HPLC–ICP-MS analysis of selenium speciation in selenium-enriched Cordyceps militaris
Abstract
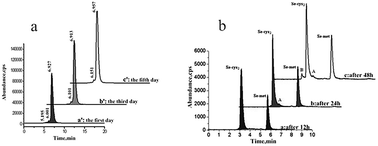
This study examined the distribution of selenium in proteins from selenium-enriched Cordyceps militaris extract using reversed-phase high performance liquid chromatography (RP-HPLC) and size-exclusion chromatography (SEC) coupled to on-line ICP-MS specific for selenium detection. An analytical method was developed for detecting the presence of selenocysteine, selenomethionine and selenopeptides. The results showed that soluble selenium was the major form of selenium in selenium-enriched C. militaris extract. About 84.4% of the selenium was present as low-molecular-weight molecules (MW < 4 kDa), whereas 7.13% of the selenium was present in the form of high-molecular-weight molecules, probably as selenoproteins. Furthermore, analysis of acid-hydrolyzed C. militaris extract revealed a higher proportion of Se-Cys2 than Se-Met (two main selenium-conjugated amino acids) in the extract, although various forms of smaller selenium-proteins or selenium-peptides were also present in low but detectable amounts. The present analysis of selenium thus combined different chromatographic techniques for efficient and rapid separation of selenoproteins and other seleno-conjugated molecules.

 Please wait while we load your content...
Please wait while we load your content...