CAS PubMed; (c) L. Cai and S. Wang, Biomaterials, 2010, 31, 7423–7434 CrossRef CAS PubMed.
- J. M. Margolis, Advanced Thermoset Composites, Industrial and Commercial Application, Van Nostrand Reinhold, New York, 1986 Search PubMed.
- (a) A. Takasu, Y. Oishi, Y. Iio, Y. Inai and T. Hirabayashi, Macromolecules, 2003, 36, 1772–1774 CrossRef CAS; (b) A. Takasu, Y. Iio, Y. Oishi, Y. Narukawa and T. Hirabayashi, Macromolecules, 2005, 38, 1048–1050 CrossRef CAS; (c) A. Takasu, A. Takemoto and T. Hirabayashi, Biomacromolecules, 2006, 7, 6–9 CrossRef CAS PubMed; (d) A. Takasu, Y. Shibata, Y. Narukawa and T. Hirabayashi, Macromolecules, 2007, 40, 151–153 CrossRef CAS; (e) A. Takasu, T. Makino and S. Yamada, Macromolecules, 2010, 43, 144–149 CrossRef CAS; (f) K. Yamamoto and A. Takasu, Macromolecules, 2010, 43, 8519–8523 CrossRef CAS.
- (a) M. Oshimura, T. Tang and A. Takasu, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 1210–1218 CrossRef CAS; (b) T. Moyori, T. Tang and A. Takasu, Biomacromolecules, 2012, 13, 1240–1243 CrossRef CAS PubMed; (c) T. Tang, M. Oshimura, S. Yamada, A. Takasu, X. P. Yang and Q. Cai, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 3171–3183 CrossRef CAS; (d) T. Tang, T. Moyori and A. Takasu, Macromolecules, 2013, 46, 5464–5472 CrossRef CAS.
- J. R. Millar, J. Chem. Soc., 1960, 1311–1317 RSC.
- (a) N. Gupta and A. K. Srivastava, Polym. Int., 1994, 35, 109–119 CrossRef CAS; (b) L. H. Sperling and V. Mishra, Polym. Adv. Technol., 1996, 7, 197–208 CrossRef CAS; (c) K. C. Frisch and D. Klempner, Polym. Eng. Sci., 1982, 22, 1143–1152 CrossRef CAS; (d) Y. S. Yang and L. J. Lee, Macromolecules, 1987, 20, 1490–1495 CrossRef CAS; (e) N. Devia, J. A. Manson and L. H. Sperling, Macromolecules, 1979, 12, 360–369 CrossRef CAS; (f) X. H. Kong and S. S. Narine, Biomacromolecules, 2008, 9, 2221–2229 CrossRef CAS PubMed; (g) M. Worzakowska, J. Mater. Sci., 2009, 44, 4069–4077 CrossRef CAS PubMed.
- D. Myung, W. Koh, J. Ko, Y. Hu, M. Carrasco, J. Noolandi, C. N. Ta and C. W. Frank, Polymer, 2007, 48, 5376–5387 CrossRef CAS PubMed.
- L. Weng, A. Gouldstone, Y. Wu and W. Chen, Biomaterials, 2008, 29, 2153–2163 CrossRef CAS PubMed.
- (a) J. P. Gong, Soft Matter, 2010, 6, 2583–2590 RSC; (b) J. P. Gong, Y. Katsuyama, T. Kurokawa and Y. Osada, Adv. Mater., 2003, 15, 1155–1158 CrossRef CAS; (c) Y. H. Na, Y. Tanaka, Y. Kawauchi, H. Furukawa, T. Sumiyoshi, J. P. Gong and Y. Osada, Macromolecules, 2006, 39, 4641–4645 CrossRef CAS; (d) T. Nakajima, H. Furukawa, Y. Tanaka, T. Kurokawa, Y. Osada and J. P. Gong, Macromolecules, 2009, 42, 2184–2189 CrossRef CAS.
- Q. Chen, L. Zhu, C. Zhao, Q. M. Wang and J. Zheng, Adv. Mater., 2013, 25, 4171–4176 CrossRef CAS PubMed.
- N. Sokoloff and P. Latschinoff, Ber. Dtsch. Chem. Ges., 1874, 7, 1384–1387 CrossRef.
- T. C. Wabnitz, J. Q. Yu and J. B. Spencer, Chem.–Eur. J., 2004, 10, 484–493 CrossRef CAS PubMed.
- (a) D. Wang, Y. Liu, Y. Z. C. Hu, C. Y. Hong and C. Y. Pang, Polymer, 2005, 46, 3507–3514 CrossRef CAS PubMed; (b) B. D. Mather, K. Viswanathan, K. M. Miller and T. E. Long, Prog. Polym. Sci., 2006, 31, 487–531 CrossRef CAS PubMed.
- (a) Enone: T. Imamoto and T. Mukaiyama, Chem. Lett., 1980, 45–46 CrossRef CAS; (b) α,β-unsaturated N-acyloxazolidinone: A. W. Hird and A. H. Hoveyda, Angew. Chem., Int. Ed., 2003, 42, 1276–1279 CrossRef CAS PubMed; (c) α,β-unsaturated esters: S.-Y. Wang, S.-J. Ji and T.-P. Loh, J. Am. Chem. Soc., 2007, 129, 276–277 CrossRef CAS PubMed; (d) M. Vuagnoux-d'Augustin and A. Alexakis, Eur. J. Org. Chem., 2007, 5852–5860 CrossRef; (e) M. G. Banwell, C. T. Bui, H. T. T. Pham and G. W. Simpson, J. Chem. Soc., Perkin Trans. 1, 1996, 967–969 RSC.
- G. Bosica and A. J. Debono, Tetrahedron, 2014, 70, 6607–6612 CrossRef CAS PubMed.
- A. M. Diciccio and G. W. Coates, J. Am. Chem. Soc., 2011, 133, 10724–10727 CrossRef CAS PubMed.
- F. Felluga, G. Pitacco, M. Prodan, S. Pricl, M. Visintina and E. Valentina, Tetrahedron: Asymmetry, 2001, 12, 3241–3249 CrossRef CAS.
- (a) K. Nozaki, J. Am. Chem. Soc., 1941, 63, 2681–2683 CrossRef CAS; (b) Z. Rappoport, C. Degani and S. Patai, J. Chem. Soc., 1963, 4513 RSC; (c) A. G. Cook, A. B. Voges and A. E. Kammrath, Tetrahedron Lett., 2001, 42, 7349–7352 CrossRef CAS.
- P. C. Sadek, The HPLC Solvent Guide, John Wiley & Sons, Inc., 2nd edn, 2002, p. 664 Search PubMed.
- (a) R. J. Cregge, J. L. Herrmann, J. E. Richman, R. F. Romanent and R. H. Schlessinger, Tetrahedron Lett., 1973, 14, 2595–2598 CrossRef; (b) J. L. Herrmann, J. E. Richman and R. H. Schlessinger, Tetrahedron Lett., 1973, 14, 2599–2602 CrossRef; (c) R. J. Cregge, J. L. Herrmann and R. H. Schlessinger, Tetrahedron Lett., 1973, 14, 2603–2606 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra13020k |
| This journal is © The Royal Society of Chemistry 2015 |